Abstract
Identifying offending pathogens is crucial for appropriate antibiotic administration for infectious spondylitis. Although computed tomography (CT)-guided biopsy for bacteriologic diagnosis is a standard procedure, it has a variable success rate. Some reports claim percutaneous endoscopic discectomy and drainage offer a sufficient amount of tissue for microbiologic examination and easy application. We therefore compared the diagnostic value of CT guidance with that of endoscope guidance in 52 patients with suspected infectious spondylitis. Twenty patients underwent percutaneous endoscopic discectomy and drainage by an orthopaedic surgeon and the other 32 patients underwent CT-guided biopsies by a radiologist. Patients were followed a minimum of 12 months after treatment. Culture results of the biopsy specimens were recorded. Causative bacteria were identified more frequently with percutaneous endoscopy than in CT-guided biopsy (18 of 20 [90%] versus 15 of 32 [47%]). We observed no biopsy-related complications or side effects in either group. The data suggest percutaneous endoscopic discectomy and drainage yield higher bacterial recovery rates than CT-guided spinal biopsy.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The primary goals of treating infectious spondylitis are to make a prompt, accurate diagnosis, isolate the responsible organism, and prescribe effective antibiotic therapy. Nonoperative treatment is adequate for most infections and those detected early. Surgery usually is reserved for patients with failed antibiotic therapy, progressive spinal deformity or instability, epidural abscess, or neurologic deficits [7, 11, 15, 16, 19]. Perioperative morbidity increases with anterior débridement or combined anterior and posterior surgery, especially for elderly patients or those in poor general condition. Thus, early diagnosis and prompt application of appropriate antibiotic therapy for cultured organisms are crucial for successful nonsurgical treatment of infectious spondylitis and prevention of further morbidity [1, 3, 4, 19].
Computed tomographic-guided biopsy for bacteriologic diagnosis has a variable rate of 36% to 91% reported accuracy in patients with spinal infections [5, 8, 17, 18, 20, 21]. Percutaneous endoscopic discectomy and drainage (PEDD), a relatively new technique, is emerging because of its simplicity and apparent diagnostic accuracy from 45% to 87% according to the organism isolated [10, 13, 22].
The purpose of our study was to compare the rate of bacterial recovery using these two strategies for management of patients with lumbar infectious spondylodiscitis.
Materials and Methods
We retrospectively reviewed the medical records of 52 patients who had lumbar infectious spondylitis treated with PEDD or CT-guided biopsy and antibiotic treatment between January 2001 and January 2006. There were 29 men (56%) and 23 women (44%) with a mean age of 63.0 years (range, 27–88 years). To be included in the study, patients had to have early-stage lumbar infectious spondylodiscitis. Infectious spondylodiscitis was diagnosed based on clinical examinations, including elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values, and radiographic and MRI findings. We excluded 18 patients with infections associated with epidural abscesses resulting in neurologic deficits, extensive bony destruction with instability or kyphotic deformities, and those with coagulopathies, consciousness disturbances, and inability to tolerate the prone position. All remaining patients were candidates for CT-guided biopsy. The detailed procedures and the comparison of PEDD and CT-guided biopsy were explained to patients and their families. We divided patients into two groups according to patients’ choices of procedure type; 20 underwent PEDD and 32 underwent CT-guided biopsy (Table 1). Patients were followed a minimum of 12 months after treatment (mean, 36.8 months; range, 12–72 months). We reviewed the admission notes, radiology reports, procedure notes, pathology reports, and microbiology laboratory results.
Table 1.
