Abstract
Unplanned excisions of soft tissue sarcomas occur with alarming frequency and result in high rates of residual disease, potentially affecting patient prognosis. To determine if unplanned excisions and residual disease status at tumor bed excision increased local recurrence rates and predicted disease-specific patient survival, we retrospectively reviewed 203 consecutive patients with high-grade soft tissue sarcomas treated operatively and followed for at least 2 years (mean, 4.8 years) or until patient death. Among the 64 patients (32%) who had undergone previous unplanned excisions, six had gross residual disease and 40 of the remaining 58 (69%) had microscopic residual disease in the tumor bed. We observed subsequent local recurrence in nine of the 139 patients (6%) after planned excision compared with 22 patients (34%) after unplanned excision. More patients with unplanned excisions who underwent limb salvage procedures required flap coverage and/or skin grafting with their definitive resection (30% versus 5%). In the unplanned excision cohort, residual disease status at tumor bed excision predicted increased rates of local recurrence and decreased disease-specific survival. Unplanned excisions of high-grade soft tissue sarcomas resulted in increased rates of local recurrence but not disease-specific survival. Residual disease at reexcision predicted the likelihood of local recurrence.
Level of Evidence: Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Reported prognostic factors for patient survival from soft tissue sarcomas include tumor stage [1, 33], grade [1, 4, 9, 22, 33], size [1, 4, 9, 22, 33, 35], depth [4], histopathology [1], and site of primary disease [1, 4, 13, 46], whereas the most important factors for local disease control include operative margin [3, 5, 7, 22, 44], grade [1, 5, 45], and radiotherapy treatment [1, 3, 34, 42, 45]. The potential influence of local disease recurrence on overall patient survival remains controversial [1, 3–5, 8, 17, 25, 34–36], but as many as 50% of all patients with high-grade soft tissue sarcomas ultimately will die of the disease [10, 12, 40].
Giuliano and Eilber [14] coined the term “unplanned total excision” in reference to the resection of presumed benign masses without appropriate preoperative imaging, biopsy, or attention to surgical margins. Despite well-publicized management and referral principles and the gravity of these diagnoses, diagnostic and therapeutic errors of soft tissue sarcomas continue, and use of suboptimal preoperative imaging, biopsy techniques, excision planning, operative margins, and adjuvant therapy remain commonplace [2, 3, 7, 11, 14, 15, 17, 18, 20, 23, 24, 27–30, 32, 33, 37, 43]. Numerous authors have described the unreliability of the reported margins after unplanned complete excisions, with residual disease rates at reexcision ranging from 24% to 91% [2, 7, 11, 14, 15, 20, 22, 24, 26, 29, 30, 32, 33, 43]. Consequently, tumor bed excision now is recommended after most cases of unplanned excision. However, relatively few authors have examined management and outcome implications of unplanned excisions beyond local residual disease rates [2, 7, 11, 24, 37].
We hypothesized unplanned excisions of high-grade soft tissue sarcomas would result in an increased risk of subsequent local recurrence with high rates of residual disease and greater reconstructive requirements after tumor bed excision. We also hypothesized residual disease status at tumor bed excision would predict local recurrence risk and disease-specific patient survival (patients succumbing to systemic or local sequelae of soft tissue sarcomas).
Materials and Methods
We retrospectively reviewed the medical records, including radiography and pathology reports, of all 203 patients with high-grade soft tissue sarcomas of the pelvis or extremities treated operatively for their primary tumor at our center between 1989 and 2005 and followed for a minimum of 2 years. To construct a more homogeneous series, we limited our study to high-grade tumors. Because of their proven greater propensity to recur locally and metastasize [1, 2, 4, 9, 22, 24, 25, 33, 44, 45], we believed an analysis of only high-grade tumors would allow us to more easily detect and clearly illustrate the potential effects of unplanned manipulation of these tumors. Exclusion criteria included patients presenting late (greater than 12 months) or treated with radiotherapy alone after unplanned excisions and those presenting with metastatic disease after planned primary treatment elsewhere. Patients were divided into two groups based on whether the initial excision had been appropriately planned and executed or was an unplanned excision of a high-grade soft tissue sarcoma. No patient underwent unplanned excision by our service during the study period; therefore, patients in the unplanned excision group were referrals to our facility after initial surgery.
