Abstract
Premature removal of the fixator after a lengthening procedure can result in gradual bending or acute fracture of the regenerate. We reviewed the records of 26 patients who underwent 28 limb lengthenings between 1997 and 2005 to assess the post lengthening regenerate fracture rate and bone healing index when using dual energy xray absorptiometry (DEXA) to aid in deciding on when to remove the fixator. Sixteen male and 10 female patients with an average age at lengthening of 12.3 years underwent an average lengthening of 5.2 cm (range, 3–9.1 cm). Nineteen femurs and nine tibiae were lengthened. Serial monthly DEXA scans were analyzed for bone mineral density. Bone healing indices and post fixator removal complications were assessed. The fixators were removed once the bone mineral density had plateaued to a less than 10% increase and plain radiographs showed no obvious defects precluding fixator removal. There were no regenerate fractures and only one fracture in the proximal segment of the lengthened bone after apparatus removal and the healing index for the series averaged 47 d/cm (range, 20–73 d/cm). Using serial DEXA scans during the consolidation phase of lengthening has a low rate (3.6%) of fractures while maintaining an acceptable bone healing index without excessively increasing fixation time.
Level of Evidence: Level IV, therapeutic retrospective study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bone lengthening has been used since the early 1900s with Codvilla [7] reporting the first lengthening procedure in 1905 (see The Classic in this issue). In the 1950s Ilizarov [16] advanced the principles of distraction osteogenesis. This technique currently is used worldwide in the treatment of limb-length discrepancies and deformities as well as in the regeneration of large segmental bony defects in cases of trauma, infection, and tumor [5]. Although distraction osteogenesis yields satisfactory results, premature removal of the fixator can result in plastic deformity or acute fracture of the regenerate [5]. As such, an adequate regenerate must be present prior to apparatus removal.
A fixator in place for extended periods is not only a considerable psychological burden on the patient [10] but also can lead to persistent pain [15], increased risk of pin tract infections [32], and osteopenia [6]. The standard method to monitor regenerate bone formation and to determine when to remove the fixator is based on orthogonal radiographs and the formation of cortices in the region of the regenerate. It is generally accepted that three to four cortices must form before removal of the fixator [14, 21]. However, this method of plain radiographic determination has poor interobserver and intraobserver reliability [30]. Fractures through the regenerate reportedly range from 3% to 50% [2, 8, 9, 14, 20, 21]. Of note, fractures through the regenerate may not necessarily be due to early removal of the fixator but instead due to poor quality of the regenerate bone that may or may not respond to extended fixation.
Several methods of assessing the quality of regenerate bone have been reported including computed tomography [31], bone scintigraphy [11, 17], and magnetic resonance [31]; however, these modalities are not used on a routine basis. Dual energy xray absorptiometry (DEXA) is a scanning technique used to determine bone mineral density (BMD) and bone mineral content (BMC). One animal study suggests bone mineral density directly correlates to biomechanical strength [23] and DEXA scans have been proposed as useful tools in assessing the regenerate quality during the distraction phase of lengthening in humans [12, 13, 18, 24]. The question of whether or not DEXA could be useful during the consolidation phase still remains.
We therefore assessed the regenerate fracture rate when DEXA scanning was used during the consolidation phase to determine the timing of fixator removal. We also assessed the bone healing index and the degree of corticalization of the regenerate at the time of fixator removal based on DEXA.
Materials and Methods
We retrospectively reviewed all 30 patients undergoing 34 distraction osteogenesis lengthenings between 1997 and 2005 with an external ring fixator or uniplanar apparatus. Patients with congenital pseudarthrosis of the tibia were excluded. We identified 30 patients undergoing 34 lengthenings. Two patients were excluded due to insufficient densitometry and radiographic data availability. One patient who underwent four lengthenings was not fully followed with DEXA data for two of the lengthenings; therefore, these two lengthenings were excluded. An additional two were excluded for failure of regenerate formation. Thus, we reviewed the remaining 26 patients (28 lengthening procedures). The average age was 12.3 years (range, 3–20 years). There were 10 female and 16 male patients. Nineteen femurs (Table 1) and nine tibiae (Table 2) were lengthened. One male patient underwent two lengthenings for a traumatic distal femoral physeal arrest. One female patient underwent bilateral femoral lengthening for hypochondroplasia (Table 3).
