Abstract
X-linked hereditary hypophosphatemic rickets can induce various multiplanar deformities of the lower limb. We evaluated our ability to correct these deformities and assessed complications and recurrence rates in 10 children (eight girls and a pair of twin boys) followed from early childhood to skeletal maturity. We performed 37 corrective operations in 10 children. Depending on the patient’s age, external fixation was used in 53 segments: Kirschner wires in 18, DynaFix® in three, the Taylor Spatial Frame® device in 13, and the Ilizarov device in 19. Internal fixation with intramedullary nailing was performed in 12. After bone consolidation, we radiographically determined the mechanical axis at an average distance of 0.5 cm medial to the center of the knee. The average mechanical lateral distal femoral angle was 85° (range, 83°–92°) and the average mechanical medial proximal tibial angle was 91° (range, 85°–92°). Deviation of the mechanical axis and knee orientation lines was increased at the followups conducted during a period of 5 to 12 months. Additional followups revealed a recurrence rate of 90% after the first corrective procedure and 60% after a second procedure.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The term hypophosphatemic rickets (HPR) denotes a group of metabolic bone diseases with common biochemical, biomechanical, and clinical features. It usually is manifested during infancy and childhood. Common biochemical characteristics are the presence of normal or slightly reduced serum calcium levels and a markedly reduced serum phosphate level.
Abnormal or delay in the mineralization of growth cartilage and in newly formed bone collagen induce various deformities commonly found around the knee.
X-linked hereditary hypophosphatemic rickets (XHPR) is the most common form of HPR [20], marked by renal phosphate wasting, impaired renal production of 1,25-dihydroxyvitamin D3, and abnormal mineralization of bone [1, 16]. XHPR is inherited in an X-linked dominant fashion, caused by a mutation in the PHEX gene (phosphate-regulating gene with homology to endopeptidases), which is located on Xp 22.1 [9]. The pathogenic mechanisms by which mutations in the PHEX gene cause XHPR are not entirely known. Some individuals reveal features of XHPR with no family history of rickets (sporadic cases), and many subsequently transmit the phenotype in an X-linked dominant manner consistent with HPR [26]. The disease occurs more commonly in girls [2, 4].
The clinical manifestations of XHPR include a disproportionately short stature and limb deformities, which become apparent after the age of 1 or 2 years. The deformities frequently are located around the knee, such as the bilateral genu varum or valgum often combined with tibial torsion, bowing of the tibia and the femur, or the so-called windswept deformity [19]. Laboratory data commonly reveal hyperphosphaturia, hypophosphatemia, eucalcemia, and elevated levels of alkaline phosphatase.
Defective bone mineralization results in rachitic changes at the growth plate and osteomalacia in trabecular and cortical bone. The traditional treatment consists of controlling hypophosphatemia to prevent deformities of long bones and achieving normal growth with phosphate supplementation and pharmacologic doses of vitamin D or its derivatives [10, 11, 17, 25]. Pharmacologic treatment frequently reverses radiographic signs of active rickets, ie, pigeon chest, rachitic rosary, asymmetric or odd-shaped skull, genu varum or valgum with distinctive cupping and widening of the growth plates (Fig. 1), in children without healing the coexistent osteomalacia [12]. Despite adequate medical treatment, the growth response may be unsatisfactory and some patients remain unresponsive [3]. In most cases, the deformities do not resolve and eventually lead to multiplanar misalignment of the lower limb. Patients with residual deformities have been treated by various methods of corrective osteotomy and fixation devices, such as Kirschner wires (Figs. 2, 3), plaster cast, plates [21], epiphysiodesis [7], Ilizarov devices [5] (Fig. 4), and unilateral external fixation or intramedullary nailing devices [8, 15, 23] (Fig. 5).
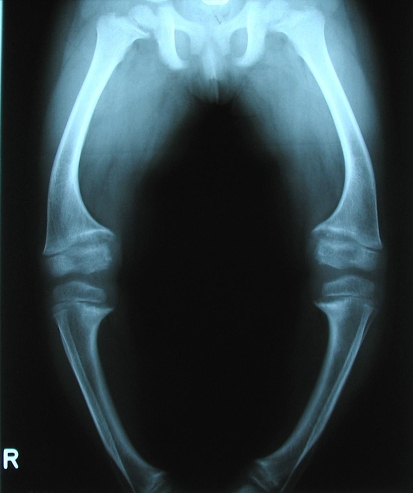
Fig. 1.