From our medical records, we obtained patient age at time of surgery, gender, tumor location, size (greatest linear dimension of the resected tumor), depth (subcutaneous or deep to the compartmental fascia), duration of symptoms, histopathologic diagnosis, and American Joint Committee on Cancer stage [16]. Surgical treatment and the use of adjuvant therapy, including chemotherapy and radiotherapy, were analyzed for each patient. Of the 203 patients with high-grade soft tissue sarcomas, 64 (32%) had an unplanned excision before referral (Table 1). There were 113 male and 90 female patients with an average age of 56 years (median, 57; range, 10–90 years). The average duration of symptoms was 10.7 months (median, 5 months; range, 0.5–132 months). The mean time from unplanned excision to presentation was 3.2 months (median, 2 months; range, 0.5–11 months). The American Joint Committee on Cancer stage of disease at presentation to our facility was II in 77 patients, III in 102 patients, and IV in 24 patients. There were no differences between the planned and unplanned excision cohorts regarding age, gender, disease site, histopathologic diagnoses, patients presenting with metastatic (Stage IV) disease, or duration of pretreatment symptoms. All included patients had a minimum 2 years (mean, 4.8 years; range, 2–18 years) postoperative followup unless death supervened. Key outcome variables were local recurrence of disease after definitive treatment, reconstructive requirements after primary resection or tumor bed excision, and disease-specific patient survival. Institutional Review Board approval was granted before study initiation.
Table 1.
Summary data*
| Patient demographics, treatment, and outcomes | All patients | Planned | Unplanned | p Value |
|---|---|---|---|---|
| Number of patients | 203 | 139 (68%) | 64 (32%) | |
| Age (years) | 56 (range, 10–90) | 57 (range, 10–90) | 53 (range, 22–87) | 0.16 |
| Gender | 0.38 | |||
| Male | 113 (56%) | 74 (53%) | 39 (61%) | |
| Female | 90 (44%) | 65 (47%) | 25 (39%) | |
| American Joint Committee on Cancer stage | ||||
| II | 77 (38%) | 45 (32%) | 32 (50%) | |
| III | 102 (50%) | 75 (54%) | 27 (42%) | |
| IV | 24 (12%) | 19 (14%) | 5 (8%) | 0.33 |
| Size† (cm) | 10.7 (SD, 7.2) | 11.6 (SD, 7.7) | 8.9 (SD, 5.4) | 0.02 |
| Duration of symptoms (months) | 5 (range, 0.5–132) | 5 (range, 0.5–132) | 6 (range, 1–120) | 0.42 |
| Depth† | < 0.0001 | |||
| Subcutaneous | 78 (38%) | 35 (25%) | 43 (67%) | |
| Deep | 125 (62%) | 104 (75%) | 21 (33%) | |
| Resection margins | ||||
| Positive | 6 (3%) | 2 (1%) | 4 (6%) | 0.08 |
| Negative (< 1 cm) | 106 (52%) | 86 (62%) | 20(31%) | 0.08 |
| Negative (≥ 1 cm) | 91 (45%) | 51 (37%) | 40 (63%) | < 0.0001 |
| Radiotherapy | 122 (60%) | 81 (58%) | 41 (64%) | 0.53 |
| Chemotherapy† | 107 (53%) | 83 (60%) | 24 (38%) | 0.003 |
| Disease at reexcision | NA | NA | NA | |
| Positive | 40 (63%) | |||
| Negative | 18 (28%) | |||
| Gross | 6 (9%) | |||
| Amputation | 37 (18%) | 29 (21%) | 8 (13%) | 0.22 |
| Flap and/or STSG† for limb salvage procedures | 22 (13%) | 5 (5%) | 17 (30%) | < 0.0001 |
| Local Recurrence† | 31 (15%) | 9 (6%) | 22 (34%) | < 0.0001 |
| Time to local recurrence (months) | 22 (range, 3–175) | 31 (range, 11–175) | 17 (range, 3–117) | 0.12 |
| Postoperative followup† (months) | 58 (range, 24–212) | 63 (range, 24–212) | 48 (range, 24–132) | 0.04 |
| Disease-specific survival | ||||
| Alive, NED | 107 (53%) | 66 (47%) | 41 (64%) | 0.07 |
| AWD | 11 (5%) | 6 (4%) | 5 (8%) | |
| DOD | 71 (35%) | 54 (39%) | 17 (27%) | |
| DOC | 14 (7%) | 13 (9%) | 1 (2%) | |
* Data provided as mean (SD), median (range), or number (percentage), as appropriate; †significant difference between cohorts; STSG = split-thickness skin graft; NED = no evidence of disease; AWD = alive with disease; DOD = died of disease; DOC = died of other causes; SD = standard deviation; NA = not applicable.
A power analysis revealed a sample size of 203 patients (139 in the primary excision/control group and 64 in the unplanned excision group) would provide a 98% power to detect a difference in survival outcomes based on the log rank test, assuming a constant hazard ratio of 2.0 during the followup and a two-sided alpha of 0.05. This means the study had a 98% chance of detecting a twofold increase in the risk of local recurrence or disease-specific mortality between study groups. However, this study had only a 68% power to detect a 50% difference (hazard ratio, 1.5) in these same outcome measures.