Table 1.
Data on femoral lengthening patients
| Case # | Diagnosis | Age (years) | Distraction gap (cm) | BHI (days/cm) | Number of cortices at removal |
|---|---|---|---|---|---|
| 1 | Congenital short femur | 7.7 | 4.5 | 65 | 2 |
| 2 | Infectious physeal arrest | 20.1 | 4.0 | 73 | N/A |
| 3 | Legg-Calve Perthes | 18.3 | 4.2 | 63 | N/A |
| 4 | Hemihypertrophy | 12.1 | 4.7 | 41 | 2 |
| 5 | Traumatic physeal arrest | 10.2 | 4.0 | 39 | 1 |
| 6 | Hypochondroplasia | 13.3 | 7.4 | 45 | 1 |
| 7 | Hypochondroplasia | 13.3 | 7.1 | 51 | 0 |
| 8 | Traumatic physeal arrest | 18.9 | 5.3 | 52 | N/A |
| 9 | Traumatic physeal arrest | 15.9 | 5.0 | 39 | N/A |
| 10 | Congenital short femur | 13.2 | 5.9 | 52 | 1 |
| 11 | Traumatic physeal arrest | 15.5 | 5.3 | 54 | 0 |
| 12 | Traumatic physeal arrest | 15.0 | 6.0 | 39 | 0 |
| 13 | Congenital short femur | 4.9 | 4.1 | 59 | 2 |
| 14 | Congenital short femur | 16.8 | 6.5 | 37 | 1 |
| 15 | Spondyloepiphyseal dysplasia | 6.0 | 7.5 | 29 | 0 |
| 16 | Infectious physeal arrest | 11.8 | 9.0 | 22 | 1 |
| 17 | Amniotic band syndrome | 5.7 | 4.0 | 39 | 2 |
| 18 | Congenital short femur | 7.2 | 5.0 | 48 | 2 |
| 19 | Congenital short femur | 17.6 | 5.8 | 48 | 1 |
| Averages | 12.9 | 5.5 | 47 |
BHI = bone healing index.
Table 2.
Data on tibial lengthening patients
| Case # | Diagnosis | Age (years) | Distraction Gap (cm) | BHI (days/cm) | Number of cortices at removal |
|---|---|---|---|---|---|
| 1 | Posteromedial tibial bow | 8.6 | 4.6 | 48 | 2 |
| 2 | Traumatic physeal arrest | 17.4 | 3.6 | 61 | 0 |
| 3 | Fibular hemimelia | 3.5 | 3.0 | 54 | 3 |
| 4 | Fibular hemimelia | 8.2 | 4.5 | 40 | 2 |
| 5 | Fibular hemimelia | 4.0 | 3.7 | 44 | N/A |
| 6 | Tibial hemimelia | 15.5 | 4.3 | 67 | 2 |
| 7 | Hemihypertrophy | 16.0 | 3.6 | 46 | 2 |
| 8 | Traumatic bone loss | 13.7 | 9.1 | 20 | 2 |
| 9 | Spondyloepiphyseal dysplasia | 12.9 | 5.4 | 49 | 1 |
| Averages | 11.1 | 4.6 | 47.6 |
BHI = bone healing index.
Table 3.
