Abstract
Hip osteoarthritis leads to chronic pain and deteriorated joint function, which affect weightbearing and balance during gait. THA effectively restores hip function but it is not known whether THA restores balance during gait. We hypothesized patients would have greater frontal plane and smaller sagittal plane center of mass-center of pressure inclination angles preoperatively compared with control subjects, and THA would improve these inclination angles by 16 weeks postsurgery. Compared with control subjects, we observed greater frontal plane inclination angles and smaller sagittal plane angles preoperatively, indicating gait imbalance. These inclination angles were improved postoperatively, providing better balance control. Despite improvement, patients differed in frontal and sagittal plane inclination angles compared with control subjects. This suggests residual deficits in dynamic balance control in patients undergoing THA before and up to 4 months after surgery.
Introduction
During gait, the hip increases stride length, leg progression, and stability during single-limb support [50]. Osteoarthritis of the hip results in chronic pain, reduced muscle strength, and reduced ability to perform daily activities [42, 45]. Patients with degenerative hips have difficulty maintaining stability [26, 32] and are at risk of falling [1, 29, 31, 46]. Falls are a major cause of death among the elderly, with an estimated 28% of adults older than 65 years experiencing one or more falls [40]. Zhan et al. suggested that of the 200,000 patients who underwent THA in the United States, 60% were older than 65 years [51]. Because decreased joint function and muscle strength are major risk factors causing falls [40, 41], concerns regarding the risk of falling might exist among patients undergoing THA [1, 29]. Arnold and Faulkner [1] screened 106 older adults with hip osteoarthritis, with 45% reporting at least one fall in the previous year. Mitchell et al. [29] reported 39% of their patients undergoing hip or knee arthroplasty experienced one or more falls during a 4-week period before study participation and concluded there is a need to implement a fall-prevention program. Furthermore, periprosthetic fracture of the femur often occurs after a fall and can lead to a high rate of postoperative complications and mortality [2, 10, 21]. Lack of bone quality, previous surgery, medical comorbidities, and age have been cited as factors associated with periprosthetic fractures [10, 22, 49].
THA effectively decreases pain and improves mobility and quality of life [9, 23]. Studies on postural balance during standing after THA showed patients had a greater medial-lateral sway [35] and reduced ability to control center of mass (CoM) movement [32] when compared with control subjects. Although walking velocity, stride length, and stride time reportedly are improved after THA [23, 47], on average, patients continue to have a slower gait speed and shorter stride length than various control subjects up to 4 years [24, 38, 43] and even 10 years [3]. Trunk pitch (forward-backward) and roll (side-to-side) have been used to reflect balance control of patients undergoing THA during gait [26]. Compared with presurgery, trunk pitch and roll angles during gait progressively decrease by 4 and 12 months postsurgery. After THA, patients also showed a slight lean of the CoM toward the operated limb during the double-support phase of gait [43]. This pattern of dynamic balance control during gait is apparently different from that of healthy older adults, but the study did not report how subjects performed preoperatively. Several studies have suggested hip position sense is largely preserved after THA [12, 16], yet it remains unknown whether patients improve balance control during gait.
A common surrogate for balance has been the interaction between the CoM and center of pressure (CoP) [6, 7, 13]. Specifically, the inclination angle formed between the line connecting the CoM and CoP and a vertical line passing through the CoP throughout the gait cycle provides a measure of balance control in the frontal and sagittal planes [20]. Elderly individuals with balance impairments have greater frontal and reduced sagittal plane CoM-CoP inclination angles than healthy elderly people during walking. In addition to these alignment measures, CoM instantaneous velocity reflects the individual’s ability in dynamic balance control [13, 15, 37]. Substantially reduced CoM velocity has been observed in the elderly at the instant when the CoM and CoP are maximally separated [13].
We therefore hypothesized, compared with age-matched control subjects, patients undergoing THA would have characteristics of CoM-CoP interactions associated with impaired balance control at presurgery, ie, greater frontal plane CoM-CoP inclination angles, smaller sagittal plane CoM-CoP inclination angles, and slower CoM instantaneous velocities at maximum CoM-CoP separation. We also hypothesized THA would allow patients to decrease the medial inclination angle and increase the sagittal plane angle by 16 weeks postsurgery.
