Abstract
Obtaining autogenous bone graft from the iliac crest can entail substantial morbidity. Alternatively, bone graft can be harvested from long bones using an intramedullary (IM) harvesting system. We measured bone graft volume obtained from the IM canals of the femur and tibia and documented the complications of the harvesting technique. Donor site pain and the union rate were compared between the IM and the traditional iliac crest bone graft (ICBG) harvest. Forty-one patients (23 male, 18 female) with an average age of 44.9 years (range, 15–78 years) had graft harvested from long bones using an IM harvest system (femoral donor site, 37 patients; tibial donor site, four patients). Forty patients (23 male, 17 female; average age, 46.4 years; range, 15–77 years) underwent anterior ICBG harvest. We administered patient surveys to both groups to determine pain intensity and frequency. IM group reported lower pain scores than the ICBG group during all postoperative periods. Mean graft volume for the IM harvest group was 40.3 mL (range, 25–75 mL) (graft volume was not obtained for the ICBG group). Using an intramedullary system to harvest autogenous bone graft from the long bones is safe provided a meticulous technique is used.
Level of Evidence: Level III, retrospective comparative study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Autogenous bone graft is the material of choice in limb reconstruction and fusion procedures of the extremities [6]. Many potential donor sites are available for graft harvest, all with recognized major and minor complications [1, 4, 7, 20, 23, 27]. Sources of relatively large amounts of bone graft (ie, 20–40 cc) are the anterior iliac crest, posterior iliac crest, and the fibula. Harvesting bone graft from these sites can result in complications such as residual pain, injury to the lateral femoral cutaneous nerve anteriorly, injury to the superficial peroneal nerves at the region of the fibula, and injury to the superior cluneal nerves in the region of the posterior iliac crest [4]. Other major complications include abdominal hernias, sacroiliac joint injuries, avulsion fractures of the anterior iliac spine, and vascular injury to the superior gluteal artery [4]. Despite all these complications, these areas provide excellent bone graft and are the only sources of tricortical bone graft when immediate structural stability is required [4].
The medullary canal of the long bones is another potential bone graft donor site [24]. The reamer/irrigator/aspirator (RIA) system (Synthes, Inc., West Chester, PA) was developed as a simultaneous reaming and aspiration system to reduce the intramedullary pressure, heat generation, operating time, and systemic effects of reaming (eg, fat embolism) [2, 10, 11, 14, 16, 17, 22]. Even though the device was conceived in the 1970s, it was not developed for clinical use until the late 1990s [3]. The RIA system was used to prepare long bones for fracture fixation with intramedullary nails. Several investigators noted that the aspirate from the reaming system was laden with bioactive substrates (eg, osteoblasts) and growth factors (eg, bone morphogenetic protein–2 [BMP-2]) after performing cell cultures and flow cytometry [8, 9, 18, 22, 25, 26]. The RIA system has been used clinically to procure bone graft from the femur; however, few studies describe its clinical use [12, 13, 15, 24].
We therefore report the (1) graft volume removed using the RIA system and the (2) union rate of the recipient sites in both the RIA and iliac crest bone graft (ICBG) harvest groups after bone grafting combined with BMP-2. We also compared the (3) complications and (4) donor site pain after bone graft harvest with the RIA system with a control group of patients who underwent anterior ICBG harvest.
Materials and Methods
We retrospectively reviewed prospectively collected data on 41 patients (RIA group) who had bone graft harvested from the femoral or tibial intramedullary canal between January 2006 and December 2007. Our historical control group included 40 patients (ICBG group) who had bone graft harvested from the anterior iliac crest between January 2005 and February 2007. Six patients in the study had ICBG harvested during one stage of treatment and bone graft harvested using the RIA system during another stage of treatment. We compared the union rates and complications including pain between the two groups. We also reported the volume of graft collected using the RIA system. Power calculations were conducted using G*Power 3 statistical software [5]. We anticipated large effect sizes in the present study; therefore, power calculations were conducted using a large estimated effect size (d = 0.80). This was used to determine an appropriate sample size needed to detect a meaningful difference between the groups for an independent samples two-tailed t test. Power calculations indicated that approximately n = 42 was required in each of the two independent treatment groups. Forty-one patients were in the RIA group and 40 patients were in the ICBG group, which yielded robust effect sizes (range, d = 1.42–2.13) for all dependent variables (ie, acute pain intensity, acute pain frequency, intermediate pain intensity, intermediate pain frequency, chronic pain intensity, chronic pain frequency). We had prior IRB approval for our retrospective comparison study. We obtained verbal informed consent from the participants when the prospective survey was administered.
