Abstract
While the concept of using polymer-based sustained-release delivery systems to maintain therapeutic concentration of protein drugs for extended periods of time has been well accepted for decades, there has not been a single product in this category successfully commercialized to date despite clinical and market demands. To achieve successful systems, technical difficulties ranging from protein denaturing during formulation process and the course of prolonged in vivo release, burst release, and incomplete release, to low encapsulation efficiency and formulation complexity have to be simultaneously resolved. Based on this updated understanding, formulation strategies attempting to address these aspects comprehensively were reported in recent years. This review article (with 134 citations) aims to summarize recent studies addressing the issues above, especially those targeting practical industrial solutions. Formulation strategies representative of three areas, microsphere technology using degradable hydrophobic polymers, microspheres made of water soluble polymers, and hydrophilic in vivo gelling systems will be selected and introduced. To better understand the observations and conclusions from different studies for different systems and proteins, physicochemical basis of the technical challenges and the pros and cons of the corresponding formulation methods will be discussed.
Key words: immunogenicity, protein, stability, sustained release
INTRODUCTION
Protein drugs represent a group of the most effective, natural, and the fastest growing medicines for treatment of nearly 150 indications including various severe chronic conditions such as cancer, diabetes, hepatitis, leukemia, and rheumatoid arthritis (1). A critical problem in protein therapy is that most protein drugs are currently administered by frequent injections due to their tissue impermeability and short in vivo life. In the case of chronic conditions, daily or multiple weekly injections for years or even lifetime have resulted in poor patient compliance. For tissue regeneration therapy on the other hand, the in vivo life of some cytokines are limited to hours or even minutes after injection, far from sufficient to exert biological functions in vivo. Sustained-release technology offers the promise for reducing dosing frequency, maximizing the efficacy–dose relationship, and decreasing adverse side effects. To achieve in vivo or in situ sustained-release of protein drugs, various polymer-based formulation strategies have been examined since 1970s (2–10).
However, developing sustained-release dosage forms of proteins has been proven to be a daunting task. Despite extensive research efforts and considerable technology advances, there has yet to be a single sustained-release protein dosage form commercialized to date since the drop off of Nutropin Depot, the only once-launched sustained-release protein drug in this category. Due to the susceptible advanced structures, sustained-release depot technologies successfully applied to peptide drugs are no longer feasible to proteins. Rather, they cause proteins to denature. Therefore, most of recent formulation strategies attempting practical dosage forms have involved efforts to avoid exposing proteins to water–oil interfaces, water–air interfaces, cross-linking reagents, and hydrophobic environment of the polymer matrix of sustained-release systems (3,4).
The primary objective of this review article is to update the advances in developing sustained-release protein dosage forms in recent years, especially those attempting practical industrial technologies or products. For fundamental discussions regarding stabilizing microencapsulated proteins, alleviating acidity generated from degradable polymers, and improving protein release kinetics from polymer-based sustained-release systems, some excellent earlier reviews are available (11–20).
While various formulation strategies have been proposed and examined to achieve a comprehensive solution for sustained-release delivery of proteins, the observations or conclusions from different researchers for different proteins and delivery systems should best be understood in a way such that they can be comparable to each other. Therefore, discussions regarding chemical bases of protein stabilizing approaches in both formulation process and prolonged sustained-release period are included in this review. However, protein PEGylation and other structural modification are considered as different long-acting strategies than sustained-release of native proteins, and, thus, studies in these areas are not discussed.
THE CHALLENGES IN DEVELOPING POLYMER-BASED PROTEIN SUSTAINED-RELEASE SYSTEMS
Protein Instability in Formulation Processes
As compared with peptide drugs, the greatest difficulty in formulating proteins into polymer-based sustained-release dosage forms is that protein molecules possess fragile advanced structures which may easily denature during formulation processes involving water–organic solvent interfaces and during a sustained-release period by protein aggregation and protein adsorption onto the hydrophobic polymers. The energy barrier for dissolved protein molecules to unfold was reported to be around 5–20 kcal/mol (16,17), similar to that of a hydrophobic interaction and water–oil or water–air interfacial tension. Because of such close energy level, protein molecules may easily be denatured due to the interfacial tension between water and organic solvents used to dissolve biodegradable polymers for sustained-release, or due to the contact with the hydrophobic polymer matrix.