Materials and Methods
We recruited 30 adults for this study and divided them into two groups: 20 patients (14 males, six females) undergoing THA and 10 age-matched control subjects (five males, five females) without orthopaedic problems (Table 1). Three-dimensional gait analysis was performed on each patient undergoing THA during three visits to assess dynamic balance control during gait using the CoM-CoP inclination angles in the frontal and sagittal planes. Control subjects were tested for identical variables during two visits. Before testing, subjects provided signed consent for the experimental procedure approved by the University of Oregon Institutional Review Board.
Table 1.
Anthropometric measures for control and THA groups
| Anthropometric measure | Control subjects (n = 10) | Patients undergoing THA (n = 20) | p Values |
|---|---|---|---|
| Age (years) | 59.9 (5.3) | 57.0 (5.2) | 0.173 |
| Height (cm) | 168.1 (7.2) | 172.5 (8.5) | 0.178 |
| Weight (kg) | 74.71 (15.1) | 95.0 (14.8) | 0.002 |
| Body mass index (kg/m2) | 26.3 (3.9) | 31.9 (4.3) | 0.002 |
Values are expressed as means, with standard deviations in parentheses.
Patients recruited in this study were scheduled for THA (mean age ± standard deviation, 57.0 ± 5.2 years) through Orthopedic Healthcare Northwest, PC of Eugene, OR (Table 1). Age-matched control subjects (mean age, 59.9 ± 5.3 years) were recruited through flyers placed on the university campus and surrounding neighborhoods. Control subjects were community-dwelling individuals without lower limb joint surgery, any history of neurologic or musculoskeletal impairment, or incidence of vertigo or arthritis (Table 1). The patients recruited reported no prior joint surgery, fracture of the lower limb, or history of neurologic impairment, and all were diagnosed with unilateral osteoarthritis of the hip. Before surgery, we evaluated all surgical patients for hip function with Harris hip scores (mean, 54.7 ± 12.4; range, 27.3–75.7) [14]. All patients underwent primary THA using the anterior (12 patients) or lateral (eight patients) surgical approach on the affected limb and received uncemented (17 patients) or cemented (three patients) Zimmer hip implants (Zimmer Inc, Warsaw, IN). Patients then underwent an identical physical therapy regimen with the same physical therapist during the study period.
Patients undergoing THA were tested at three times: presurgery, 6 weeks, and 16 weeks postsurgery. By 6 weeks postsurgery, patients no longer used crutches, and by 16 weeks postsurgery, the muscles affected during THA presumably would be sufficiently healed to allow patients to resume their usual activities of daily living after their rehabilitation protocol [4, 19]. Thus, short-term recovery of balance control at this time was investigated in this study. During each visit, we asked subjects to walk at a self-selected pace along a 10-m walkway. Practice walking trials were first performed so subjects could become comfortable with the environment and the markers. We collected four level walking trials during each visit as subjects ambulated along the walkway. Control subjects were tested during two visits 1 month apart to ensure test repeatability. Among the control subjects, no time effects were identified between the two visits. Therefore, average values for each dependent variable were used for comparison with the patient groups.
Whole-body motion data were collected using an eight-camera motion system (Motion Analysis Corp, Santa Rosa, CA) with a set of 29 reflective markers placed on bony landmarks [13]. Two force plates (Advanced Mechanical Technologies Inc, Watertown, MA) were placed in series at the center of the walkway to collect ground reaction forces and moments. We predetermined the separation distance between force plates based on visual inspection of a subject’s step length.
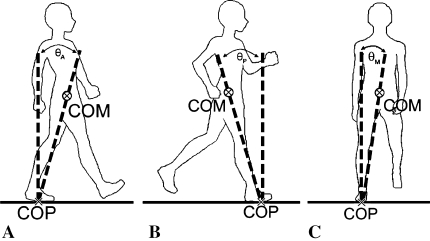
Marker trajectories and data from the force plates were sampled at 60 Hz and 960 Hz, respectively, for 6 seconds in each trial. Data collected during one complete stride were analyzed from heel strike of the involved limb onto the first force plate to the subsequent heel strike of the same foot. Motion data were reconstructed using EVA/RT software (Motion Analysis Corp) and filtered using a low-pass, fourth-order Butterworth filter with an 8-Hz cutoff frequency. Virtual markers at joint centers were created and combined with anthropometric data to determine the CoM locations for 13 body segments [6]. Laboratory-written programs (MATLAB® Version 7.0; The Mathworks Inc, Natick, MA) were used for further data processing. The whole-body CoM was calculated using the weighted sum of all segmental CoMs. The CoP was computed with the measured ground reaction forces and moments. We calculated linear CoM velocities using a generalized crossvalidated spline algorithm [48]. Instantaneous CoM velocities in the frontal and sagittal planes at the maximum CoM-CoP separation were identified. The CoM-CoP inclination angles were defined as the angles formed between the line connecting the CoP and CoM with a vertical line through the CoP (Fig. 1) and were calculated for each instant throughout the gait cycle [20]. The maximum medial, anterior, and posterior CoM-CoP inclination angles then were identified for analysis.