The goal of surgical treatment was complete tumor removal with an appropriately wide or radical margin of resection. The decision to perform a limb-sparing procedure versus amputation was determined by the anticipated ability to achieve adequate operative margins while maintaining a functional limb based on lesion depth, size, involvement of critical neurovascular structures, and location and extent of the initial surgery for patients with unplanned excisions. All patients referred after unplanned excisions underwent tumor bed excision: a wide reresection of the region of actual or apparent tumor involvement, including the entire prior operative incision with an adjacent cuff of skin and soft tissue, based on the operative and pathology reports of the unplanned procedure, preoperative physical examination, and preoperative MRI of the region (including areas of unplanned excision hematoma or postoperative edema). Flap and/or skin graft reconstruction was performed whenever primary closure could not be achieved without undue tension, particularly in patients likely to receive radiotherapy or whenever critical neurovascular structures or bone lacked adequate soft tissue coverage after definitive resection. Final operative margins were considered positive if the tumor extended to the inked specimen on any slide and negative if any normal tissue intervened on all slides. Twenty patients (17 in the planned excision and three in the unplanned excision cohorts, respectively) had definitive pathology reports that explicitly reported negative margins but did not provide adequate detail regarding the linear width of the closest margins. These patients were considered to have negative margins less than 1 cm for purposes of data analysis. Patients undergoing tumor bed excision with no residual disease evident were considered to have negative margins of 1 cm or greater.
Because of the broad time span of the study, administration of chemotherapy and radiotherapy was not uniform. Adjuvant therapy treatment decisions were made by the treating surgeon based on recommendations of a multidisciplinary sarcoma conference tumor board. Chemotherapy was administered to 107 patients (53%) and was used with greater frequency (p = 0.003) with planned versus unplanned excisions (60% versus 38%). Patients with larger, deeper tumors or more advanced stages of disease at presentation were more likely to receive chemotherapy. Patients who had greater than 90% tumor necrosis after neoadjuvant chemotherapy had additional postoperative chemotherapy with similar agents, whereas patients with less than 90% tumor necrosis underwent a modified postoperative chemotherapeutic regimen. There were no toxic, chemotherapy-related deaths in either group. Radiotherapy was used in 122 patients (60%), including preoperative radiotherapy in a minority (six of 122), with no difference between treatment groups. Adjuvant radiation therapy also was administered preferentially to patients with larger, deeper tumors and with close wide or wide-contaminated margins or microscopically positive margins (72% of patients with final margins less than 1 cm; 81% of patients with final margins less than 0.5 cm). Radiotherapy generally was not administered to patients undergoing amputation, wide excisions with adequate margins associated with either an excellent response (greater than 95% necrosis) to neoadjuvant chemotherapy [19] or small (4 cm or less), subcutaneous tumors [38, 39, 44], or select patients presenting with widespread metastatic disease in whom chemotherapy was the only practicable chance of extending survival and local recurrence was considered unlikely in the patient’s anticipated remaining life span.
We performed a clinical examination and MRI for surveillance of local tumor recurrence 3 months after surgery and then every 3 to 4 months for 2 years and every 6 months up to Year 5 with annual clinical examinations thereafter. Surveillance for distant metastases included alternate chest radiographs and CT scans every 3 months postoperatively for 5 years and annual chest radiographs thereafter.
Descriptive statistics were analyzed for both cohorts. Differences in potentially confounding continuous variables (tumor size and duration of postoperative followup) between the two cohorts were determined using Student’s t test, after assessing data distribution with quantile-quantile plots and equality of variance with Levene’s test. Otherwise, the Mann-Whitney U test was used for nonnormally distributed data (age, duration of symptoms, operative margins). Differences between rates and proportions of occurrences (patient gender, American Joint Committee on Cancer stage, lesion depth, resection margins, reconstructive requirements, adjuvant treatment, amputation, and actuarial local recurrence rates) were assessed using chi square analysis or Fisher’s exact test, as appropriate. All reported p values are two-tailed. Local recurrence-free and disease-specific patient survival were analyzed with the Kaplan-Meier method [21], with differences in survivorship between cohorts and subgroups (residual disease status at tumor bed excision, margin status, radiotherapy treatment) assessed through the log rank (Mantel-Cox) test [31]. We performed multivariate analyses for factors affecting local recurrence-free and disease-specific survival using stepwise Cox proportional-hazards regression analysis [6]. Statistical analysis was performed using SPSS® Version 15.0 (SPSS Inc, Chicago, IL). Power analysis calculations were determined using the PASS 2008 software package (NCSS, Kaysville, UT).