Data collected during the course of treatment included the amount of bone graft harvested from patients in the RIA group, the union rate of the area receiving the bone graft, and complications. Prospectively collected data also included immediate postoperative pain (within 48 hours), intermediate postoperative pain, and long-term (more than 3 months) postoperative pain using a subjective scoring system. The historical comparison group who had bone graft harvested from the anterior iliac crest were interviewed by telephone to determine the immediate, intermediate, and long-term pain scores. We obtained data on demographics, diagnosis, indication for surgery, type of procedure, donor site for bone graft harvest, diameter of the reamer used, volume of the graft harvested, and complications from the medical records, operative notes, and imaging studies.
The RIA Bone Graft Harvest Group consisted of 41 patients (23 male and 18 female). The average age at surgery was 44.9 years (range, 15–78 years). The primary procedure was performed to treat nonunion of the femur (nine patients), nonunion of the tibia (12 patients), knee fusion nonunion (six patients), nonunion of ankle fusion (five patients), nonunion of the humerus (two patients), nonunion at the regenerate site following lengthening or deformity correction (two patients), docking site nonunion (two patients), congenital pseudarthrosis of the tibia (two patients), and cervical spine fusion (one patient). The bone graft was harvested from the femur in 37 patients (ipsilateral femur, 23 patients; contralateral femur, 14 patients) and the ipsilateral tibia in four patients. A single reamer head was used in 36 patients, and two reamer heads were used in five patients. The median diameter of the reamers was 14 mm (range, 12–16 mm). BMP-2 was used in addition to the bone graft to augment the nonunion site in 39 of 41 patients. The minimum followup was 3 months (average, 9.1 months; range, 3–17 months).
The Iliac Crest Bone Graft Harvest Group served as the control group and consisted of 40 patients (23 male and 17 female) who underwent anterior iliac crest bone graft harvest. We selected these patients as controls because this was the most common iliac crest donor region used at our center before we began using the intramedullary harvesting technique. In the ICBG group, the average age at surgery was 46.4 years (range, 15–77 years). In the majority of the ICBG cases, harvesting anterior ICBG helped to expedite the surgical procedures because harvesting the posterior ICBG would have required separate positioning, prepping, and draping before the procedure for repair of the nonunion. The primary procedure was performed to treat nonunion of the femur (seven patients), nonunion of the tibia (11 patients), knee fusion nonunion (seven patients), nonunion of foot and ankle fusion (five patients), nonunion of the humerus (three patients), nonunion of the radius (one patient), nonunion at the regenerate site following lengthening or deformity correction (three patients), docking site nonunion (two patients), and congenital pseudarthrosis of the tibia (one patient). The minimum followup period was 5 months (average, 20.2 months; range, 5–30 months).
The patients were not matched for age, sex, or diagnosis to the RIA group. Patients in both the RIA and ICBG groups were at least 15 years old, and the bone graft was harvested for similar indications as in the RIA group.