An anteroposterior radiograph shows the lower limbs of a 2.5-year-old girl with XHPR at initial presentation. The knees show the distinctive cupping and widening of the growth plates. Laboratory data: phosphate, 0.58 mg/dL; alkaline phosphatase, 935 U/L.
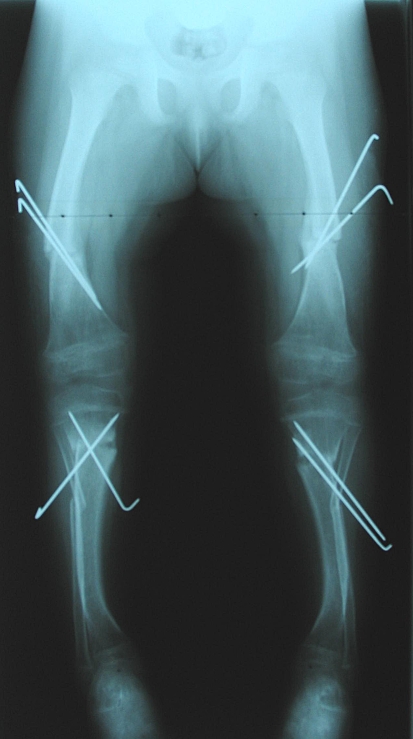
Fig. 2.
An anteroposterior radiograph shows the lower limbs of the patient in Fig. 1 after surgical correction in both lower legs (femur, tibia, and fibular osteotomies and Kirschner wire fixation) at the age of 4 years. Laboratory data: phosphate, 2.2 mg/dL; calcium, alkaline phosphatase, 776 U/L.
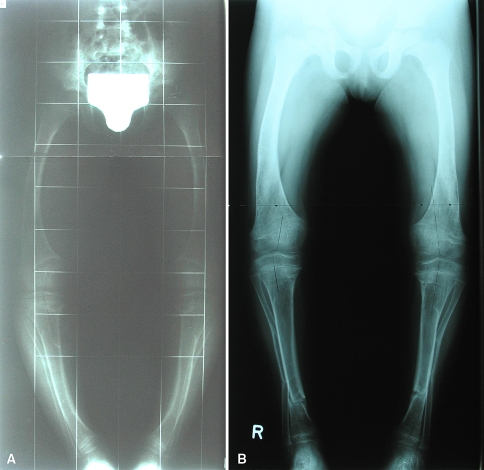
Fig. 3A–B.
Anteroposterior radiographs show the lower limbs of the patient in Fig. 1 (A) with obvious recurrent deformity and (B) 4 months after correction of both legs at the age of 5 years (distal tibial and fibular osteotomies). Laboratory data: phosphate, 2.4 mg/dL; alkaline phosphatase, 641 U/L.
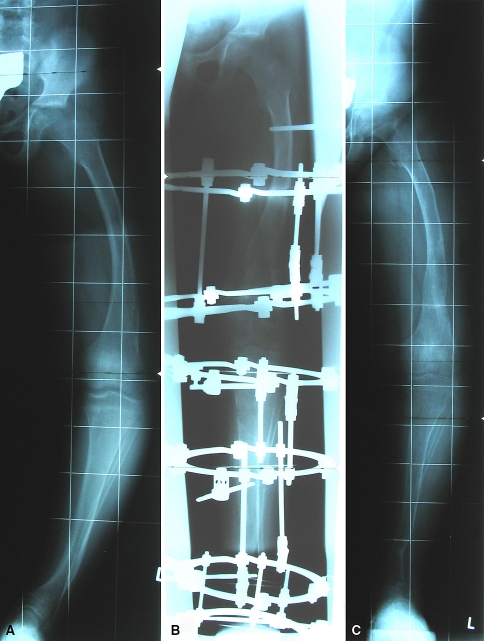
Fig. 4A–C.