Diagnoses
| Diagnosis | Number of patients | Number of femurs | Number of tibiae |
|---|---|---|---|
| Congenital short femur | 6 | 6 | |
| Fibular hemimelia | 3 | 3 | |
| Posttraumatic growth arrest | 5 | 5 | 1 |
| Posttraumatic segmental defect | 1 | 1 | |
| Infectious physeal arrest | 2 | 2 | |
| Spondyloepiphyseal dysplasia | 2 | 1 | 1 |
| Posteromedial tibial bow | 1 | 1 | |
| Tibial hemimelia | 1 | 1 | |
| Hemihypertrophy | 2 | 1 | 1 |
| Hypochondroplasia | 1 | 2 | |
| Amniotic band syndrome | 1 | 1 | |
| Legg-Calve-Perthes | 1 | 1 | |
| Total | 26 | 19 | 9 |
All surgeries were performed by the senior author (RCH). Patients with multiplanar deformities or deformities in the tibia underwent application of an Ilizarov fixator (Smith & Nephew Inc, Memphis, TN) and the rest underwent application of an Orthofix fixator (LRS Limb Reconstruction System; Orthofix SRL, Verona, Italy). Low-energy subperiosteal osteotomies were performed in all cases. Three femoral and nine tibial Ilizarov distractors and 16 femoral Orthofix distracters were used.
Patients were encouraged to weightbear starting on postoperative day two. Patients undergoing tibial lengthenings were fitted with a modified ankle-foot orthosis for nighttime use to prevent hindfoot equinus. We began distraction at a rate of 0.25 mm every 6 hours 5–7 days after surgery.
All patients followed a standardized physical therapy regimen. Patients were seen by a physiotherapist on postoperative day two and given active and passive range of motion exercises to be performed daily. The patients were seen by our outpatient physiotherapy team on a weekly basis to monitor range of motion and to encourage home exercise routines. Two weeks after the surgery patients underwent a weekly pool-based physical therapy session.
All patients were followed with standard AP and lateral radiographs weekly during the distraction phase to assess the amount lengthened and quality of the regenerate and monthly during the consolidation phase for regenerate progression and consolidation. Both authors (RCH, NS) analyzed plain radiographs for number of cortices on AP and lateral radiographs as well as for percentage of bony bridging. Full radiographic analysis for the number of cortices of the regenerate was available in only 23 of the 28 lengthenings. Radiographs were not available for 5 of the earlier patients as these had been lost in the archiving process. The number of cortices formed on orthogonal radiographs (Tables 1, 2) as well as the percentage of bony bridging was calculated.
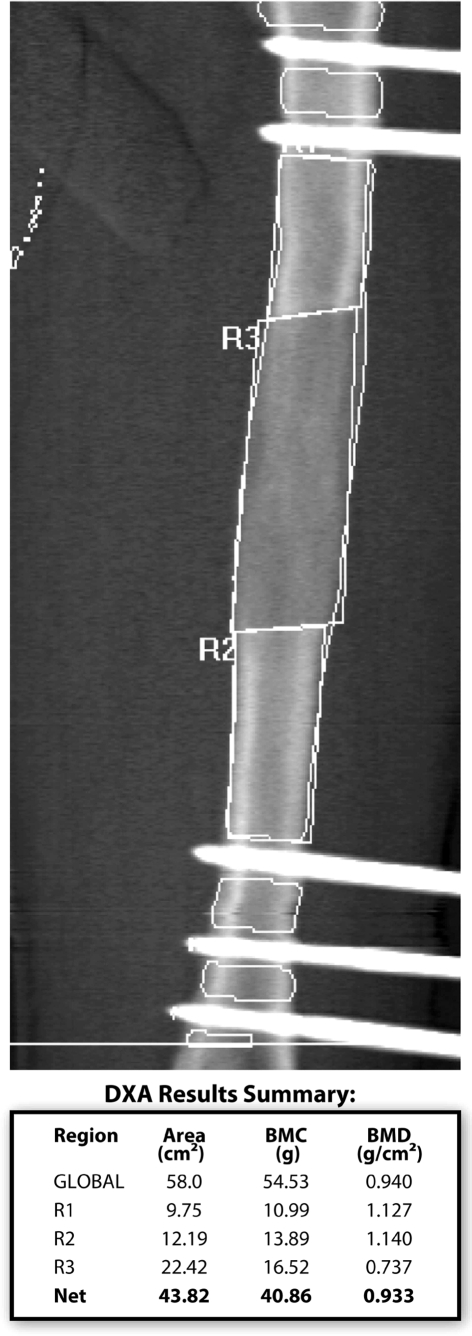
We obtained DEXA scans (Hologic QDR 4500 BMD machine with Discovery A version 12.01:03 software, Hologic Inc, Bedford, MA) at monthly intervals during the consolidation phase. The BMC was measured for the entire regenerate and a BMD was calculated from this measurement (Fig. 1). The change in BMD was followed monthly. A minimum of three data points was required starting with the first scan once the distraction phase was complete. Subsequent scans were taken monthly. Once the increase in BMD between monthly DEXA scans had plateaued to less than 10%, the fixator was removed provided the plain radiographs did not show any major deficiencies or transverse lucencies in the regenerate bone. Dynamization of the fixator was not part of this routine prior to removal of the fixator.