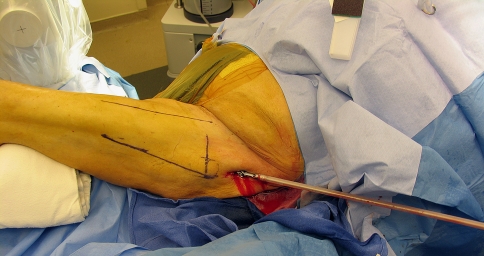
The same surgeons (JDC, DP, JEH) harvested bone graft from both groups. Our surgical technique is similar to that described by Stafford and Norris [24] with a few exceptions. We positioned the patient supine on a radiolucent table with a bump under the buttock. We flexed and adducted the lower limb over the contralateral lower limb to obtain access to the femur. The anatomic axis of the femur was identified on both coronal and sagittal planes. We used a 2-mm Steinmann pin to identify the entry point to the femoral canal. We prefer to use a trochanteric tip entry point as opposed to a piriformis entry to avoid damage to the femoral neck. We then used a minimally invasive approach and made a 2- to 3-cm incision centered on this pin. The 8-mm cannulated acorn reamer was passed over the Steinmann pin to broach the greater trochanter. A 2.5-mm ball-tipped guide wire was then positioned in the femur. The position of the guide wire was confirmed to be central on both views of the femur. The diameter of the reamer head was chosen after the guide wire was inserted into the femoral canal. The isthmus of the femur was identified radiographically, and a radiopaque ruler with millimeter increments was used to measure the diameter of the femur at the isthmus. Our preference is to choose a reamer head size that overlaps the inner cortical diameter of the bone by one millimeter on each side. The reamer head diameter was determined by measuring the diameter of the femoral canal at the isthmus. As the reamer head entered the femur, an approach/withdrawal technique was used to slowly advance the reamer through the femur (Fig. 1). The first pass was directed towards the lateral femoral condyle and the second pass to the medial femoral condyle. It is very important to visualize the entire process with fluoroscopy to avoid eccentric reaming of the canal. Also, with the approach/withdrawal technique, it is important to ensure the guide wire is still in place in the distal femur. The intramedullary bone graft harvest was performed with the RIA system (Synthes, Inc.) in this group (Figs. 2, 3). The graft was harvested using a minimally invasive technique in all patients. When the collection filter was full, the reaming was stopped and the filter emptied before beginning any additional reaming (Fig. 4A–B). If more bone graft was required, the next size reamer head was used. The reamer cutting head and drive shaft tubing are single-use devices. Therefore, it can be difficult to replace the reamer head with a larger-diameter reamer head. Care should be taken not to over-thin the femoral cortices. The effect of cortical thinning on mechanical strength of the femoral diaphysis is negligible when overreamed by 2 mm [19]. The wound was closed in two layers. If the RIA graft was harvested from the contralateral limb, full weight bearing was allowed on that limb. If the graft was harvested from the ipsilateral limb, weight bearing was dictated by the condition of the recipient site. The RIA system was also used to harvest intramedullary bone graft from the tibia. The technique is similar to the one that is used to ream the tibial shaft for fracture fixation. A 12.5- to 14-mm diameter reamer head was used to ream the tibia.
Fig. 1.
The patient is positioned supine on the radiolucent table with the leg adducted, and the anatomic axis of the femur is marked in both planes. The level of the greater trochanter is marked. The minimally invasive approach is shown.
Fig. 2.
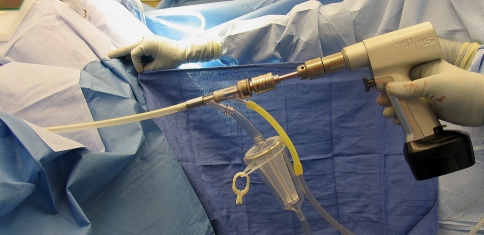
The Reamer/Irrigator/Aspirator (RIA) system consists of: A, a reamer head; B, collection tube outside the drive shaft; C, aspiration port; D, irrigation port; E, drive shaft seal.
Fig. 3.
Photograph shows the RIA system assembled with the capturing system and filter.
Fig. 4A–B.
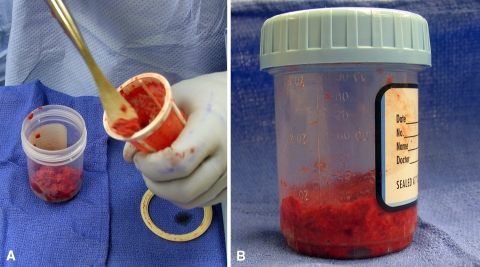
(A) The intramedullary bone graft is transferred from the capturing system to a (B) measuring bottle.
Anterior iliac crest cancellous bone graft was harvested through a separate incision made over the anterior iliac crest beginning 2 cm posterior to the anterior superior iliac spine and carried posteriorly. A window was made in the cortex of the cephalad aspect of the iliac crest, and a curette was used to harvest the cancellous bone between the iliac tables. BMP-2 was used in 32 of 40 patients in this group. No substantial defect in the outer iliac crest was created, and no backfill material was implanted. The incision was closed in three layers over a small closed wound suction drain (C.R. Bard, Inc., Covington, GA, USA). Bone graft was harvested from the anterior iliac crest in all 40 patients in the ICBG group, but the graft volume obtained was not recorded.