Fig. 1A–C.
The CoM-CoP inclination angles in the (A) anterior (θA), (B) posterior (θP), and (C) medial (θM) directions are defined as angles formed by a line connecting the CoM and CoP, and a vertical line passing through the CoP.
We calculated temporal distance gait measures during a single stride. Stride length and stride time were computed from the position change of the heel marker. Average step width was calculated as the distance between the ankle centers at heel strike of each foot. Stride length was normalized by the subject’s height, whereas step width was normalized by the distance between the two anterior superior iliac spines. Both measures were normalized to account for differences in individual anthropometrics and allow for comparison between subjects and groups. We determined gait velocity by the position and time change of the whole-body CoM.
We used a mixed-model analysis of variance with repeated measures to detect the effects of group (control versus THA) and time (presurgery versus 6 weeks and 16 weeks postsurgery) on gait temporal-distance parameters, CoM-CoP inclination angles, and CoM velocity. Analyses were performed with SAS® 9.0 (SAS Institute Inc, Cary, NC).
Results
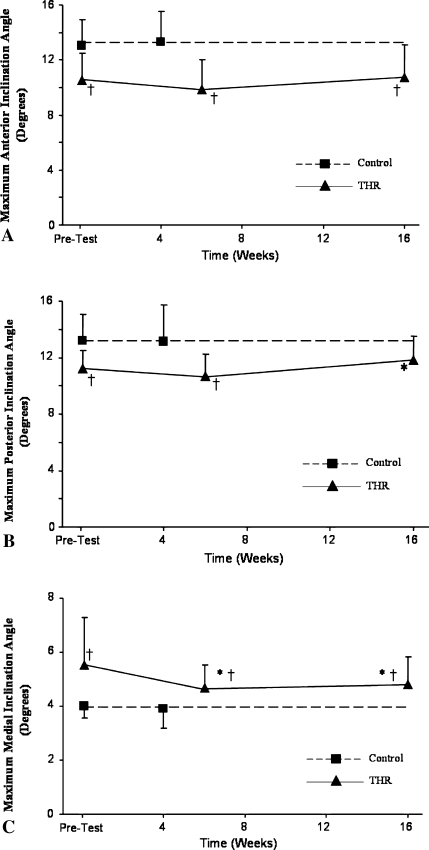
Before THA we observed patterns of CoM-CoP interactions associated with impaired balance control in patients (Fig. 2). When compared with control subjects, the peak anterior and posterior CoM-CoP inclination angles (Fig. 3A–B) were smaller (p = 0.0004 and p = 0.0012, respectively), whereas the peak medial CoM-CoP inclination angle (Fig. 3C) was greater (p = 0.0006) in patients undergoing THA. At the instants of maximal anterior and posterior CoM and CoP separation, patients undergoing THA had slower (p = 0.0023 and p = 0.0028, respectively) CoM forward velocities (Table 2). Furthermore, when compared with control subjects, patients undergoing THA walked with a slower gait velocity (p = 0.0024), shorter stride length (p = 0.0005), and larger step width (p = 0.0066) (Table 3).
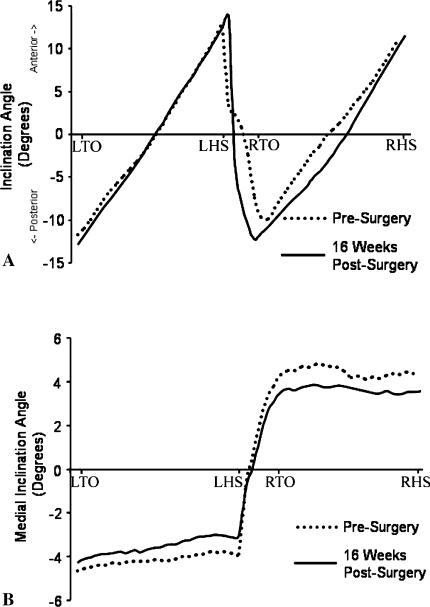
Fig. 2A–B.
The graph shows typical CoM-CoP inclination angles from left toe-off (LTO) to left heel strike (LHS) to right toe-off (RTO) to right heel strike (RHS) in (A) the sagittal and (B) frontal planes before and after THA.