Fig. 1.
A dual energy xray absorptiometry scan shows the areas extrapolated and bone mineral content and density values.
The bone healing index (BHI) was calculated by the number of days the fixator was on per centimeter of lengthening.
Results
There were no fractures of the regenerate in this study; however, there was one fracture in an adjacent segment of the lengthened bone. This patient fractured through one of the proximal half pin holes one day after removal of a femoral unilateral external fixator. The patient was treated with a hip spica for 10 weeks with no long-term effects. None of the patients fractured or deformed through the regenerate after removal of the fixator despite being allowed to fully weight bear immediately after fixator removal without cast or brace.
The average bone healing index was 47 days/cm (range, 20–73 days/cm). The average distraction length was 5.2 cm (range, 3–9.1 cm). The average time to removal of the fixator once BMD had plateaued was 3.4 weeks (range, 0–10 weeks). On average, six (range, 3–11) DEXA scans were obtained during the consolidation period.
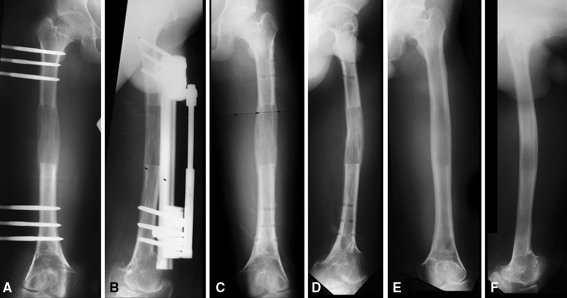
No cases had corticalization of all four cortices of the regenerate bone. On average there were 1.3 cortices (range, 0–3 cortices) at time of removal. The average percentage of bony bridging in the distracted zone was 93% (range, 70–100%). There were five cases with no cortex, seven with one cortex, 10 with two cortices, and only one with three cortices. There was one case in which there were no cortices at the time of removal but by the 1-year followup there was full corticalization without sequelae (Fig. 2A–F).
Fig. 2A–F.
The following are radiographs of a patient showing no cortices at the time of fixator removal. These include anteroposterior (A) and lateral (B) radiographs taken immediately before removal of the fixator, anteroposterior (C) and lateral (D) radiographs taken immediately following removal of the fixator, and anteroposterior (E) and lateral (F) radiographs taken 12 months after removal of fixator showing no cortices at the time of removal of the fixator and full recorticalization of the regenerate at followup without plastic deformity or fracture through the lengthened segment.