Results
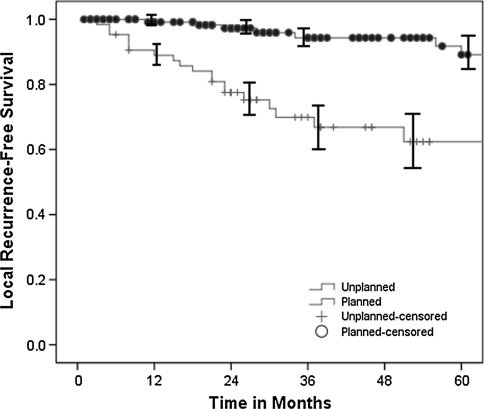
Patients with unplanned excisions had a greater (p < 0.0001) risk of local recurrence than those with planned excisions (34% versus 6%, respectively) despite attempted wide tumor bed excision in all cases and radiotherapy treatment in nearly 2/3 of patients (Table 1). The Kaplan-Meier 5-year local recurrence-free survival estimate (Fig. 1) was 89.7% (95% confidence interval [CI], 83.1%–94.0%) for the planned excision group and 63.7% (95% CI, 50.7%–75.1%) for the unplanned excision group. This difference in local recurrence rates between study cohorts remained when data were stratified by lesion depth and size (0–5 cm, 5–10 cm, and greater than 10 cm). There was no difference (p = 0.12) between time to local recurrence after tumor bed excision versus planned primary excisions (median, 17 months versus 31 months). Of patients with evaluable final operative margins, unplanned excisions undergoing reexcision had a greater (p < 0.0001) proportion of operative margins greater than 1 cm, but there was no difference (p = 0.84) in the width of final operative margins after tumor bed excision versus planned excision (median, 1.2 cm versus 0.7 cm). Likewise, planned and unplanned excisions had similar (p = 0.08) percentages of microscopically positive final margins (1.4% versus 6.3%). Of patients with microscopically positive final margins, two patients who had undergone unplanned initial resections had subsequent local recurrences despite radiotherapy treatment. There was no difference (p = 0.13) in local recurrence rates between patients with margins less than 1 cm and 1 cm or greater. The mean tumor size was greater (p = 0.02) in the planned excision group than in the unplanned group (11.6 cm versus 8.9 cm). Overall, there were 125 deep and 78 superficial soft tissue sarcomas, with a greater proportion (p < 0.0001) of deep tumors in the appropriately planned and performed excision cohort. Accordingly, unplanned excision occurred in 55% of subcutaneous tumors versus only 17% of deep tumors. Limb salvage with wide local or radical excision was performed in 166 patients, and 37 patients (18.2%) underwent amputation, with no difference in amputation rate based on the status (planned versus unplanned) of the initial resection. More (p < 0.0001) patients with prior unplanned excisions and undergoing limb salvage procedures required flap coverage and/or split-thickness skin grafting concomitant with their definitive resection (30% versus 5%). Planned initial resection (relative risk, 0.10; p < 0.0001), final operative margins of 1 cm or greater (relative risk, 0.30; p = 0.003), and radiotherapy treatment (relative risk, 0.37; p = 0.009) decreased the risk of local recurrence, whereas tumor size and depth, histopathologic subtype, and disease site did not predict local recurrence. However, in the unplanned excision cohort, radiotherapy did not protect (p = 0.09) against local recurrence, with a counterintuitive trend showing a decreased local recurrence rate in the unplanned subgroup not treated with radiotherapy.
Fig. 1.
The Kaplan-Meier 5-year survivorship estimate curves show decreased (p < 0.0001) local recurrence-free survival after unplanned excision and tumor bed reexcision (63.7%; 95% CI, 50.7%–75.1%) versus primary planned excision (89.7%; 95% CI, 83.1%–94.0%). The solid circles (planned excisions) and plus signs (unplanned excisions) along the curves represent censored patients.