Fig. 3A–C.
Maximum (A) anterior, (B) posterior, and (C) medial CoM-CoP inclination angles are shown for the two subject groups. †At all times, the THA group displayed a smaller anterior inclination angle (p = 0.0004, 0.0002, and 0.0064, respectively) and greater medial inclination angle (p = 0.0006, 0.0167, and 0.0101, respectively) when compared with the control group, indicating decreased ability in balance control. The posterior inclination angle approached the level of control subjects by 16 weeks postsurgery (p = 0.0562), whereas it was smaller before surgery (p = 0.0012) and 6 weeks postsurgery (p = 0.0005). *Compared with presurgery, patients undergoing THA had a smaller medial inclination angle at 6 (p = 0.0078) and 16 (p = 0.0084) weeks and a greater posterior inclination angle (p = 0.0247) at 16 weeks postsurgery.
Table 2.
Instantaneous CoM velocities at maximum CoM-CoP separation in control and THA groups
| CoM velocity | Control subjects | Patients undergoing THA | p Values | ||
|---|---|---|---|---|---|
| Presurgery | 6 weeks postsurgery | 16 weeks postsurgery | |||
| At maximum anterior separation (m/second) | 1.26 (0.17) | 0.99 (0.26) | 1.05 (0.21) | 1.17 (0.17) | < 0.0001* |
| 0.0023† | |||||
| At maximum posterior separation (m/second) | 1.38 (0.21) | 1.07 (0.30) | 1.11 (0.24) | 1.27 (0.20) | < 0.0001* |
| 0.0028† | |||||
| At maximum mediolateral separation (m/second) | 0.05 (0.02) | 0.07 (0.03) | 0.06 (0.04) | 0.06 (0.03) | 0.2075* |
| 0.0589† | |||||
Values are expressed as means, with standard deviations in parentheses; *time effects; †group effects; CoM = center of mass; CoP = center of pressure.
Table 3.
Temporal-distance gait measures for the control and THA groups
| Temporal-distance measure | Control subjects | Patients undergoing THA | p Values | ||
|---|---|---|---|---|---|
| Presurgery | 6 weeks postsurgery | 16 weeks postsurgery | |||
| Gait velocity (m/second) | 1.28 (0.17) | 1.00 (0.26) | 1.06 (0.22) | 1.19 (0.16) | < 0.0001‡ |
| 0.0024§ | |||||
| Stride length* | 0.80 (0.08) | 0.64 (0.14) | 0.67 (0.10) | 0.73 (0.07) | < 0.0001‡ |
| 0.0005§ | |||||
| Step width† | 0.36 (0.07) | 0.44 (0.09) | 0.43 (0.07) | 0.41 (0.07) | 0.1205‡ |
| 0.0066§ | |||||
| Stride time (seconds) | 1.06 (0.09) | 1.13 (0.11) | 1.12 (0.13) | 1.07 (0.09) | 0.0073‡ |
| 0.0949§ | |||||
Values are expressed as means, with standard deviations in parentheses; *normalized to body height; †normalized to anterior superior iliac spine width; ‡time effects; §group effects.
Patients having THA had improved gait and balance control by 16 weeks postsurgery. Patients undergoing THA had a smaller (p = 0.0084) medial inclination angle with a greater (p = 0.0247) posterior inclination angle 16 weeks postsurgery when compared with before surgery (Fig. 3B–C). The CoM velocity also increased (p < 0.0001) for patients undergoing THA at maximum anterior and posterior CoM-CoP separation by 16 weeks postsurgery. However, at 6 weeks postsurgery, only the medial inclination angle improved (p = 0.0078) from before surgery. When compared with presurgery, the THA group walked faster (p < 0.0001), with a larger (p < 0.0001) stride length and a shorter (p = 0.0045) stride time at 16 weeks postsurgery (Table 3). Values of these parameters at 6 weeks postsurgery were similar to those before surgery (Table 3).
When compared with the control subjects at 16 weeks postsurgery, patients undergoing THA performed similarly in gait velocity (p = 0.2383), stride length (p = 0.0576) (Table 3), and CoM velocities at maximum anterior and posterior CoM-CoP separations (p = 0.2204 and p = 0.1979, respectively) (Table 2). However, their CoM-CoP inclination angles in anterior (p = 0.0064) and medial (p = 0.0101) directions failed to reach control levels (Fig. 3).