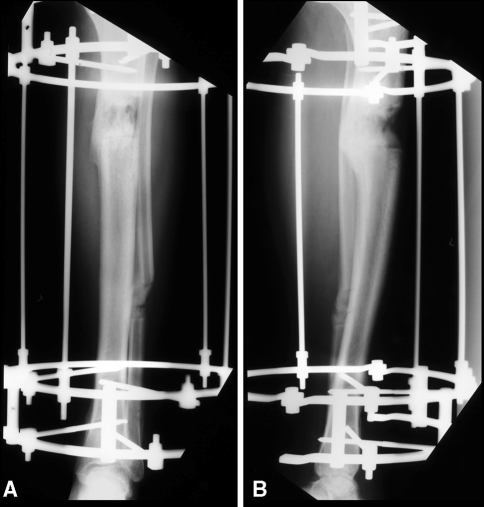
All patients had their external fixators removed once the BMD plateaued except one. Patient 7 (Table 2) had the frame removed while the BMD was still increasing. In that case, it was decided to remove the fixator because radiographs (Fig. 3) revealed sufficient bony bridging.
Fig. 3A–B.
The following anteroposterior (A) and lateral (B) radiographs are of a patient whose fixator was removed based on adequate bridging despite increasing bone mineral density.
There were three major complications apart from the fracture discussed above. One patient who underwent tibial lengthening for fibular hemimelia who previously had a Syme’s amputation underwent a lengthening of her stump and developed a knee subluxation that after multiple reconstructive efforts went on to a knee disarticulation. There was one patient with residual coronal plane deformity of 15° of valgus at the tibial osteotomy site that did not change after removal of the fixator. One patient who underwent tibial lengthening developed a premature bony bridge requiring a revision osteotomy. Another patient who underwent a tibial lengthening sustained an ipsilateral femur fracture from a major fall two weeks after removal of the fixator. She was treated with a hip spica for 6 weeks followed by a long-leg cast for 3 weeks before being allowed to resume full weight bearing. There were no serious pin site infections in our series requiring operative intervention.
Discussion
Distraction osteogenesis has become a universally accepted method of treating leg-length inequality and segmental bone loss secondary to trauma, infection, and tumor. Although many advances have been made there still remain many problems with this procedure. One difficulty is determining when to remove the fixator. Although early removal of the fixator is ideal for the patient, family, and medical caregivers, it may lead to deformity or fracture at the lengthened site if the biomechanical properties of the regenerate are inadequate. The purposes of this study were to assess the fracture rate of the regenerate and the bone healing index, and determine the number of cortices present on radiographs at the time of fixator removal when DEXA is used to aid in the decision making process of fixator removal.
The major limitation of this study is that there is no control group for comparison and therefore it is difficult to make a firm conclusion on how DEXA influences the time in the frame or the rate of complications. However, the parameters of regenerate fractures and bone healing indices have been reported in multiple other studies [2–5, 10, 20, 29, 32] and therefore adequate historical controls are available. Also, assessing the number of cortices formed at the time of fixator removal in a way acts as an internal control: if the number of cortices is less than three then this indicates that our bone healing index would have been higher had we waited for adequate cortices to form based on the usual criteria for fixator removal. Second, serial DEXA scans are required on regular intervals. The ideal method would be a one time DEXA that compares the bone density to either preoperative or contralateral segments; however, such a model has not yet been worked out. Third, we made an attempt to report the minor complications; however, because this was a retrospective review, it was very difficult to ascertain the actual number of minor complications especially for pin tract infections.