Anteroposterior radiographs show the left lower limb of the patient in Figure 1 (A) at the age of 10 years and (B) after surgical correction using an external fixation device and (C) at the 12-month followup after removal of the fixation device. Laboratory data: phosphate, 2.7 mg/dL; alkaline phosphatase, 721 U/L.
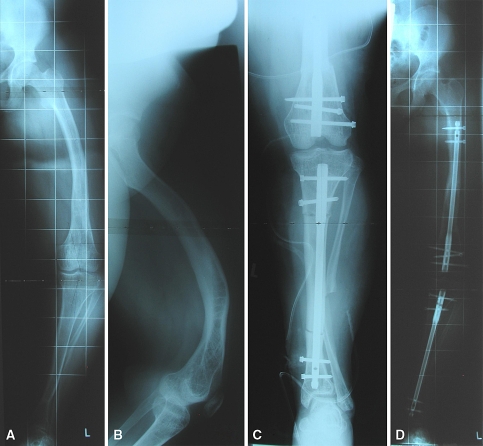
Fig. 5A–D.
(A, B) Anteroposterior radiographs show the left leg of the patient in Fig. 1 at the age of 15 years. Anterior radiographs show the legs (C) after surgical correction and (D) at the 24-month followup. Laboratory data: phosphate, 2.3 mg/dL; alkaline phosphatase, 189 U/L.
In this descriptive study, we followed 10 patients with XHPR from early childhood to maturity. We evaluated our ability to correct lower limb multiplanar deformities by different methods depending on the children’s age, complications, and recurrence rate after restoration of alignment of the lower limb under adequate supplementation therapy.
Materials and Methods
We retrospectively reviewed 33 patients (15 males, 18 females) with HPR treated between 1978 and 2005. The patients were on average 13 years of age (range, 3–48 years) at the time of presentation. Of these 33 patients, 10 children with XHPR had been treated and followed for a minimum of 10 years, were older than 15 years at the last followup, and were skeletally mature as evidenced by a closed physis on radiographic examination. These 10 children, eight girls and a pair of fraternal twin boys, constituted the patients for this study. At last assessment they were an average of 18.2 years of age (range, 15–23 years). The diagnosis of XHPR was established at an average age of 2 years 9 months (range, 2–4 years) by physical examination, radiographic signs of rickets, complete metabolic testing, and a family history consistent with X-linked dominant inheritance. At initial presentation, we observed varus deformity in four patients, valgus deformity in five patients located in the entire lower limb, and a rotational deformity in one patient. The minimum followup was 10 years (range, 10–17 years).
At initial presentation, the diagnosis was confirmed by the following laboratory tests: hyperphosphaturia (average, 112.3 mmol/day; range, 79–120 mmol/day; normal range, 15–45 mmol/day), hypophosphatemia (average, 2.0 mg/dL; range, 0.58–2.2 mg/dL; normal range, 2.5–4.9 mg/dL), normocalcemia and normal electrolyte levels, and increased alkaline phosphatase activity (average, 884 U/L; range, 607–1162 U/L; normal range, 91–385 U/L). We examined the patients every 6 months.
Treatment to control hypophosphatemia was started after the diagnosis had been established according to a standard medical protocol, including oral supplementation therapy with phosphate (Reducto®; Temmler Pharma GmbH & Co KG, Marburg, Germany) and vitamin D (Rocaltrol®; Roche, Mannheim, Germany). The goal of medical treatment was metabolic stability established by laboratory tests. Preoperative laboratory data were as follows: normophosphaturia (average, 18.4 mmol/day; range, 16.1–40.6 mmol/day), hypophosphatemia (average, 2.1 mg/dL; range, 2.0–2.7 mg/dL), normocalcemia and normal electrolyte levels, and increased alkaline phosphatase activity (average, 661 U/L; range, 189–810 U/L). Normal alkaline phosphatase activity was registered preoperatively in six of 37 cases.