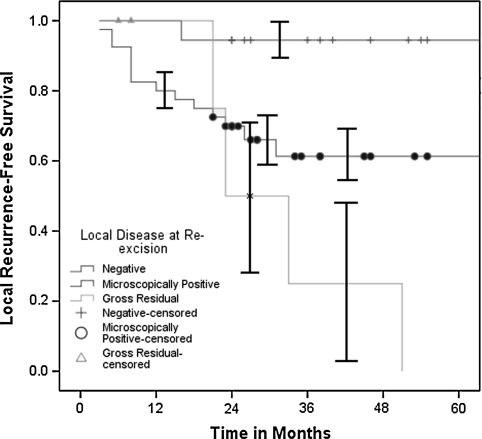
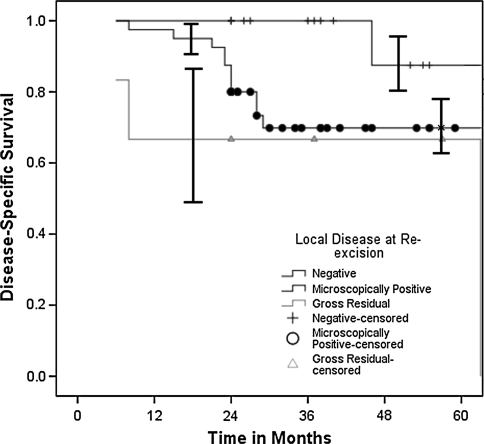
Patients in the unplanned excision cohort who had positive residual disease at tumor bed excision had an increased (p = 0.015) risk of subsequent local recurrence and decreased (p = 0.06) disease-specific survival. In addition to six patients with gross clinical residual disease after unplanned excision, microscopic residual disease was identified in 40 of 58 (69%) pathologic specimens of tumor bed excisions. Subsequent local recurrences developed in 17% (three of 18) of patients without evidence of disease at tumor bed excision, 38% (15 of 40) of patients with microscopic residual disease, and 67% (four of six) of patients presenting with gross residual disease. The 5-year local recurrence-free survival estimates were 94.4% (95% CI, 70.6%–99.7%) for patients with no residual disease, 61.3% (95% CI, 44.7%–75.8%) for patients with microscopically positive disease, and 0% (95% CI, 0%–48.3%) for patients with gross residual disease (Fig. 2). The 5-year disease-specific survival estimates were 87.5% (95% CI, 62.3%–97.5%) for patients with no residual disease, 69.8% (95% CI, 53.1%–82.8%) for patients with microscopically positive disease, and 66.7% (95% CI, 24.1%–94.0%) for patients with gross residual disease (Fig. 3). There was no difference in disease-specific survival (p = 0.27) between patients with unplanned excisions who presented early (less than 3 months) or late after the unplanned procedures. American Joint Committee on Cancer stage of disease (relative risk, 3.7; p < 0.0001) and lesion depth (relative risk, 1.7; p = 0.04) independently predicted decreased disease-specific survival. Initial excision status, lesion size, local recurrence, adjuvant treatments, age, gender, duration of symptoms, histopathologic subtype, disease site, and operative margins were not predictive. Actuarial disease-specific survival was 65.0% during the study period. Without controlling for confounding variables, the 5-year disease-specific survival estimate favored (p = 0.07) the unplanned excision cohort (60% versus 74%).
Fig. 2.
The Kaplan-Meier 5-year survivorship estimate curves among patients with unplanned excisions based on disease status at tumor bed excision show decreased (p = 0.015) local recurrence-free survival in patients with microscopic (61.3%; 95% CI, 44.7%–75.8%) or gross residual disease (0%; 95% CI, 0%–48.3%) versus no residual disease (94.4%; 95% CI, 70.6%–99.7%). The plus signs (no residual disease), solid circles (microscopically positive residual disease), and solid triangles (gross residual disease) along the curves represent censored patients.
Fig. 3.
The Kaplan-Meier 5-year survivorship estimate curves among patients with unplanned excisions based on disease status at tumor bed excision show decreased (p = 0.06) disease-specific survival in patients with microscopic (69.8%; 95% CI, 53.1%–82.8%) or gross residual disease (66.7%; 95% CI, 24.1%–94.0%) versus no residual disease (87.5%; 95% CI, 62.3%–97.5%). The plus signs (no residual disease), solid circles (microscopically positive residual disease), and solid triangles (gross residual disease) along the curves represent censored patients.
Discussion
Unplanned excisions of soft tissue sarcomas occur frequently, but controversy remains regarding the degree to which these unplanned manipulations may negatively affect definitive treatment or the local and/or systemic prognosis of patients with these tumors [3, 11, 17, 18, 20, 24, 26, 32, 33, 37]. We hypothesized unplanned excisions of high-grade soft tissue sarcomas would result in an increased risk of subsequent local recurrence and greater reconstructive requirements, and proven residual disease at tumor bed excision would be prognostic for local recurrence-free and disease-specific patient survival.
A principle limitation of this retrospective study is the lack of standardization regarding adjuvant therapy treatments across the time frame of treatment. However, these treatment decisions were determined using the criteria outlined previously and in accordance with our multidisciplinary tumor board recommendations. Because our radiotherapy use rates of 60% of cases overall and 70% of cases undergoing limb salvage are consistent with or greater than in numerous other series [2, 3, 7, 11, 17, 18, 26, 29, 32, 33], we believe this represents treatment consistent with the evolving standard of care for management of soft tissue sarcomas. Despite this, local recurrence findings may imply our tumor bed excisions were inadequate to achieve local disease control. Despite the greater flap and skin graft reconstructive requirements noted after tumor bed excision in our patients (30% versus 5% for limb salvage cases), Manoso et al. [29] reported a flap rate of 82% after tumor bed excision. However, we noted no difference in positive margins between cohorts and a greater proportion of margins 1 cm or greater in the unplanned excision cohort and 6% rate of positive margins after tumor bed excision compare favorably with the 12% to 19% rates reported by others [7, 26, 32]. We lacked a control group of patients treated with radiotherapy alone after unplanned excision (likely a reflection of referral bias to our surgical service), but our findings suggest this area may be worthy of additional study. Finally, although the mean duration of followup was longer after planned excisions, this ostensibly would bias our results in favor of the unplanned cohort. We therefore believe our study design and treatments were adequate for the analysis performed. Because unplanned excisions were performed on 32% of high-grade lesions in our series, and may occur in as much as 50% of all soft tissue sarcomas [11, 13, 24, 33, 37], this topic and our conclusions are highly relevant to orthopaedic oncologists and general orthopaedic surgeons.