The decision to remove the fixator is generally based on plain radiography and clinical examination at the time of removal of the fixator; however, using these criteria fracture rates of 30–50% [2, 8] have been reported although rates of 10–15% [5, 20] are more common. Using DEXA to determine when to remove the fixator provides an objective tool in making this decision. In our series of 28 lengthenings using DEXA as an aid to determine when to remove the fixator, there were no fractures or deformities in the regenerate zone and only one in an adjacent bone segment resulting in a fracture rate of 3.6%. One animal study examining biomechanical regenerate strength after distraction osteogenesis in sheep suggested a direct correlation with BMD using DEXA [23]. Although several other radiological techniques have been described to assess the regenerate [11, 17, 31], none are used on a routine basis. The ideal in vivo technique in assessing the mechanical strength of the regenerate bone remains elusive. A recent paper studied the use of in vivo regenerate axial stiffness testing after limb lengthening to determine when to remove the fixator [1]. This may prove a valuable tool in decreasing post fixator removal fracture rates; however, this technique only tests axial strength of the regenerate and not torsional or bending strengths. Other methods of lengthening have also been developed to avoid such problems with external fixators. One method is lengthening over a nail to prevent this type of complication [22, 25, 27]. Aside from risks of infection, in many limb lengthening cases, the insertion of a nail or correction of a deformity over a nail is often precluded by coronal or sagittal plane deformity.
The extended period of external fixation is perhaps the most difficult part of the lengthening procedure for the patient [10]. A shorter consolidation phase is ideal; however, early removal of the fixator increases the risk of regenerate fractures. Currently, there is no objective way to determine when the regenerate is strong enough to tolerate fixator removal without resulting in plastic deformation or acute fracture of the lengthened segment. Our lengthening index of 47 d/cm appears to be at the upper limit of the range of healing indices seen in the literature for bone lengthening in children. Noonan et al. [20] reported a healing index of 24 d/cm in femoral lengthenings on average of 11 cm and 32 d/cm in tibial lengthenings on average of 9 cm. Our healing index was closer to that seen by Aldegheri [3] who reported a healing index of 44 d/cm with an average lengthening of 6.6 cm. Our healing index may in part be due to our relatively shorter average lengthening of 5.2 cm as healing indices decrease as the amount lengthened increases [9, 14]. Also important to note is that in our study there was no postoperative immobilization or protected weightbearing; whereas, in the study by Noonan et al. [20], 36% of femoral lengthening patients were protected for an average of 48 days and 61% of tibial lengthening patients were protected for an average of 37 days in above-knee casts. Another explanation for our slightly high healing index could be a function of using the DEXA to determine timing of fixator removal. We speculate the DEXA results encouraged us to persist with frames in certain cases that were at risk of fracturing, and by doing so our fracture rate decreased but our healing index increased. A low healing index by itself, however, can not be considered a success. The benefits of early removal of the fixator need to be weighed against its potential pitfalls. The goal is to optimize the bone healing index such that the regenerate is biomechanically strong enough to withstand fixator removal.
We did not follow the standard guidelines of removing the fixator only once there was formation of three or four cortices in the regenerate bone [14, 21]. Two studies suggest there are less post fixator removal fractures or regenerative deformity when radiographs show the development of adequate cortices [14, 21] and there is stability in the operating room under manual stress when the fixation is uncoupled [4]. Also, in femur fractures treated with external fixation, refracture rates correlate with the number of cortices seen on orthogonal radiographs [28]. With an average of 1.3 cortices formed at the time of fixator removal we observed no postoperative fractures. This suggests there is more to the biomechanical strength of the regenerate than primarily the number of cortices. This further strengthens the need for an imaging modality such as the DEXA that assesses bone density and therefore indirectly assesses mechanical strength of the regenerate bone. This assessment of the number of cortices formed prior to fixator removal acts as an internal control in assessing our bone healing index. If the frames were left on until a minimum of three cortices were formed, it is very likely that our bone healing index could have been higher than 47 d/cm. Though the assessment of cortices on radiographs is subject to substantial variability [30], we believe it is imperative to follow plain radiographs during the consolidation phase and not rely solely on bone mineral density. There are cases in which a regenerate fails to progress as well as cases in which there are substantial regenerate cysts, transverse lucencies in the distracted zone or cortical fractures. If this occurs and only bone mineral density is followed, the fixator will likely be removed prematurely; therefore, DEXA cannot be used as the sole method in determining when to remove the fixator but rather in combination with plain radiographs to optimize timing of fixator removal.