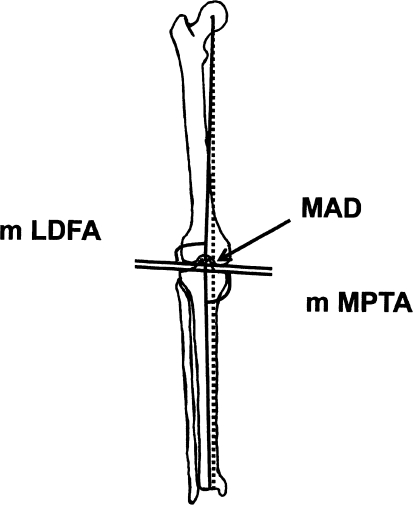
Alignment of the lower limb (preoperatively and postoperatively) was assessed by measuring the mechanical axis deviation (MAD) and the knee orientation lines according to the method described by Paley et al. [18] (Fig. 6). We assessed malrotation of the lower limb by clinical examination in all patients and on rotational CT in one patient. Preoperatively, we observed varus deformity of the lower limb in 19 legs, with the MAD on average 5.8 cm medial to the center of the knee (range, 2.5–11.5 cm). The average mechanical lateral distal femoral angle (mLDFA) was 106° (range, 93°–123°) and the average mechanical medial proximal tibial angle (mMPTA) was 76° (range, 69°–94°). We observed valgus deformity in 16 legs, with the MAD on average 5.4 cm lateral to the center of the knee (range, 1.5–10.7 cm). The average mLDFA was 80° (range, 58°–100°) and the average mMPTA was 87° (range, 75°–118°). One patient had a uniplanar external rotation deformity of 30°.
Fig. 6.
Alignment of the lower limb was assessed by measuring the MAD, the knee orientation lines, the mLDFA, and the mMPTA according to the method described by Paley et al. [18].
Surgical correction procedures were planned according to the patient’s clinical assessment and the extent of deformity in the lower limb (eg, MAD [13] beyond the knee). Radiographs were assessed preoperatively, postoperatively, and at followups. Anteroposterior images under full weightbearing, with the patella centered forward, and lateral radiographs of the lower limb were obtained.
Thirty-seven corrective operations of the lower limb, involving 98 osteotomies and two epiphysiodeses with staples, were performed in the 10 patients (30 femoral osteotomies, 35 tibial osteotomies, 33 fibular osteotomies). Percutaneous osteotomies were performed preferably in the metaphyseal or the metadiaphyseal region. In patients who underwent tibial correction osteotomy, an additional osteotomy at the middle third of the fibula was performed.
For fixation in patients 3 to 4 years of age, we used Kirschner wires and a pelvic leg plaster cast. The cast extended downward from the chest and included the abdomen and the entire affected leg. In patients aged 5 to 14 years, we used an external device. The Ilizarov device (Smith & Nephew, Inc, Memphis, TN) was used from 1987 to 1990 and the Taylor Spatial Frame® (Smith & Nephew) after 1990. For uniplanar deformities, particularly in the femur, unilateral fixation devices (DynaFix®; EBI, Parsippany, NJ) were given preference. Depending on the extent of the deformity, some patients older than 14 years underwent two or three osteotomies fixed by intramedullary nailing. In two patients with a valgus deformity, a distal femoral epiphysiodesis with staples was performed. Corrections of angular and rotational deformities were performed either in the acute or the gradual setting, depending on the severity of the deformity. Bone lengthening of approximately 1 cm was performed only in cases of a gradual corrective procedure to avoid early bone consolidation before the corrective procedure had been completed.
We applied external fixation in 53 segments (the Taylor Spatial Frame® in 13 segments, the Ilizarov system in 19 segments, DynaFix® in three segments), Kirschner wires in 18 segments, and internal fixation by intramedullary nailing in 12 segments. Six patients underwent simultaneous deformity correction in both limbs. Seven correction procedures were performed in four patients before the age of 6 years, 11 procedures in seven patients between the ages of 8 and 11 years, and 19 correction procedures in 10 patients after the age of 12 years. The patients’ average age was 8 years 3 months (range, 4–11 years) at the first correction and 14 years 5 months (range, 13–17 years) at the last correction.
A recurrence was defined as increased MAD extending beyond the knee either laterally or medially.