We observed a nearly sixfold increased risk of local recurrence (34% versus 6%) after unplanned excision of high-grade soft tissue sarcomas despite tumor bed excisions in all cases and a greater proportion of larger, deep tumors in the planned excision cohort. Although some authors have reported no difference in local recurrence after primary excision versus tumor bed excision [11, 24], others have reported increased rates of local recurrence ranging from 22% to 27% [3, 18, 26, 32]. In a small series of myxofibrosarcomas, Manoso et al. [30] reported a 57% local recurrence rate after unplanned excisions and found, in keeping with our results, radiotherapy was not able to mitigate against this increased risk of local disease failure. Likewise, Lin et al. [26] reported radiotherapy did not affect local recurrence rates among patients undergoing unplanned excision with sarcomas of the hand or foot who underwent tumor bed excision with negative margins. They also reported better local control in patients who underwent tumor bed excision than in patients who did not. However, not all of the latter group was treated with radiotherapy and radiotherapy did improve local control in patients not treated with tumor bed excision. In contrast, Delaney et al. [8] reported a 24% local recurrence rate in patients with soft tissue sarcomas with positive margins at resection treated with radiotherapy, and Kepka et al. [23] reported only a 12% incidence of local failure after radiotherapy treatment without tumor bed excision after unplanned excisions. These findings suggest treatment with radiotherapy alone may be adequate for patients in whom tumor bed excision is medically or anatomically impracticable. Our data with high-grade soft tissue sarcomas suggest tumor bed excisions, when performed, should be aggressive and tumor bed excision with or without subsequent radiotherapy still does not provide local control equivalent to planned primary excision and adjuvant treatment per the standard of care.
The overall residual disease rate of 72% after tumor bed excision in our patients is consistent with rates in prior reports ranging from 24% to 91% [2, 7, 11, 14, 15, 20, 22, 24, 26, 29, 30, 32, 33, 43]. We also noted residual disease status at tumor bed excision correlated with local recurrence risk, an intuitive finding noted by others [2, 7, 32]. We also observed residual disease status at tumor bed excision affected disease-specific patient survival. Although our p value of 0.06 was greater than the conventionally accepted standard of 0.05, this criterion is arbitrary [41], and our study had only a 24% power to detect a 50% survival difference between subgroups with and without residual disease. We believe the magnitude of our finding (an 18% to 21% difference in disease-specific survival for residual disease versus no residual disease) represents a clinically important difference. Other authors have suggested residual disease status may be prognostic in similar instances and proposed this finding may be reflective of biologic tumor aggressiveness as opposed to the inadequacy of the initial, unplanned procedure [2, 11].
Despite confirming the prognostic value of residual disease after tumor bed excision for local recurrence-free and disease-specific survival, we did not find unplanned excision had a major effect on disease-specific survival in multivariable analysis. Some series have had findings similar to ours regarding survival [17, 18, 33, 37], but Lewis et al. [24] reported improved survival after unplanned excision treated with tumor bed excision versus primary excision after attempting to control for confounding variables. Others have questioned the validity and reproducibility of these findings [18, 20, 37], and the putative survival difference occurred despite no difference in local recurrence rates between groups. However, there is general agreement that tumor bed excision is indicated after most cases of unplanned excision [2, 7, 11, 14, 15, 18, 20, 24, 29, 30, 32, 37].
We found an increased rate of local recurrence and high residual disease rates at tumor bed excision after unplanned excision of high-grade soft tissue sarcomas. Unplanned excisions also required greater reconstructive measures after definitive operative treatment. Local disease status after tumor bed excision predicted the subsequent risk of local recurrence. We recommend tumor bed excision and liberal use of adjuvant radiotherapy after most unplanned excisions, although these interventions were not able to achieve local control of disease equivalent to that with planned excisions. We therefore emphasize the best treatment of unplanned excision of soft tissue sarcomas is prevention.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that this or her institution has approved the reporting of these cases, that all investigations were conducted in conformity with ethical principles of research, and that a waiver of informed consent for participating in this study was obtained.