DEXA scanning is a relatively safe procedure with low radiation exposure. The radiation exposure for fan beam DEXA scanners such as the Hologic QDR 4500 BMD Scanner have been measured to be comparable to posteroanterior chest xray [19] with an effective dose of 17uSv[26] or approximately two days of background radiation.
Using DEXA in conjunction with plain radiography to determine when the regenerate BMD plateaued resulted in a post fixator removal regenerate fracture and deformity rate of 0% and a fracture rate of 3.6% in the lengthened bone despite encouraging all patients to weight bear as tolerated without cast or splint immobilization. With an acceptable bone healing index of 47 d/cm, no fractures or bending of the regenerate and a low fracture rate of 3.6% in adjacent segments of the lengthened bone, we believe DEXA is a useful adjunct in deciding on when to remove the fixator.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved or waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aarnes GT, Steen H, Ludvigsen P, Waanders NA, Huiskes R, Goldstein SA. In vivo assessment of regenerate axial stiffness in distraction osteogenesis. J Orthop Res. 2005;23:494–498. [DOI] [PubMed]
- 2.Abe M, Shirai H, Okamoto M, Onomura T. Lengthening of the forearm by callus distraction. J Hand Surg [Br]. 1996;21:151–163. [DOI] [PubMed]
- 3.Aldegheri R. Distraction osteogenesis for lengthening of the tibia in patients who have limb-length discrepancy or short stature. J Bone Joint Surg Am. 1999;81:624–634. [DOI] [PubMed]
- 4.Antoci V, Ono CM, Antoci V, Jr, Raney EM. Bone lengthening in children: how to predict the complications rate and complexity? J Pediatr Orthop. 2006;26:634–640. [DOI] [PubMed]
- 5.Birch JG, Samchukov ML. Use of the Ilizarov method to correct lower limb deformities in children and adolescents. J Am Acad Orthop Surg. 2004;12:144–154. [DOI] [PubMed]
- 6.Cattermole HC, Cook JE, Fordham JN, Muckle DS, Cunningham JL. Bone mineral changes during tibial fracture healing. Clin Orthop Relat Res. 1997;339:190–196. [DOI] [PubMed]
- 7.Codivilla A. On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. 1905. J Bone Joint Surg Am. 1905;s2–2:353–369.
- 8.Danziger MB, Kumar A, DeWeese J. Fractures after femoral lengthening using the Ilizarov method. J Pediatr Orthop. 1995;15:220–223. [PubMed]
- 9.Dinah AF. Predicting duration of Ilizarov frame treatment for tibial lengthening. Bone. 2004;34:845–848. [DOI] [PubMed]
- 10.Eldridge JC, Bell DF. Problems with substantial limb lengthening. Orthop Clin North Am. 1991;22:625–631. [PubMed]
- 11.Eski M, Ilgan S, Cil Y, Sengezer M, Ozcan A, Yapici K. Assessment of distraction regenerate using quantitative bone scintigraphy. Ann Plast Surg. 2007;58:328–334. [DOI] [PubMed]
- 12.Eyres KS, Bell MJ, Kanis JA. Methods of assessing new bone formation during limb lengthening. Ultrasonography, dual energy X-ray absorptiometry and radiography compared. J Bone Joint Surg Br. 1993;75:358–364. [DOI] [PubMed]
- 13.Eyres KS, Bell MJ, Kanis JA. New bone formation during leg lengthening. Evaluated by dual energy X-ray absorptiometry. J Bone Joint Surg Br. 1993;75:96–106. [DOI] [PubMed]
- 14.Fischgrund J, Paley D, Suter C. Variables affecting time to bone healing during limb lengthening. Clin Orthop Relat Res. 1994;301:31–37. [PubMed]