We (GJ, MVB) assessed outcomes using a telephone survey (Appendix 1) that was created by the authors. During the telephone survey, the patients were asked to report their pain intensity and frequency of pain before 48 hours, from 48 hours to 3 months, and after 3 months. A pain evaluation score consisted of two components: one for measuring intensity of pain and the other for frequency of the pain. For the intensity scale, a rating of 0 points meant no pain and 10 points was “as bad as it could be.” For the frequency scale, a rating of 0 points meant that pain was present “none of the time” and 10 points meant it was present “all the time.” A total score of 20 points indicated the worst pain was present “all the time.” The patients were asked whether they had difficulty with ambulation or sensation at the donor site during the acute (< 3 months) and chronic (> 3 months) postoperative period. The patients were also asked about complications (ie, persistent drainage from the donor site, wound dehiscence) and their satisfaction with the cosmetic result after 3 months. Forty of 41 patients in the RIA group were contacted by telephone using the survey described above. One patient had died due to causes unrelated to the bone graft harvest. We were able to contact all patients in the ICBG group.
All data were approximately normally distributed. We performed independent samples t tests to compare pain levels at three separate time periods for both the RIA and ICBG groups. All data were analyzed using SPSS for Windows, version 10.0 (SPSS Inc., Chicago, IL).
Results
The average volume of graft for the whole RIA group was 40.3 mL (range, 25–75 mL). The average volume of graft harvested from the femur (37 patients) was 41.1 mL (range, 25–75 mL). The average volume of graft harvested from the tibia (four patients) was 32.5 mL (range, 25–50 mL).
In the RIA group, the recipient site healed in 37 of 41 patients. In the ICBG group, 32 of 40 patients achieved union at the nonunion site.
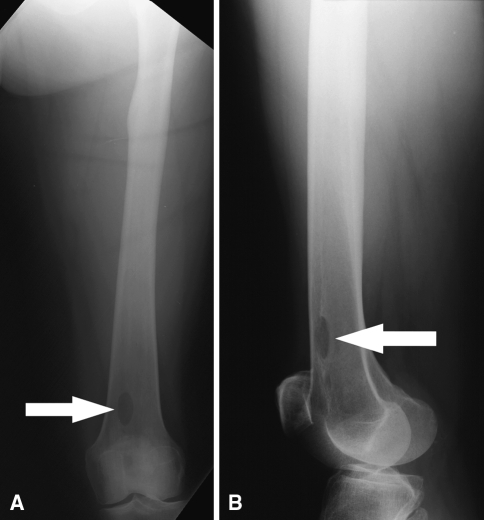
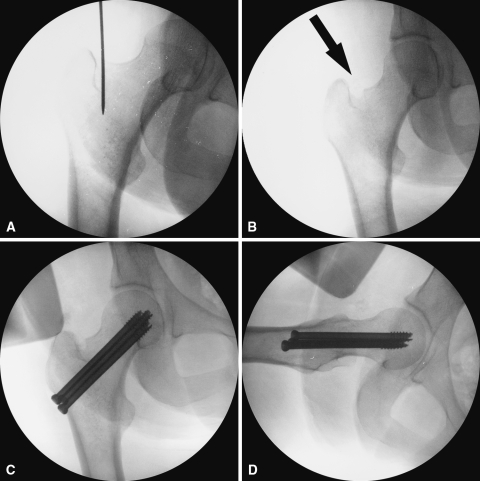
Two technical complications occurred in the RIA group that were related to the harvesting technique. In one patient, the distal anterior femoral cortex was perforated because of eccentric reaming of the canal (Fig. 5A–B). Only partial weight bearing was allowed on the lower limb for 3 months. The patient had persistent pain in the distal anterior thigh for approximately 4 months after the procedure and then the pain resolved. The second patient had a piriformis entry point rather than a greater trochanteric entry point to the femoral canal. A 15-mm reamer head was used to harvest the graft. This resulted in excessive reaming of the femoral neck. The patient underwent prophylactic fixation of the femoral neck to prevent a femoral neck fracture (Fig. 6A–D). No technical complications occurred in the ICBG group. Two mechanical difficulties were encountered in the RIA group. In two patients, the capturing system disengaged from the reamer during the reaming process. The system was reattached without losing any of the harvested graft. There were no mechanical failures in the ICBG group. In the RIA group, we observed no instances of deep infection, hematoma, femoral fracture, or fat embolism related to the harvest procedure. In the ICBG group, we observed four donor-site related complications. Three patients developed deep infection of the donor site, and one patient developed a hematoma. These patients underwent incision, drainage, and débridement of the donor site. None of the patients in the RIA group had numbness related to the donor site. Eight patients in the ICBG group had numbness related to the donor site.