Results
Postoperatively, when bone consolidation was achieved, we radiographically determined a mean MAD of 0.5 cm medial to the center of the knee (range, 0–2 cm; standard deviation [SD], 0.7 cm). The mean mLDFA was 85° (range, 83°–92°; SD, 1.9°) and the mean mMPTA was 91° (range, 83°–92°; SD, 3°). At the followup performed between 5 and 12 months after the osteotomy had healed, the MAD and knee orientation lines were increased in all patients. In patients who had a varus deformity preoperatively, the MAD increased to a mean value of 3.1 cm medial to the center of the knee (range, 0–6.5 cm; SD, 2.4 cm). The mean mLDFA was 98° (range, 78°–110°; SD, 2.1°) and the mean mMPTA was 93° (range, 80°–94°; SD, 1°). In patients who had a valgus deformity preoperatively, the MAD increased to a mean value of 2.1 cm lateral to the center of the knee (range, 0–7.5 cm; SD, 3.1 cm). The mean mLDFA was 91° (range, 81°–106°; SD, 4.2°) and the mean mMPTA was 90° (range, 80°–102°; SD, 8.4°).
Two patients had femoral fractures after removal of the external fixation devices. At least one pin or Kirschner wire infection occurred in every patient who underwent correction using an external fixation device (Taylor Spatial Frame®, Ilizarov system, DynaFix®). Therefore, all of these patients received antibiotic therapy. Among those who underwent correction involving Kirschner wire fixation and a plaster cast, at least one Kirschner wire infection occurred in each patient. In general, no corrective procedure appeared clearly superior to any other.
Followups revealed a recurrence rate in 90% of patients after the first corrective procedure and 60% of patients after the second procedure.
Discussion
Deformities of the lower limb in children with XHPR commonly located around the knee are marked by a specific clinical appearance, laboratory data, and biomechanical features. In this descriptive study, we followed 10 patients from early childhood to maturity to evaluate our ability to correct lower limb multiplanar deformities by different methods depending on the children’s age, the complications, and the recurrence rate after restoration of the alignment of the lower limb under adequate supplementation therapy.
One limitation of our study is that we have no current data for children showing an association among misalignment of the lower limb, severity of knee deformity, and long-term consequences at the onset of arthritis. This association is well documented for adults [22]. Long-term followups will clarify this association and the long-term sequelae of rachitic deformity. Another limitation of our study is that we do not have sufficient data to compare our results with results of a retrospective series of control subjects treated solely by adequate supplementation therapy alone. As the incidence of XHPR is low, there are not enough patients to statistically identify any predictive factors or to form a control group. We believe not offering operative correction for severe multiplanar deformities of the lower limb would not have been in the best interest of our patients.
Traditionally, treatment of XHPR consists of controlling hypophosphatemia by supplementation of phosphate and pharmacologic doses of vitamin D before surgical correction [6, 14, 24, 25]. All of our patients were given such therapy. According to the medical protocol, laboratory testing was performed every 6 months to avoid side effects such as hypervitaminosis D, nephrocalcinosis, and secondary or tertiary hyperparathyroidism. Nevertheless, all but one patient (Fig. 4) had hypophosphatemia, with an average value of 2.1 mg/dL before the corrective procedure. Supplementation of phosphate and pharmacologic doses of vitamin D from early childhood onward reportedly curtailed progression of bowleg deformity. However, our data do not confirm this finding. Our patients received supplementation once the diagnosis of XHPR had been established at an average age of 2 years 9 months but they did not respond in a similar way as did patients evaluated by Evans et al. [7]. Deformity of the lower limb worsened under supplementation therapy. Although surgery may markedly improve limb deformities and medication may reduce active rickets, the coexisting osteomalacia is not cured [14]. Consequently, the deformities may recur later despite metabolic control of the disease. Some authors claim it may be safer to continue medical treatment and bracing until adolescence, at which time the deformity can be corrected with a minimum risk of recurrence [7]. Choi et al. [5] reported the results of correction using the Ilizarov method in 14 patients with an average age of 13 years 9 months. The deformity recurred in one patient who was 3 years old. The mean duration of followup was 5 years 7 months. The authors concluded a serum phosphate level lower than 2.5 mg/dL is a risk factor for recurrence of deformity [5]. Some of our patients had greater deformity of the limb develop despite normal laboratory data under substitution therapy (Fig. 4). The preoperative average serum phosphate level of 2.1 mg/dL in our series is comparable with the 2.0 mg/dL reported by Song et al. [23].