- 15.Garcia-Cimbrelo E, Olsen B, Ruiz-Yague M, Fernandez-Baillo N, Munuera-Martinez L. Ilizarov technique. Results and difficulties. Clin Orthop Relat Res. 1992;283:116–123. [PubMed]
- 16.Ilizarov GA. Clinical application of the tension-stress effect for limb lengthening. Clin Orthop Relat Res. 1990;250:8–26. [PubMed]
- 17.Kawano M, Taki J, Tsuchiya H, Tomita K, Tonami N. Predicting the outcome of distraction osteogenesis by 3-phase bone scintigraphy. J Nucl Med. 2003;44:369–374. [PubMed]
- 18.Maffulli N, Cheng JC, Sher A, Lam TP. Dual-energy X-ray absorptiometry predicts bone formation in lower limb callotasis lengthening. Ann R Coll Surg Engl. 1997;79:250–256. [PMC free article] [PubMed]
- 19.Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK. Radiation exposure in bone mineral density assessment. Appl Radiat Isot. 1999;50:215–236. [DOI] [PubMed]
- 20.Noonan KJ, Leyes M, Forriol F, Canadell J. Distraction osteogenesis of the lower extremity with use of monolateral external fixation. A study of two hundred and sixty-one femora and tibiae. J Bone Joint Surg Am. 1998;80:793–806. [DOI] [PubMed]
- 21.Paley D. Problems, obstacles, and complications of limb lengthening by the Ilizarov technique. Clin Orthop Relat Res. 1990;250:81–104. [PubMed]
- 22.Paley D, Herzenberg JE, Paremain G, Bhave A. Femoral lengthening over an intramedullary nail. A matched-case comparison with Ilizarov femoral lengthening. J Bone Joint Surg Am. 1997;79:1464–1480. [DOI] [PubMed]
- 23.Reichel H, Lebek S, Alter C, Hein W. Biomechanical and densitometric bone properties after callus distraction in sheep. Clin Orthop Relat Res. 1998;357:237–246. [DOI] [PubMed]
- 24.Reiter A, Sabo D, Pfeil J, Cotta H. Quantitative assessment of callus distraction using dual energy X-ray absorptiometry. Int Orthop. 1997;21:35–40. [DOI] [PMC free article] [PubMed]
- 25.Saraph V, Roposch A, Zwick E-B, Linhart WE. Tibial lengthening over nails in children using modified Ender nails: preliminary results of a new treatment. J Pediatr Orthop B. 2004;13:383–388. [DOI] [PubMed]
- 26.Shrimpton PC, Wall BF, Hart D. Diagnostic medical exposures in the U.K. Appl Radiat Isot. 1999;50:261–269. [DOI] [PubMed]
- 27.Simpson AH, Cole AS, Kenwright J. Leg lengthening over an intramedullary nail. J Bone Joint Surg Br. 1999;81:1041–1045. [DOI] [PubMed]
- 28.Skaggs DL, Leet AI, Money MD, Shaw BA, Hale JM, Tolo VT. Secondary fractures associated with external fixation in pediatric femur fractures. J Pediatr Orthop. 1999;19:582–586. [DOI] [PubMed]
- 29.Stanitski DF, Shahcheraghi H, Nicker DA, Armstrong PF. Results of tibial lengthening with the Ilizarov technique. J Pediatr Orthop. 1996;16:168–172. [DOI] [PubMed]
- 30.Starr KA, Fillman R, Raney EM. Reliability of radiographic assessment of distraction osteogenesis site. J Pediatr Orthop. 2004;24:26–29. [DOI] [PubMed]
- 31.Tjernstrom B, Thoumas KA, Pech P. Bone remodeling after leg lengthening: evaluation with plain radiographs, and computed tomography and magnetic resonance imaging scans. J Pediatr Orthop. 1992;12:751–755. [PubMed]
- 32.Velazquez RJ, Bell DF, Armstrong PF, Babyn P, Tibshirani R. Complications of use of the Ilizarov technique in the correction of limb deformities in children. J Bone Joint Surg Am. 1993;75:1148–1156. [DOI] [PubMed]