Patient demographic data
| Parameters | Method | p Value | |
|---|---|---|---|
| PEDD | CT-guided biopsy | ||
| Number of patients | 20 | 32 | |
| Age (years) | 62.9 ± 14.9 | 63.0 ± 14.6 | 0.975* |
| Male/female (number of patients) | 12/8 | 17/15 | 0.627† |
| Back pain | 20 (100%) | 32 (100%) | |
| Sciatica | 7 (35%) | 12 (37.5%) | 0.855† |
| Erythrocyte sedimentation rate (mm/hour) | 59.7 ± 20.4 | 49.4 ± 17.6 | 0.060* |
| C-reactive protein level (mg/L) | 52.1 ± 26.5 | 56.8 ± 20.8 | 0.476* |
| Positive imaging finding | 20 (100%) | 32 (100%) | |
PEDD = percutaneous endoscopic discectomy and drainage; CT = computed tomography; *analyzed using an independent t-test; †analyzed using a chi square test.
CT-guided biopsies were performed by experienced interventional radiologists familiar with CT-guided needle puncture and aspiration (Temno Biopsy System, 18G × 15 cm, Cardinal Health, McGaw Park, IL). The aspirated and retrieved material, either using PEDD or CT-guided biopsy, was sent to the laboratory for Gram and acid-fast staining and for culturing for aerobic and anaerobic microorganisms and mycobacteria tuberculosis. Histopathologic examination and additional laboratory studies, including ESR, leukocyte count, and CRP were performed for all patients. Thereafter, patients with positive culture findings received a specific antibiotic treatment or chemotherapy accordingly. Patients with negative culture findings received empirical antibiotics according to the suggestions of infection physicians.
We (SCY, TSF) performed the PEDD procedures through the posterolateral approach in the operating room. The patient was situated prone on a radiolucent frame suitable for fluoroscopy. We performed all procedures with local anesthesia and conscious sedation. Under fluoroscopic guidance, the target site was located, and the entry site was marked on the skin at a point 8 to 12 cm from the midline. After sterile preparation, draping, and local anesthesia, we inserted a spinal needle directly into the center of the targeted disc. A guidewire was introduced through the spinal needle into the central disc space, and the spinal needle then was withdrawn. After creating a small stab wound incision (approximately 1 cm), a dilator and cannulated sleeve were guided over the wire and passed sequentially into the disc center. We repeated fluoroscopy in two orthogonal planes to verify the correct position of the endoscope tip. The tissue dilator then was removed, and the cutting tool was inserted to harvest a core of impacted biopsy specimen. Discectomy forceps then were inserted through the cannulated sleeve to extract additional tissue from the infected disc under fluoroscopic monitoring. After biopsy and débridement, normal saline solution was used for irrigation and the intradiscal lesion was checked endoscopically. Finally, a drainage tube was inserted into the débrided disc space and connected to a negative-pressure pump Hemovac (Zimmer, 3.2 mm diameter, Dover, OH). All tubes were left in place until drainage stopped or was less than 10 mL within 24 hours for 3 consecutive days (Figs. 1–3). The patients were allowed to walk postoperatively wearing a rigid spinal orthosis.
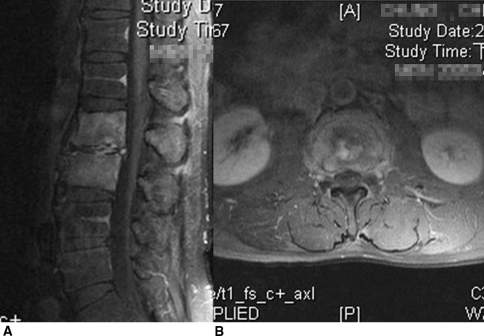
Fig. 2A–B.
Preoperative (A) sagittal and (B) axial MR scans with contrast showed L2 to L3 pyogenic vertebral osteomyelitis.
Fig. 1A–B.
A 46-year-old man with infectious spondylitis was treated with percutaneous endoscopic discectomy and drainage. Preoperative (A) anteroposterior and (B) lateral radiographs show narrowing of the L2 to L3 disc space and end-plate erosion.
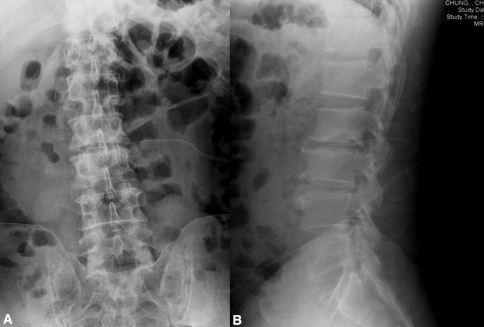
Fig. 3A–B.