The study of Song et al. [23] included 20 patients with an average age of 20 years who underwent surgical correction by external fixation and/or intramedullary nailing. The patients had 18 major complications (recurrent deformity in eight cases, repeat fracture in two cases, equinus contracture in two cases, peroneal nerve palsy in one case) and 13 minor complications [23]. In our series of 98 osteotomies, two patients had a femoral fracture after removal of the external devices. In contrast to Song et al. [23], we performed bone lengthening of approximately 1 cm. In our patients, surgical correction procedures were planned according to the patient’s clinical assessment and the extent of deformity in the lower limb (eg, MAD [13] beyond the knee). The severity of the deformity disturbs gait and jeopardizes knee mechanics and alignment. The method of surgical correction was selected according to the patient’s age because the use of fixation techniques depends on the dimensions of bone and the maturity of the growth plate. Four of our patients were younger than 6 years when they underwent the first corrective procedure.
The size of our population (10 patients) is comparable with the series by Evans et al. [7] and Rubinovitch et al. [21], who reported a recurrence rate of 27% depending on the control of vitamin D metabolism. Followups in our patients revealed a recurrence rate of 90% after the first operation and 60% after the second procedure, with recurrence defined as MAD beyond the knee. In patients who underwent surgical correction after the age of 14 years, the recurrence rate was 30%. All of the recurrent deformities reported by Song et al. [23], who corrected 53 segments, occurred in children (13 segments [23%]). Like Song et al. [23], we also found surgical correction by external fixation associated with a uniform recurrence rate at the metaphysis and the diaphysis, whereas intramedullary nailing was associated with recurrence at the metaphysis.
We believe substitution therapy should be administered to control hypophosphatemia in the presence of XHPR. Deformity of the lower limb in children increases under supplementation therapy. Our investigation suggested severe deformities of the lower limb can be improved by surgery at all ages independent of the method used. Recurrence of deformities in the lower limb is independent of the correction procedure used. Furthermore, the deformity progression tends to decline after physical maturity is attained. Based on data for 10 patients with XHPR from early childhood to maturity, we currently perform corrective procedures at an early stage of deformity, anticipate two or three steps of corrective procedures during growth, and avoid prolonged corrective procedures in cases of severe deformity because the deformity may be expected to worsen with time.
Footnotes
Each author certifies that he has no commercial concerns (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained.
References
- 1.Albright F, Butler AM, Bloomberg E. Rickets resistant to vitamin D therapy. Am J Dis Child. 1937;54:529–547.
- 2.Burnett CH, Dent CE, Harper C, Warland BJ. Vitamin D-resistant rickets: analysis of twenty-four pedigrees with hereditary and sporadic cases. Am J Med. 1964;36:222–232. [DOI] [PubMed]
- 3.Chesney RW, Mazess RB, Rose P, Hamstra AJ, DeLuca HF, Breed AL. Long-term influence of calcitriol (1,25-dihydroxyvitamin D) and supplemental phosphorous in X-linked hypophosphatemic rickets. Pediatrics. 1983;71:559–567. [PubMed]
- 4.Cho HY, Lee BH, Knag JH, Ha IS, Cheong HI, Choi Y. A clinical and molecular genetic study of hypophosphatemic rickets in children. Pediatr Res. 2005;58:329–333. [DOI] [PubMed]
- 5.Choi IH, Kim JK, Chung CY, Cho TJ, Lee SH, Suh SW, Whang KS, Park HW, Song KS. Deformity correction of knee and leg lengthening by Ilizarov method in hypophosphatemic rickets: outcomes and significance of serum phosphate level. J Pediatr Orthop. 2002;22:626–631. [DOI] [PubMed]