Fig. 5A–B.
(A) Anteroposterior and (B) lateral view radiographs show the distal femur. The arrows show the perforation in the anterior femoral cortex.
Fig. 6A–D.
(A) Anteroposterior view fluoroscopic image of the proximal femur shows the piriformis entry point. (B) Anteroposterior view fluoroscopic image shows aggressive reaming of the femoral neck (arrow). (C) Anteroposterior and (D) lateral view fluoroscopic images show prophylactic fixation with cannulated cancellous screws.
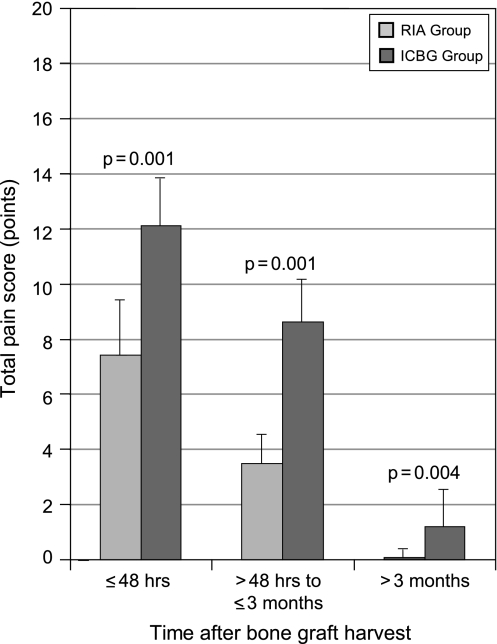
The total pain score (frequency and intensity) within 48 hours was greater (p = 0.001) for the ICBG than for the RIA group (Fig. 7). The average total pain score for the RIA group was 7.41 points (range, 0–12 points). The average total pain score for the ICBG group was 12.13 points (range, 9–18 points). The pain score less than 3 months postoperatively was also higher (p = 0.001) among patients in the ICBG group (Fig. 7). The average intermediate pain score for the RIA group was 3.51 points (range, 0–9 points) and for the ICBG group was 8.61 points (range, 3–15 points). Chronic pain scores more than 3 months postoperatively were higher (p = 0.004) among patients in the ICBG group (Fig. 7). For the RIA and ICBG groups, the average chronic pain scores were 0.10 points (range, 0–4 points) and 1.30 points (range, 0–10 points), respectively.
Fig. 7.
The total pain scores reported by patients in the iliac crest bone graft (ICBG) group were compared with the scores reported by the reamer/irrigator/aspirator (RIA) group. Patients were asked during a telephone survey to report their pain intensity and frequency of pain less than 48 hours, from 48 hours to 3 months, and more than 3 months after the procedure. Patients in the RIA group experienced substantially less pain during all three time periods.
Discussion
Obtaining autogenous bone graft from the iliac crest often entails substantial morbidity. Graft material may also be harvested from long bones using an intramedullary (IM) harvesting system. We therefore documented the volume of bone graft harvested from the intramedullary canals of the tibia and the femur, the union rate of the recipient sites in both the intramedullary and iliac crest bone graft harvest groups, the complications of the intramedullary harvest technique, and the donor site pain of the intramedullary harvest versus the traditional iliac crest bone graft harvest.