Postoperative (A) anteroposterior and (B) lateral radiographs revealed a negative-pressure drainage tube originated in the L2 to L3 disc space for further drainage.
The microbiology reports comprised microscopy and culture findings, and any specific pathogens identified by either method were recorded and assessed. We analyzed differences in culture rate and infection control between the two groups using Pearson chi square test. Clinically, final infection control was assessed by careful physical examination and imaging studies during admission and at least 3 months after discharge. Any procedure-related complication or side effect also was recorded.
Results
Among the 52 patients, we identified 36 causative microorganisms. Two patients in the CT-guided biopsy group had mixed flora infections. Most infections were caused by Gram-positive bacteria, predominantly Staphylococcus species (Table 2). No biopsy-related complication or side effect was observed in either group during followup. The positive culture rate was higher (p = 0.002) in the PEDD group. The most prominent clinical sign of infectious spondylodiscitis was back pain, which was reported by all 52 patients. In the PEDD group, 18 patients (90%) reported immediate relief of back pain after PEDD. In the CT-guided biopsy group, no patients reported immediate improvement of back pain after the biopsy (Table 3).
Table 2.
Summary of causative microorganisms
| Pathogens | Method | |
|---|---|---|
| PEDD | CT-guided biopsy | |
| Oxacillin-resistant Staphylococcus aureus | 5 | 4 |
| Oxacillin-sensitive Staphylococcus aureus | 4 | 4 |
| Tuberculosis | 3 | 1 |
| Streptococcus viridans | 2 | 1 |
| Candida albicans | 1 | 2 |
| Propionibacterium acnes | 0 | 2 |
| Pseudomonas aeruginosa | 1 | 1 |
| Prevotella | 1 | 0 |
| Proteus mirabilis | 0 | 1 |
| Enterococcus faecalis | 1 | 1 |
| Enterococcus coli | 0 | 1 |
PEDD = percutaneous endoscopic discectomy and drainage; CT = computed tomography.
Table 3.
Comparison of patient groups
| Parameters | Method | p Value | |
|---|---|---|---|
| PEDD (n = 20) | CT-guided biopsy (n = 32) | ||
| Immediate back pain relief | 18 | 0 | |
| Positive culture | 18 (90%) | 15 (46.9%) | 0.002* |
| Infection control | 15 (75%) | 14 (43.8%) | 0.027* |
*Statistically significant by Pearson chi square test; PEDD = percutaneous endoscopic discectomy and drainage; CT = computed tomography.
Causative bacteria were identified in 18 (90%) of 20 biopsy specimens in the PEDD group. Systemic antibiotics and antituberculous or antifungal chemotherapy were administered according to sensitivity studies for identified pathogens. Fifteen of the 20 patients responded successfully to PEDD along with at least a 6-week course of parenteral antibiotic therapy or a full course of antimicrobial chemotherapy (Figs. 4, 5). Five of 20 patients underwent surgery for persistent infection (ie, despite medical treatment) and osteolytic destruction of the vertebral body with spinal instability or kyphotic deformity. Elevated CRP values returned to a normal range within 7 days to 5 weeks among these patients. Elevated ESR irregularly decreased to half of the original pretreatment values within 3 to 9 weeks.
Fig. 4A–B.
(A) Anteroposterior and (B) lateral radiographs show an intact end plate of the L2 and L3 vertebral body with good lordotic alignment 8 months postoperatively.
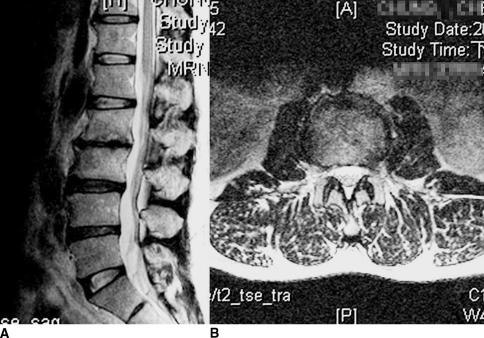
Fig. 5A–B.