Discussion
Given that greater than 60% of patients undergoing THA in the United States are older than 65 years and deteriorated joint function and reduced muscle strength are major risk factors of falling in the elderly, a better understanding of the presence or resolution of gait imbalance for patients undergoing THA is needed. We therefore hypothesized patients undergoing THA would have characteristics associated with impaired balance control before surgery and THA would allow patients to decrease the medial inclination angle and increase the sagittal plane angle by 16 weeks postsurgery.
We note several limitations. First is the small sample size. We performed a post hoc power analysis using the medial CoM-CoP inclination angle to compare differences between groups. Our sample size of 30 subjects had 79.7% power to detect differences at the 0.05 level between the THA and control groups with an effect size of 1.2 [18]. This indicates patients having THA sway with an angle that is 1.2 standard deviations above the mean value of control subjects. The magnitude and pattern of the medial inclination angle for patients undergoing THA were similar to those reported for a group of elderly patients experiencing falls [20]. Second, the difference in body mass index (BMI) among patients having THA and control subjects could be another limitation of this study. A high BMI is reportedly a risk factor for THA owing to its possible association with osteoarthritis [17]. The BMIs for our patients having THA and control subjects are consistent with typically reported values [33, 36]. Although obese men walk slower with shorter strides and wider steps than nonobese individuals [44] and obese children have greater sway areas in the medial lateral direction [28], we observed improvement in gait performance after THA. By 16 weeks postsurgery, our patients walked with a similar gait velocity and stride length as control subjects while continuing to maintain a greater BMI. Third, the outcomes could be affected by the short-term nature of this investigation. Although patients might show additional improvement by 6 and 12 months after THA, gait imbalance and the subsequent health complications remain a concern during this time. The average time from implant insertion to periprosthetic fracture ranged from 0.3 years for ingrowth stems to 8.5 years for loose cemented stems, with a majority of fractures resulting from falls [2]. Of 16 fractures after THA investigated by Wu et al. [49], five occurred within 1 month after surgery. In other studies, patients undergoing THA exhibited asymmetry in step length, load-bearing [8], and reduced walking efficiency compared with healthy patients [34] 4 weeks after surgery. Therefore, understanding the gait patterns 6 weeks and 16 weeks postsurgery could allow for proper postoperative rehabilitation intervention and return patients to control levels quickly.
Given that the peak medial CoM-CoP inclination angle occurs during the single-support phase (Fig. 2B), an additional analysis was performed to detect any differences between the operated and nonoperated limbs in patients having THA. No between-limb differences were detected across all three testing times. At 6 weeks postsurgery, the peak medial CoM-CoP inclination angle was the only measure that showed substantial improvement as compared with before surgery. This frontal plane balance measure reportedly better distinguishes elderly patients who experience falls than typical gait temporal-distance parameters [20]. It therefore might show more sensitively the effectiveness of THA in improving frontal plane balance control. Given that these patients stopped using crutches 6 weeks postsurgery, such improvement in balance control would be needed.
Although we observed improvements in all balance control measures 16 weeks postsurgery compared with before surgery, patients undergoing THA still had substantially larger medial and smaller anterior CoM-CoP inclination angles than control subjects, indicating evidence of residual deficits in dynamic balance control [20]. These differences in the medial inclination angles agree with findings on hip abductor muscle strength, suggesting persistently diminished abductor moments postsurgery [30, 38]. Patients with abductor weakness or hip pain attempt to laterally shift the CoM over the affected hip to minimize the net joint moments. This perturbs CoM motion in the frontal plane and limits progression in the sagittal plane [25, 39]. Patients undergoing THA had some improvement in their ability to control the CoM momentum as evidenced through the instantaneous velocity of the CoM at maximum CoM-CoP separation (Table 2). It has been suggested dynamic gait stability requires control of CoM position and velocity [37]. Such improvement could be indicative of increased confidence and control of the sagittal plane CoM during gait for patients undergoing THA.
Before surgery, subjects undergoing THA had slower gait velocity, shorter stride length, and wider step width than control subjects. Consistent with the literature, by 16 weeks postsurgery, patients undergoing THA had an increase in gait velocity and stride length compared with before surgery (Table 4) [3, 5, 23, 24, 30, 38, 43, 47]. We observed no major differences between patients and control subjects at 16 weeks postsurgery for these variables, suggesting improved range of motion and/or hip muscle strength after surgery and more efficient leg progression during gait [32]. Although gait velocity is slower up to 4 years after THA [24], this patient group may have regained normal temporal-distance gait patterns at a faster rate as a result of differences in rehabilitation protocols, specific surgical procedures, or postoperative activity levels.