References
- 1.Brennan MF, Casper ES, Harrison LB, Shiu MH, Gaynor J, Hajdu SI. The role of multimodality therapy in soft-tissue sarcoma. Ann Surg. 1991;214:328–336. [DOI] [PMC free article] [PubMed]
- 2.Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of unplanned excision of a soft tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90:203–208. [DOI] [PubMed]
- 3.Clasby R, Tilling K, Smith MA, Fletcher CD. Variable management of soft tissue sarcoma: regional audit with implications for specialist care. Br J Surg. 1997;84:1692–1696. [DOI] [PubMed]
- 4.Collin C, Godbold J, Hajdu S, Brennan MF. Localized extremity soft tissue sarcoma: an analysis of factors affecting survival. J Clin Oncol. 1987;5:601–612. [DOI] [PubMed]
- 5.Collin C, Hajdu SI, Godbold J, Friedrich C, Brennan MF. Localized operable soft tissue sarcoma of the upper extremity: presentation, management, and factors affecting local recurrence in 108 patients. Ann Surg. 1987;205:331–339. [DOI] [PMC free article] [PubMed]
- 6.Cox DR. Regression models and life-tables. J R Stat Soc Ser B. 1972;34:187–220.
- 7.Davis AM, Kandel RA, Wunder JS, Unger R, Meer J, O’Sullivan B, Catton CN, Bell RS. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1987;66:81–87. [DOI] [PubMed]
- 8.Delaney TF, Kepka L, Goldberg SI, Hornicek FJ, Gebhardt MC, Yoon SS, Springfield DS, Raskin KA, Harmon DC, Kirsch DG, Mankin HI, Rosenberg AE, Nielsen GP, Suit HD. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2003;56:21–27. [DOI] [PubMed]
- 9.Eilber FR, Morton DL, Eckardt J, Grant T, Weisenburger T. Limb salvage for skeletal and soft tissue sarcomas: multidisciplinary preoperative therapy. Cancer. 1984;53:2579–2584. [DOI] [PubMed]
- 10.Enneking WF, Spanier SS, Malawer MM. The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer. 1981;47:1005–1022. [DOI] [PubMed]
- 11.Fiore M, Casali PG, Miceli R, Mariani L, Bertulli R, Lozza L, Collini P, Olmi P, Mussi C, Gronchi A. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13:110–117. [DOI] [PubMed]
- 12.Gerner RE, Moore GE, Pickren JW. Soft tissue sarcomas. Ann Surg. 1975;181:803–808. [DOI] [PMC free article] [PubMed]
- 13.Gerrand CH, Bell RS, Wunder JS, Kandel RA, O’Sullivan B, Catton CN, Griffin AM, Davis AM. The influence of anatomic location on outcome in patients with soft tissue sarcoma of the extremity. Cancer. 2003;97:485–492. [DOI] [PubMed]
- 14.Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol. 1985;3:1344–1348. [DOI] [PubMed]
- 15.Goodlad JR, Fletcher CD, Smith MA. Surgical resection of primary soft-tissue sarcoma: incidence of residual tumour in 95 patients needing re-excision after local resection. J Bone Joint Surg Br. 1996;78:658–661. [PubMed]
- 16.Greene FL, Page DL, Fleming ID, Fritz A, Balch CM, Haller DG, Morrow M, eds. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer; 2002.
- 17.Gronchi A, Miceli R, Fiore M, Collini P, Lozza L, Grosso F, Mariani L, Casali PG. Extremity soft tissue sarcoma: adding to the prognostic meaning of local failure. Ann Surg Oncol. 2007;14:1583–1590. [DOI] [PubMed]
- 18.Gustafson P, Dreinhofer KE, Rydholm A. Soft tissue sarcoma should be treated at a tumor center: a comparison of quality of surgery in 375 patients. Acta Orthop Scand. 1994;65:47–50. [DOI] [PubMed]
- 19.Henshaw RM, Priebat DA, Perry DJ, Shmookler BM, Malawer MM. Survival after induction chemotherapy and surgical resection of high-grade soft tissue sarcoma: is radiation necessary? Ann Surg Oncol. 2001;8:484–495. [DOI] [PubMed]
- 20.Hoeber I, Spillane AJ, Fisher C, Thomas JM. Accuracy of biopsy techniques for limb and limb girdle soft tissue tumors. Ann Surg Oncol. 2001;8:80–87. [DOI] [PubMed]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [DOI]
- 22.Karakousis CP, Proimakis C, Walsh DL. Primary soft tissue sarcoma of the extremities in adults. Br J Surg. 1995;82:1208–1212. [DOI] [PubMed]
- 23.Kepka L, Suit HD, Goldberg SI, Rosenberg AE, Gebhardt MC, Hornicek FJ, Delaney TF. Results of radiation therapy performed after unplanned surgery (without re-excision) for soft tissue sarcomas. J Surg Oncol. 2005;92:39–45. [DOI] [PubMed]