- 6.Costa T, Marie PJ, Scriver CR, Cole DE, Reade TM, Nogrady B, Glorieux FH, Delvin EE. X-linked hypophosphatemia: effect of calcitriol on renal handling of phosphate, serum phosphate, and bone mineralization. J Clin Endocrinol Metab. 1981;52:463–472. [DOI] [PubMed]
- 7.Evans GA, Arulanantham K, Gage JR. Primary hypophosphatemic rickets: effect of oral phosphate and vitamin D on growth and surgical treatment. J Bone Joint Surg Am. 1980;62:1130–1138. [PubMed]
- 8.Eyres KS, Brown J, Douglas DL. Osteotomy and intramedullary nailing for the correction of progressive deformity in vitamin D-resistant hypophosphataemic rickets. J R Coll Surg Edinb. 1993;38:50–54. [PubMed]
- 9.Francis F, Hennig S, Korn B, Reinhardt R, De Jong P, Poustka A, Lehrach H, Rowe PS, Goulding JN, Summerfield T, Mountford R, Read AP, Popowska E, Pronicka E, Davies KE, O’Riordan JL, Econs MJ, Nesbitt T, Drezner MK, Oudet C, Pannetier S, Hanauer A, Strom TM, Meindl A, Lorenz B, Cagnoli B, Mohnike KL, Murken J, Meitinger T. A gene (PEX) with homologies to endopeptidase is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11:130–136. [DOI] [PubMed]
- 10.Friedman NE, Lobaugh B, Drezner MK. Effect of calcitriol and phosphorous therapy on the growth of patients with X-linked hypophosphatemia. J Clin Endocrinol Metab. 1993;76:839–844. [DOI] [PubMed]
- 11.Glorieux F, Scriver CR. Loss of a parathyroid hormone-sensitive component of phosphate transport in X-linked hypophosphatemia. Science. 1972;175:997–1000. [DOI] [PubMed]
- 12.Glorieux FH, Marie PJ, Pettifor JM, Delvin EE. Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med. 1980;303:1023–1031. [DOI] [PubMed]
- 13.Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femora valgus and varus deformity. J Bone Joint Surg Am. 2003;85:1229–1237. [DOI] [PubMed]
- 14.Harrell RM, Lyles KW, Harrelson JM, Friedman NE, Drezner MK. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia: induction and maintenance with phosphorus and calcitriol. J Clin Invest. 1985;75:1858–1868. [DOI] [PMC free article] [PubMed]
- 15.Kanel JS, Price CT. Unilateral external fixation for corrective osteotomies in patients with hypophosphatemic rickets. J Pediatr Orthop. 1995;15:232–235. [PubMed]
- 16.Lobaugh B, Drezner MK. Measurement of 25-hydroxyvitamin D–1 alpha-hydroxylase activity in mammalian kidney. Anal Biochem. 1983;129:416–424. [DOI] [PubMed]
- 17.Miyamoto J, Koto S, Hasegawa Y. Final height of Japanese patients with X-linked hypophosphatemic rickets: effect of vitamin D and phosphate therapy. Endocr J. 2000;47:163–167. [DOI] [PubMed]
- 18.Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planing for frontal and sagittal plan corrective osteotomies. Orthop Clin North Am. 1994;25:425–465. [PubMed]
- 19.Pierce DS, Wallace WM, Herndon CH. Long-term treatment of vitamin-D resistant rickets. J Bone Joint Surg Am. 1964;46:978–997. [PubMed]
- 20.Rasmussen H, Tenenhouse HS. Mendelian hypophosphatemias. In: Sciver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Basis of Inherited Disease. 7th ed. New York, NY: McGraw-Hill; 1995:3717–3745.
- 21.Rubinovitch M, Said SE, Glorieux FH, Cruess RL, Rogala E. Principles and results of corrective lower limb osteotomies for patients with vitamin D-resistant hypophosphatemic rickets. Clin Orthop Relat Res. 1988;237:264–270. [PubMed]
- 22.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. [DOI] [PubMed]
- 23.Song HR, Soma Raju VV, Kumar S, Lee SH, Suh SW, Kim JR, Hong JS. Deformity correction by external fixation and/or intramedullary nailing in hypophosphatemic rickets. Acta Orthop. 2006;77:307–314. [DOI] [PubMed]
- 24.Vaisbich MH, Koch VH. Hypophosphatemic rickets: results of a long-term followup. Pediatr Nephrol. 2006;21:230–234. [DOI] [PubMed]
- 25.Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med. 1991;325:1843–1848. [DOI] [PubMed]
- 26.Winters RW, McFalls VW, Graham JB. “Sporadic” hypophosphatemia and vitamin D-resistant rickets. Pediatrics. 1960;25:959–966. [PubMed]