Table 4.
Published studies of temporal-distance gait measures
| Study | Followup | Gait velocity (m/second) | Stride length (m) | Step width (cm) | |||
|---|---|---|---|---|---|---|---|
| Presurgery | Postsurgery | Presurgery | Postsurgery | Presurgery | Postsurgery | ||
| Current study | 16 weeks | 1.00 (0.26) | 1.19 (0.16) | 1.11 (0.25) | 1.26 (0.15) | 13.9 (4.0) | 13.0 (2.6) |
| Bennett et al. [3] | 10 years | NA | 1.07 (0.24) | NA | 1.14 (0.19) | NA | NA |
| Boardman et al. [5] | 12 months | 0.99 (0.24) | 1.17 (0.20) | NA | NA | NA | NA |
| Lindemann et al. [23] | 3 months | 1.06 (0.20) | 1.16 (0.13) | 1.2 (0.15) | 1.26 (0.12) | NA | NA |
| Loizeau et al. [24] | 4 years | NA | 0.71* (0.13) | NA | 1.02* (0.16) | NA | NA |
| Mont et al. [30] | 6–15 months | NA | 0.96 (0.13) | NA | NA | NA | NA |
| Perron et al. [38] | 6–18 months | NA | 1.07 (0.2) | NA | 1.2 (0.1) | NA | NA |
| Sliwinski et al. [43] | 2–24 months | NA | 1.09 (0.19) | NA | NA | NA | NA |
| Wall et al. [47] | 6 months | 0.34† (0.17) | 0.61† (0.18) | 0.78 (0.20) | 1.21 (0.20) | NA | NA |
Values are expressed as means, with standard deviations in parentheses; *values provided are for the affected limb; †normalized to body height; NA = data not available.
Wide step widths have been associated with lateral extensions of the base of support and a need to laterally control the position and velocity of the CoM [11, 15]. Although there have been reports of changes before and after surgery in gait velocity and stride length [23, 47], changes in step width have not been reported. By maintaining a larger step width presurgery when compared with control subjects, patients showed strategy to accommodate a greater medial CoM-CoP inclination angle during gait. A wider step width may be a preventive measure to ensure the CoM remains inside the base of support, and such a strategy may compensate for existing instability [11, 15]. However, we observed no major narrowing of the step width among the THA group postsurgery.
Differing gait patterns between patients undergoing THA and control subjects may be the result of residual antalgic gait [27]. McCrory et al. [27] hypothesized lingering differences might be the result of physiologic deficits such that patients have inability to restore hip strength and range of motion to normal levels, and adaptation of a habitual gait pattern. Surgery reduced pain at the hip, but because of fundamental differences in BMI and residual antalgic gait, it is possible patients will always maintain balance control different from that of control subjects.
Our data suggest a short-term improvement in gait performance after THA. Although balance control has improved, as measured by the CoM-CoP inclination angles, patients undergoing THA still have not reached the level of control subjects by 16 weeks postsurgery.
Acknowledgments
We thank Crystal Mills for assisting in the recruitment of patients and Robin High for consulting with us on the statistical analysis.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Arnold CM, Faulkner RA. The history of falls and the association of the timed up and go test to falls and near-falls in older adults with hip osteoarthritis. BMC Geriatr. 2007;7:17. [DOI] [PMC free article] [PubMed]
- 2.Beals RK, Tower SS. Periprosthetic fractures of the femur: an analysis of 93 fractures. Clin Orthop Relat Res. 1996;327:238–246. [DOI] [PubMed]
- 3.Bennett D, Humphreys L, O’Brien S, Kelly C, Orr JF, Beverland DE. Gait kinematics of age-stratified hip replacement patients: a large scale, long-term follow-up study. Gait Posture. 2008;28:194–200. [DOI] [PubMed]
- 4.Bertocci GE, Munin MC, Frost KL, Burdett R, Wassinger CA, Fitzgerald SG. Isokinetic performance after total hip replacement. Am J Phys Med Rehabil. 2004;83:1–9. [DOI] [PubMed]
- 5.Boardman DL, Dorey F, Thomas BJ, Lieberman JR. The accuracy of assessing total hip arthroplasty outcomes: a prospective correlation study of walking ability and 2 validated measurement devices. J Arthroplasty. 2000;15:200–204. [DOI] [PubMed]