This study has several limitations. The followup period for the RIA group is of short duration (average, 9.1 months; range, 3–17 months). The patients in the ICBG group might have been susceptible to “memory bias” when responding to the survey because they had a longer followup time (average, 20.2 months) than the RIA group (average, 9.1 months). It is unclear whether this memory bias would have made their pain scores higher or lower. Although our effect size is greater than or equal to d = 1.42, our power analysis was based on numerical rating scales for pain intensity and frequency that are not validated. Another limitation of this study is the use of anterior iliac crest bone graft instead of posterior iliac crest bone graft for comparison. Patients who undergo posterior iliac crest graft harvest have fewer complications and higher graft volumes than patients who undergo anterior iliac crest harvest [1]. Patients who underwent posterior iliac crest harvest could have provided a better control group. Also, union rates in the RIA and ICBG groups cannot be solely attributed to bone graft because BMP-2 was used in almost all patients and we had other unmatched variables (eg, smoking history, diabetes).
The volume of bone graft (average, 40.3 mL; range, 25–75 mL) reported in this study is slightly lower than the range reported in the literature (range, 30–90 mL) [21, 24]. We are unaware of comparative figures for graft harvested from the tibia (average, 32.5 mL; range, 25–50 mL).
The rate of recipient site healing was comparable in both the ICBG and RIA groups although we did not match the groups for potentially confounding variables (eg, smoking history, diabetes) and we used BMP–2 in most cases.
Iatrogenic femoral fracture was the most catastrophic complication cited in Stafford and Norris’s [24] technical review paper. This complication did not occur in our series, but eccentric reaming did lead to an anterior cortical breach and an impending femoral neck fracture in two patients.
Patients in our RIA group experienced less pain during all three time periods—acute, intermediate, and chronic. The donor site complications of hematoma and infection that were present in the iliac crest group were not observed in the intramedullary harvesting group. This is possibly because of the very percutaneous technique used to harvest the intramedullary bone as well as the constant irrigation of the harvesting system.
This study confirms that intramedullary harvesting of bone graft has minimal donor site morbidity and pain when compared with iliac crest bone graft harvest. Comparable healing rates to iliac crest bone graft can be inferred, but a future prospective study evaluating the two techniques might yield more definitive results. Future research should include comparison with a control group who underwent posterior iliac crest harvest.
Acknowledgments
We thank Dror Paley, MD, for the contribution of his cases to this study. We also thank Stacy C. Specht, MPA, for her assistance with statistical analysis, Joy Marlowe, MA, for her graphic expertise, Alvien Lee for his photography and graphic expertise, and Amanda E. Chase, MA, for her editing expertise.
Appendix
Appendix 1.
RIA/iliac crest questionnaire
| Acute Symptoms | ||
| All questions pertain to the first 3 months after surgery: | ||
| 1. Did you have persistent drainage coming out of your donor site? | Yes | No |
| 2. Did you require antibiotics after surgery? | Yes | No |
| If yes, how long did you take the antibiotics for? | _________ | |
| 3. Did you experience any opening or splitting of the incision at the donor site? | Yes | No |
| 4. Have you had to go back to have surgery for an incision and drainage? | Yes | No |
| 5. Did you have difficulty walking or getting around? | Yes | No |
| Chronic Symptoms | ||
| All questions pertain to the time periodafterthe first 3 months following surgery: | ||
| 1. Are you satisfied with the cosmetic result of surgery? | Yes | No |
| 2. Are you still using pain medication? | Yes | No |
| If yes, what type of medication are you using? | _________ | |
| 3. Do you have any numbness at the donor site? | Yes | No |
| 4. Do you experience any discomfort from clothes rubbing against the donor site? | Yes | No |
| 5. What is your pain level on a 1-to-10-point scale (with 10 being the most severe)? | _________ | |
| Functional Assessment | ||
| All questions pertain to your current situation: | ||
| 1. Do you have difficulty walking or getting around? | Yes | No |
| 2. Has your ability to participate in recreational activities been affected? | Yes | No |
| 3. Have you had to restrict activities of daily living due to pain at the donor site? | Yes | No |
| 4. Have you been limited in your ability to complete household chores? | Yes | No |
| 5. Have you been limited in your ability to complete work/professional duties? | Yes | No |
| 6. Has your ability to participate in sexual activity been limited because of pain at the donor site? | Yes | No |
Footnotes
One of the authors (JDC) has or will receive funding from Synthes for research relevant to the RIA reamer.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtman P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84:716–720. [DOI] [PubMed]
- 2.Bedi A, Karunakar MA. Physiologic effects of intramedullary reaming. Instr Course Lect. 2006;55:359–366. [PubMed]
- 3.Danckwardt-Lillieström G. Reaming of the medullary cavity and its effect on diaphyseal bone. A fluorochromic, microangiographic and histologic study on the rabbit tibia and dog femur. Acta Orthop Scand Suppl. 1969;128:1–153. [DOI] [PubMed]
- 4.Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Ortho Surg. 2001;9:210–218. [DOI] [PubMed]
- 5.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. [DOI] [PubMed]
- 6.Friedlaender GE, Mankin HJ, Goldberg VM, eds. Bone grafts and bone graft substitutes. AAOS Monograph series 32. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006.