(A) Sagittal and (B) axial MR scans show a healing response of the débrided subchondral bone and good control of the infection 8 months postoperatively.
In the CT-guided biopsy group, 15 (47%) of 32 biopsy specimens were positive for 18 microorganisms, including two specimens with mixed flora and five causative bacteria. Of the 15 patients with positive culture findings, eight (53%) underwent subsequent surgery and of the 17 patients with negative biopsies, 10 (59%) underwent surgery. Overall, 18 of 32 patients underwent anterior surgery because of failure to identify the pathogens causing persistent infection or progressive spinal instability. The remaining 14 patients who did not have surgery recovered after at least 2 months of antimicrobial therapy. Their elevated CRP levels returned to a near normal range by 4 or more weeks after therapy, whereas elevated ESR levels took 7 weeks or more to decrease to half of the original pretreatment values.
Discussion
Spinal infections are diagnostic and treatment challenges to clinicians because of their early subtle and vague course. Advances in imaging studies, laboratory tests, and a high degree of suspicion can alert spinal surgeons to the correct diagnosis in a timely fashion to avoid the severely debilitating complications of delayed or inadequate treatment [1, 3, 4, 19]. Identifying the offending pathogen is critical for providing appropriate antibiotic therapy to patients with infectious spondylitis. CT-guided needle biopsy has been recommended for isolating causative pathogens; however, the aspirate often is inadequate and sometimes no organism has been cultured. Limitation in detecting the organism often leads spine surgeons to perform more invasive surgery. Percutaneous endoscopic discectomy was first used for treating uncomplicated herniated discs in the early 1980s [14]. Numerous minimally invasive percutaneous endoscopic procedures for lumbar disc herniation have been developed for spinal surgery with clinical outcomes comparable to those of conventional open surgery [12, 23]. The minimal invasiveness and simplicity of the technique led us to use it in managing infectious spondylodiscitis. A sufficient amount of material for microbiologic examination can be obtained directly from the infected disc region, which provides a higher diagnostic accuracy, thereby enabling prompt and appropriate antibiotic therapy to the offending pathogen. Additionally, the opportunity to débride some of the infected disc material may hasten the time of infection control and spontaneous healing.
Ideally, a prospective, randomized study should be performed to examine the optimal techniques for biopsy of infectious spondylodiscitis. However, clinical studies must consider ethical and legal restrictions imposed by the patients’ right to be informed and make choices. Although not randomized, our study groups were similar. CT-guided biopsies were indicated for all enrolled patients, who were screened by strict criteria and were self-selected for either procedure. By carefully selecting the patient population, we attempted to minimize confounding factors and concentrate on one variable (the procedure [PEDD or CT-guided biopsy]) and only included patients with true infectious spondylodiscitis. However, early and effective control of the infection by either endoscopic débridement or corrective antibiotics cannot be clearly distinguished. Additionally, the mixed flora cultured from the biopsy specimens in the CT-guided biopsy group can be biased by a relatively septic environment in the CT room.