- 6.Chou LS, Kaufman KR, Brey RH, Draganich LF. Motion of the whole body’s center of mass when stepping over obstacles of different heights. Gait Posture. 2001;13:17–26. [DOI] [PubMed]
- 7.Chou LS, Kaufman KR, Hahn ME, Brey RH. Medio-lateral motion of the center of mass during obstacle crossing distinguishes elderly individuals with imbalance. Gait Posture. 2003;18:125–133. [DOI] [PubMed]
- 8.Cichy B, Wilk M, Sliwinski Z. Changes in gait parameters in total hip arthroplasty patients before and after surgery. Med Sci Monit. 2008;14:CR159–CR169. [PubMed]
- 9.Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86:963–974. [DOI] [PubMed]
- 10.Franklin J, Malchau H. Risk factors for periprosthetic femoral fracture. Injury. 2007;38:655–660. [DOI] [PubMed]
- 11.Grabiner MD, Troy KL. Attention demanding tasks during treadmill walking reduce step width variability in young adults. J Neuroeng Rehabil. 2005;2:25. [DOI] [PMC free article] [PubMed]
- 12.Grigg P, Finerman GA, Riley LH. Joint-position sense after total hip replacement. J Bone Joint Surg Am. 1973;55:1016–1025. [PubMed]
- 13.Hahn ME, Chou LS. Age-related reduction in sagittal plane center of mass motion during obstacle crossing. J Biomech. 2004;37:837–844. [DOI] [PubMed]
- 14.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 15.Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking: experimental findings in normal subjects and above-knee amputees. Gait Posture. 2007;25:250–258. [DOI] [PubMed]
- 16.Karanjia PN, Ferguson JH. Passive joint position sense after total hip replacement surgery. Ann Neurol. 1983;13:654–657. [DOI] [PubMed]
- 17.Karlson EW, Mandl LA, Aweh GN, Sangha O, Liang MH, Grodstein F. Total hip replacement due to osteoarthritis: the importance of age, obesity, and other modifiable risk factors. Am J Med. 2003;114:93–98. [DOI] [PubMed]
- 18.Keppel G, Wickens T. Design and Analysis: A Researcher’s Handbook. Upper Saddle River, NJ: Pearson Prentice Hall; 2004.
- 19.Laupacis A, Bourne R, Rorabeck C, Feeny D, Wong C, Tugwell P, Leslie K, Bullas R. The effect of elective total hip replacement on health-related quality of life. J Bone Joint Surg Am. 1993;75:1619–1626. [DOI] [PubMed]
- 20.Lee HJ, Chou LS. Detection of gait instability using the center of mass and center of pressure inclination angles. Arch Phys Med Rehabil. 2006;87:569–575. [DOI] [PubMed]
- 21.Lindahl H, Garellick G, Regnér H, Herberts P, Malchau H. Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am. 2006;88:1215–1222. [DOI] [PubMed]
- 22.Lindahl H, Oden A, Garellick G, Malchau H. The excess mortality due to periprosthetic femur fracture: a study from the Swedish national hip arthroplasty register. Bone. 2007;40:1294–1298. [DOI] [PubMed]
- 23.Lindemann U, Becker C, Unnewehr I, Muche R, Aminin K, Dejnabadi H, Nikolaus T, Puhl W, Huch K, Dreinhöfer KE. Gait analysis and WOMAC are complementary in assessing functional outcome in total hip replacement. Clin Rehabil. 2006;20:413–420. [DOI] [PubMed]
- 24.Loizeau J, Allard P, Duhaime M, Landjerit B. Bilateral gait patterns in subjects fitted with a total hip prosthesis. Arch Phys Med Rehabil. 1995;76:552–557. [DOI] [PubMed]
- 25.MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26:633–644. [DOI] [PubMed]
- 26.Majewski M, Bischoff-Ferrari HA, Grüneberg C, Dick W, Allum JH. Improvements in balance after total hip replacement. J Bone Joint Surg Br. 2005;87:1337–1343. [DOI] [PubMed]
- 27.McCrory JL, White SC, Lifeso RM. Vertical ground reaction force: objective measures of gait following hip arthroplasty. Gait Posture. 2001;14:104–109. [DOI] [PubMed]
- 28.McGraw B, McClenaghan BA, Williams HG, Dickerson J, Ward DS. Gait and postural stability in obese and nonobese prepubertal boys. Arch Phys Med Rehabil. 2000;81:484–489. [DOI] [PubMed]