- 7.Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76–81. [DOI] [PubMed]
- 8.Hammer TO, Wieling R, Green JM, Südkamp NP, Schneider E, Müller CA. Effect of re-implanted particles from intramedullary reaming on mechanical properties and callus formation. A laboratory study. J Bone Joint Surg Br. 2007;89:1534–1538. [DOI] [PubMed]
- 9.Hoegel F, Mueller CA, Peter R, Pfister U, Suedkamp NP. Bone debris: dead matter or vital osteoblasts. J Trauma. 2004;56:363–367. [DOI] [PubMed]
- 10.Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21:192–197. [DOI] [PubMed]
- 11.Husebye EE, Lyberg T, Madsen JE, Eriksen M, Røise O. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37:935–940. [DOI] [PubMed]
- 12.Kobbe P, Tarkin IS, Frink M, Pape HC. Voluminous bone graft harvesting of the femoral marrow cavity for autologous transplantation: An indication for the “Reamer-Irrigator-Aspirator-” (RIA-) technique [in German]. Unfallchirurg. 2008;111:469–472. [DOI] [PubMed]
- 13.Kobbe P, Tarkin IS, Pape HC. Use of the ‘reamer irrigator aspirator’ system for non-infected tibial non-union after failed iliac crest grafting. Injury. 2008;39:796–800. Epub 2008 Jun 9. [DOI] [PubMed]
- 14.Müller CA, Green J, Südkamp NP. Physical and technical aspects of intramedullary reaming. Injury. 2006;37:S39–S49. [DOI] [PubMed]
- 15.Nichols TA, Sagi HC, Weber TG, Guiot BH. An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 2008;62(3 Suppl 1):E179; discussion E179. [DOI] [PubMed]
- 16.Pape HC, Dwenger A, Grotz M, Kaever V, Negatsch R, Kleemann W, Regel G, Sturm JA, Tscherne H. Does the reamer type influence the degree of lung dysfunction after femoral nailing following severe trauma? An animal study. J Orthop Trauma. 1994;8:300–309. [DOI] [PubMed]
- 17.Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87:2515–2522. [DOI] [PubMed]
- 18.Porter RM, Liu F, Pilapil C, Betz OB, Vrahas MS, Harris MB, Evans CH. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J Orthop Res. 2008 Jul 24. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 19.Pratt DJ, Papagiannnopoulos G, Rees PH, Quinnell R. The effects of medullary reaming on the torsional strength of the femur. Injury. 1987;18:177–179. [DOI] [PubMed]
- 20.Sasso RC, LeHuec JC, Shaffrey C, Spine Interbody Research Group Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(Suppl 1):S77–S81. [DOI] [PubMed]
- 21.Schmidmaier G, Herrmann S, Green J, Weber T, Scharfenberger A, Haas NP, Wildemann B. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39:1156–1163. [DOI] [PubMed]
- 22.Schult M, Küchle R, Hofmann A, Schmidt-Bräkling T, Ortmann C, Wassermann E, Schmidhammer R, Redl H, Joist A. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24:1186–1192. [DOI] [PubMed]
- 23.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, Vaccaro AR, Albert TJ. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134–139. [DOI] [PubMed]
- 24.Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6:100–107. [DOI]
- 25.Tydings JD, Martino LJ, Kircher M, Alfred R, Lozman J. The osteoinductive potential of intramedullary canal bone reamings. Curr Surg. 1986;43:121–124. [PubMed]
- 26.Tydings JD, Martino LJ, Kircher M, Alfred RH, Lozman J. Viability of intramedullary canal bone reamings for continued calcification. Am J Surg. 1987;153:306–309. [DOI] [PubMed]
- 27.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. [DOI] [PubMed]