The reported accuracy of spinal biopsy varies from 36% to 91% according to the organism isolated [5, 8, 17, 18, 20, 21]. In a retrospective study of postoperative spondylodiscitis, Fouquet et al. obtained positive bacteriologic diagnoses in nine (36%) of 25 patients who had biopsies [8]. They concluded the features of septic or chemical postoperative discitis can be distinguished by ESR, CRP, and percutaneous discal biopsy. To evaluate the diagnostic yield of CT-guided percutaneous needle aspiration in spontaneous (nonpostoperative) infectious discitis, Chew and Kline divided 105 consecutive patients having CT-guided biopsies into two groups, 43 with active disc space infection and 62 without active disc space infection [5]. Microbiologic analyses of the CT-guided percutaneous aspiration specimens were positive in 39 (91%) of 43 patients with active infections. They found CT-guided biopsy is an accurate method for identifying pathogens in active bacterial disc space infections, but is less reliable for identifying fungal infections. Rankine et al. analyzed 20 patients undergoing percutaneous spinal biopsies for spinal infections [18]. They isolated organisms in six of 12 patients not taking antibiotics, whereas an organism was isolated in only two of eight patients taking antibiotics. They argued many clinicians treat spinal infection empirically with antibiotics before biopsy, but this reduces the chance of isolating an organism and determining antibiotic sensitivity. We consulted an interventional radiologist to perform the CT-guided biopsy if we suspected a spinal infection and needed a specimen for definite diagnosis. Causative pathogens were identified in 15 (47%) of 32 biopsy specimens, which is in the reported range. The results of CT-guided biopsy can be negative for various reasons, including an inadequate amount of specimen, sampling error, previous antibiotic therapy, or initial misdiagnosis.
Our study revealed causative pathogens in 18 (90%) of 20 patients in the PEDD group. The positive culture rate was comparable to that obtained with open biopsy and superior to that of the CT-guided biopsy group. The size of the biopsy needle or discectomy forceps determines the sample size, which correlates to the positivity of the culture rate [2, 6, 22]. A sufficient amount of specimen can be obtained through PEDD with the patient under local anesthesia, avoiding the potential morbidity associated with an extensive surgical procedure. In 1994, Gebhard and Brugman first used percutaneous discectomy to diagnose and treat a patient with L4–5 bacterial discitis [9]. This technique was successful in obtaining a bacteriologic diagnosis, relieving the patient’s symptoms, and assisting in eradication of the infection, although they stated additional cases would be needed to determine the overall effectiveness of this technique. Haaker et al. treated 16 patients with lumbar disc infection using percutaneous lumbar discectomy [10]. Diagnostic biopsy identified the specific infections in 45% of patients, and only three were treated surgically. They concluded percutaneous lumbar discectomy is a very useful, minimally invasive procedure for conservative treatment of lumbar discitis. Ito et al. evaluated clinical results of posterolateral spinal endoscopic débridement and irrigation followed by percutaneous drainage in patients with pyogenic spondylodiscitis [13]. They suggested this procedure could be used in patients with multiple comorbidities who are not candidates for major spinal surgery under general anesthesia.
Twenty-three of our patients (five in the PEDD group and 18 in the CT-guided biopsy group) eventually underwent surgery for poorly controlled infections and considerable mechanical back pain caused by progressive destruction. After at least a 6-week course of parenteral antibiotic therapy or a full-course of antimicrobial chemotherapy, infection control was 75% in the PEDD group and 44% in the CT-guided biopsy group. Relief of intradiscal pressure, irrigation of inflammatory factors, endoscopic visual débridement, and minimal invasiveness that preserved adequate stability contributed to immediate back pain improvement and favorable outcomes for 15 patients in the PEDD group. With postoperative negative-pressure Hemovac suction, the pathogens in the infected tissue can continue to be removed. The mentioned reasons combined with early diagnosis and prompt antibiotic treatment of the causative cultured organisms explain the higher rates of infection control using PEDD.
In comparison with CT-guided biopsy for management of lumbar infectious spondylodiscitis, PEDD provided retrieval of much more positive specimens, yielding higher diagnostic efficacy. Therefore, the rate of secondary surgical intervention was reduced using PEDD combined with prompt application of adequate antimicrobial therapy.
Based on our data, we believe PEDD is a better alternative to CT-guided biopsy for diagnosis of early stage infectious spondylodiscitis.