- 24.Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231:655–663. [DOI] [PMC free article] [PubMed]
- 25.Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF. Association of local recurrence with subsequent survival in extremity soft tissue sarcoma. J Clin Oncol. 1997;15:646–652. [DOI] [PubMed]
- 26.Lin PP, Guzel VB, Pisters PW, Zagars GK, Weber KL, Feig BW, Pollock RE, Yasko AW. Surgical management of soft tissue sarcomas of the hand and foot. Cancer. 2002;95:852–861. [DOI] [PubMed]
- 27.Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121–1127. [PubMed]
- 28.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. For the members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78:656–663. [DOI] [PubMed]
- 29.Manoso MW, Frassica DA, Deune EG, Frassica FJ. Outcomes of re-excision after unplanned excision of soft-tissue sarcomas. J Surg Oncol. 2005;91:153–158. [DOI] [PubMed]
- 30.Manoso MW, Pratt J, Healey JH, Boland PJ, Athanasian EA. Infiltrative MRI pattern and incomplete initial surgery compromise local control of myxofibrosarcoma. Clin Orthop Relat Res. 2006;450:89–94. [DOI] [PubMed]
- 31.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemo Reports. 1966;50:163–170. [PubMed]
- 32.Noria S, Davis AM, Kandel R, Levesque J, O’Sullivan B, Wunder J, Bell R. Residual disease following unplanned excision of a soft tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78:650–656. [DOI] [PubMed]
- 33.Peabody TD, Monson D, Montag A, Schell MJ, Finn H, Simon MA. A comparison of the prognoses for deep and subcutaneous sarcomas of the extremities. J Bone Joint Surg Am. 1994;76:1167–1173. [DOI] [PubMed]
- 34.Pisters PWT, Harrison LB, Leung DHY, Woodruff JM, Casper ES, Brennan MF. Long term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14:859–868. [DOI] [PubMed]
- 35.Potter DA, Kinsella T, Glatstein E, Wesley R, White DE, Seipp CA, Chang AE, Lack EE, Costa J, Rosenberg SA. High-grade soft tissue sarcomas of the extremities. Cancer. 1986;58:190–205. [DOI] [PubMed]
- 36.Rosenberg S, Tepper J, Galstein E, Costa J, Baker A, Brennan M, DeMoss EV, Seipp C, Sindelar WF, Sugarbaker P, Wesley R. The treatment of soft tissue sarcoma of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196:305–315. [DOI] [PMC free article] [PubMed]
- 37.Rougraff BT, Davis K, Cudahy T. The impact of previous surgical manipulation of subcutaneous sarcoma on oncologic outcome. Clin Orthop Relat Res. 2005;438:85–91. [DOI] [PubMed]
- 38.Rydholm A, Gustafson P, Rööser B, Willén H, Akerman M, Herrlin K, Alvegård T. Limb-sparing surgery without radiotherapy based on anatomic location of soft tissue sarcoma. J Clin Oncol. 1991;9:1757–1765. [DOI] [PubMed]
- 39.Rydholm A, Gustafson P, Rööser B, Willén H, Berg NO. Subcutaneous sarcoma: a population-based study of 129 patients. J Bone Joint Surg Br. 1991;73:662–667. [DOI] [PubMed]
- 40.Shiu MH, Castro EB, Hajdu SI, Fortner JG. Surgical treatment of 297 soft tissue sarcomas of the lower extremity. Ann Surg. 1975;182:597–602. [DOI] [PMC free article] [PubMed]
- 41.Sterne JA, Davey Smith G. Sifting the evidence: what’s wrong with significance tests? BMJ. 2001;322:226–231. [DOI] [PMC free article] [PubMed]
- 42.Suit HD, Proppe KH, Mankin HJ, Wood WC. Preoperative radiation therapy for sarcoma of soft tissue. Cancer. 1981;47:2269–2274. [DOI] [PubMed]
- 43.Ward WG, Rougraff B, Quinn R, Damron T, O’Connor MI, Turcotte RE, Cline M. Tumors masquerading as hematomas. Clin Orthop Relat Res. 2007;465:232–240. [DOI] [PubMed]
- 44.Wilson AN, Davis A, Bell RS, O’Sullivan B, Catton C, Madadi F, Kandel R, Fornasier VL. Local control of soft tissue sarcoma of the extremity: the experience of a multidisciplinary sarcoma group with definitive surgery and radiotherapy. Eur J Cancer. 1994;30A:746–751. [DOI] [PubMed]
- 45.Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM, Merino MJ, Rosenberg SA. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16:197–203. [DOI] [PubMed]
- 46.Zeytoonjian T, Mankin HJ, Gebhardt MC, Hornicek FJ. Distal lower extremity sarcomas: frequency of occurrence and patient survival. Foot Ankle Int. 2004;25:325–330. [DOI] [PubMed]