- 29.Mitchell S, McCaskie A, Francis R, Peaston R, Birrell F, Lingard E. The need for a falls prevention programme for patients undergoing hip and knee replacement surgery. J Orthop Nursing. 2007;11:98–103. [DOI]
- 30.Mont MA, Seyler TM, Ragland PS, Starr R, Erhart J, Bhave A. Gait analysis of patients with resurfacing hip arthroplasty compared with hip osteoarthritis and standard total hip arthroplasty. J Arthroplasty. 2007;22:100–108. [DOI] [PubMed]
- 31.Nahit ES, Silman AJ, Macfarlane GJ. The occurrence of falls among patients with a new episode of hip pain. Ann Rheum Dis. 1998;57:166–168. [DOI] [PMC free article] [PubMed]
- 32.Nallegowda M, Singh U, Bhan S, Wadhwa S, Handa G, Dwivedi SN. Balance and gait in total hip replacement: a pilot study. Am J Phys Med Rehabil. 2003;82:669–677. [DOI] [PubMed]
- 33.Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty. 2005;20(7 suppl 3):46–50. [DOI] [PubMed]
- 34.Nankaku M, Tsuboyama T, Kakinoki R, Kawanabe K, Kanzaki H, Mito Y, Nakamura T. Gait analysis of patients in early stages after total hip arthroplasty: effect of lateral trunk displacement on walking efficiency. J Orthop Sci. 2007;12:550–554. [DOI] [PubMed]
- 35.Nantel J, Termoz N, Centomo H, Lavigne M, Vendittoli PA, Prince F. Postural balance during quiet standing in patients with total hip arthroplasty and surface replacement arthroplasty. Clin Biomech (Bristol, Avon). 2008;23:402–407. [DOI] [PubMed]
- 36.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960–2002. Adv Data. 2004;347:1–17. [PubMed]
- 37.Pai YC, Patton J. Center of mass velocity-position predictions for balance control. J Biomech. 1997;30:347–354. [DOI] [PubMed]
- 38.Perron M, Malouin F, Moffet H, McFadyen BJ. Three-dimensional gait analysis in women with a total hip arthroplasty. Clinical Biomech (Bristol, Avon). 2000;15:504–515. [DOI] [PubMed]
- 39.Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: Slack Inc; 1992.
- 40.Prudham D, Evans JG. Factors associated with falls in the elderly: a community study. Age Ageing. 1981;10:141–146. [DOI] [PubMed]
- 41.Robbins AS, Rubenstein LZ, Josephson KR, Schulman BL, Osterweil D, Fine G. Predictors of falls among elderly people: results of two population-based studies. Arch Intern Med. 1989;149:1628–1633. [DOI] [PubMed]
- 42.Salaffi F, Carotti M, Grassi W. Health-related quality of life in patients with hip or knee osteoarthritis: comparison of generic and disease-specific instruments. Clin Rheumatol. 2005;24:29–37. [DOI] [PubMed]
- 43.Sliwinski MM, Sisto SA, Batavia M, Chen B, Forrest GF. Dynamic stability during walking following unilateral total hip arthroplasty. Gait Posture. 2004;19:141–147. [DOI] [PubMed]
- 44.Spyropoulos P, Pisciotta JC, Pavlou KN, Cairns MA, Simon SR. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil. 1991;72:1065–1070. [PubMed]
- 45.Steultjens MP, Dekker J, van Baar ME, Oostendorp RA, Bijlsma JW. Muscle strength, pain and disability in patients with osteoarthritis. Clin Rehabil. 2001;15:331–341. [DOI] [PubMed]
- 46.Sturnieks DL, Tiedemann A, Chapman K, Munro B, Murray SM, Lord SR. Physiological risk factors for falls in older people with lower limb arthritis. J Rheumatol. 2004;31:2272–2279. [PubMed]
- 47.Wall JC, Ashburn A, Klenerman L. Gait analysis in the assessment of functional performance before and after total hip replacement. J Biomed Eng. 1981;3:121–127. [DOI] [PubMed]
- 48.Woltring HJ. A FORTRAN package for generalized, cross-validatory spline smoothing and differentiation. Adv Eng Software. 1986;8:104–113.
- 49.Wu CC, Au MK, Wu SS, Lin LC. Risk factors for postoperative femoral fracture in cementless hip arthroplasty. J Formos Med Assoc. 1999;98:190–194. [PubMed]
- 50.Wykman A, Olsson E. Walking ability after total hip replacement: a comparison of gait analysis in unilateral and bilateral cases. J Bone Joint Surg Br. 1992;74:53–56. [DOI] [PubMed]
- 51.Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J, Bright RA. Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89:526–533. [DOI] [PubMed]