Acknowledgments
We thank Yu-Hsien Kao and Chin-Hsien Wu for their support.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27–33. [DOI] [PubMed]
- 2.Arya S, Crow WN, Hadjipavlou AG, Nauta HJ, Borowski AM, Vierra LA, Walser E. Percutaneous transpedicular management of discitis. J Vasc Interv Radiol. 1996;7:921–927. [DOI] [PubMed]
- 3.Bonfiglio M, Lange TA, Kim YM. The Classic: Pyogenic vertebral osteomyelitis: disk space infections. 1973. Clin Orthop Relat Res. 2006;444:4–8. [DOI] [PubMed]
- 4.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874–880. [DOI] [PubMed]
- 5.Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218:211–214. [DOI] [PubMed]
- 6.Crow WN, Borowski AM, Hadjipavlou AG, Walser EM, Arya S, Calme MB, Amps J, Jensen R, Somisetty S, Alford B, Adesokan A. Percutaneous transpedicular automated nucleotomy for debridement of infected discs. J Vasc Interv Radiol. 1998;9:161–165. [DOI] [PubMed]
- 7.Emery SE, Chan DP, Woodward HR. Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine. 1989;14:284–291. [PubMed]
- 8.Fouquet B, Goupille P, Jattiot F, Cotty P, Lapierre F, Valat JP, Amouroux J, Benatre A. Discitis after lumbar disc surgery: features of “aseptic” and “septic” forms. Spine. 1992;17:356–358. [DOI] [PubMed]
- 9.Gebhard JS, Brugman JL. Percutaneous discectomy for the treatment of bacterial discitis. Spine. 1994;19:855–857. [DOI] [PubMed]
- 10.Haaker RG, Senkal M, Kielich T, Kramer J. Percutaneous lumbar discectomy in the treatment of lumbar discitis. Eur Spine J. 1997;6:98–101. [DOI] [PMC free article] [PubMed]
- 11.Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine. 2000;25:1668–1679. [DOI] [PubMed]
- 12.Hermantin FU, Peters T, Quartararo L, Kambin P. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am. 1999;81:958–965. [DOI] [PubMed]
- 13.Ito M, Abumi K, Kotani Y, Kadoya K, Minami A. Clinical outcome of posterolateral endoscopic surgery for pyogenic spondylodiscitis: results of 15 patients with serious comorbid conditions. Spine. 2007;32:200–206. [DOI] [PubMed]
- 14.Kambin P, Schaffer JL. Percutaneous lumbar discectomy: review of 100 patients and current practice. Clin Orthop Relat Res. 1989;238:24–34. [PubMed]
- 15.Krodel A, Sturz H, Siebert CH. Indications for and results of operative treatment of spondylitis and spondylodiscitis. Arch Orthop Trauma Surg. 1991;110:78–82. [DOI] [PubMed]
- 16.Ozuna RM, Delamarter RB. Pyogenic vertebral osteomyelitis and postsurgical disc space infections. Orthop Clin North Am. 1996;27:87–94. [PubMed]
- 17.Parker LM, McAfee PC, Fedder IL, Weis JC, Geis WP. Minimally invasive surgical techniques to treat spine infections. Orthop Clin North Am. 1996;27:183–199. [PubMed]
- 18.Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80:607–609. [DOI] [PMC free article] [PubMed]
- 19.Rezai AR, Woo HH, Errico TJ, Cooper PR. Contemporary management of spinal osteomyelitis. Neurosurgery. 1999;44:1018–1025; discussion 1025-1026. [DOI] [PubMed]
- 20.Staatz G, Adam GB, Keulers P, Vorwerk D, Gunther RW. Spondylodiskitic abscesses: CT-guided percutaneous catheter drainage. Radiology. 1998;208:363–367. [DOI] [PubMed]
- 21.Vinicoff PG, Gutschik E, Hansen SE, Karle A, Rieneck K. [CT-guided spinal biopsy in spondylodiscitis][in Danish]. Ugeskr Laeger. 1998;160:5931–5934. [PubMed]
- 22.Yang SC, Fu TS, Chen LH, Niu CC, Lai PL, Chen WJ. Percutaneous endoscopic discectomy and drainage for infectious spondylitis. Int Orthop. 2007;31:367–373. [DOI] [PMC free article] [PubMed]
- 23.Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine. 2002;27:722–731. [DOI] [PubMed]