To circumvent solvent-induced protein denaturing, contact of protein solutions with organic solvents or hydrophilic/hydrophobic interfaces should be avoided (21). There could be two ways to achieve this: to convert proteins to solid particles or other stabilized forms prior to encapsulating these fragile molecules into polymeric systems (22) and to avoid using polymeric materials which need organic solvents to dissolve (23). For either of the approaches, however, technical challenges still exist. First of all, to convert protein solutions to solid protein particles, the process itself must be mild enough to ensure that the native conformation of proteins will not be altered. While many protein stabilizers (such as salts, sugars, surfactants, and bivalent metal ions) have been applied to form protein-containing solid particles prior to microencapsulation or to stabilize protein droplets suspending in organic polymer solution (24–28), most of them are compromised with burst release (28) protein aggregation (29,30), reduced efficacy (31), and formulation complexity (32). The all-hydrophilic systems, on the other hand, have experienced limited duration of protein release, burst release, or exposing proteins to reactive cross-linkers.
Protein Aggregation During In Vivo Release
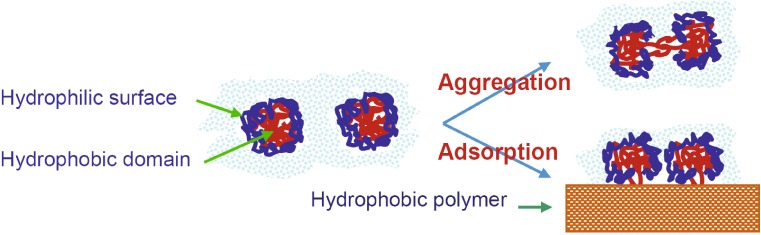
Maintaining the native conformation of protein during the sustained-release process may be an even greater challenge. Being packed in a sustained-release depot at high concentration and at body temperature for a prolonged period of time, unprotected protein molecules may have an increased probability to aggregate with each other or to adsorb on the inner surface of the polymer matrix (33). As flexible macromolecules, proteins may denature from their native state by reversible and irreversible conformation changes (16,18). For water soluble proteins, aggregation through the hydrophobic domains of proteins or adsorption of proteins onto the polymer matrix facilitate irreversible conformation changes of proteins (see Fig. 1; 16,18). A successful sustained-release system must prevent, or at least reduce, protein–polymer contact and interaction.
Fig. 1.
Schematic description of protein aggregation and adsorption
Most degradable polymers used for protein sustained-release are polyesters that generate acidic species during degradation. The acidic species (which may still be polymers or oligomers) may be entrapped in sustained-release depots and result in a localized pH drop which is another cause for protein denaturing (33–38).
Immunogenicity by Denatured Proteins
Proteins denaturing from their native state are often antigenic and sometimes result in severe immunogenicity and serious clinical consequences. Neutralizing antibodies resulting from denatured proteins cannot only attenuate the efficacy of protein therapy but also induce significant side effects if the antibodies cross-react with patients’ endogenous proteins. For example, protein-induced neutralizing antibodies to erythropoietin (EPO) result in red cell aplasia (39) and induced antifactor VIII (FVIII) antibodies worsen the pathology associated with hemophilia (40). While immunogenicity induced by denatured or aggregated proteins has been a long-standing concern, there has not yet been a regulatory guideline for acceptable levels of protein aggregation. In the development of new protein delivery systems, avoiding any increased protein aggregation, as compared with already approved products, is crucial.
Encapsulation Efficiency and Formulation Complexity
For microencapsulation using the so-called “double emulsion” method, proteins in solution state may easily leak to the outer aqueous continuous phase, resulting in unacceptable low encapsulation efficiency (41,42). Replacing the inner protein solution with solidified protein particles may substantially improve encapsulation efficiency, but protein particles still have the chance to contact with the outer aqueous continuous phase, leading to considerable loss of proteins. In general, higher encapsulation efficiency may be obtained by atomizing a protein-in-polymer suspension through a drying (or solidification) atmosphere prior to entering a collecting buffer (43). However, spray drying associates with complicated microencapsulation processes, equipment, and sterilization conditions (32). The technical challenges in developing sustained-release systems for proteins are summarized in Table 1.
Table 1.
Technical Challenges for Developing Sustained-Release Protein Dosage Forms
| Challenges | Causes | References |
|---|---|---|
| Protein denaturing in formulation processes | High temperature, shear, cavitation, interface, cross-linking reagent | (44–53) |
| Protein denaturing, aggregation, adsorption to polymer matrix during release | Environment factors like temperature, moisture | (54–56) |
| Immunogenicity of denatured proteins | Denaturation, aggregation | (57–62) |
| Burst release | In vivo conditions like body temperature, pH, buffer | (63) |
| Incomplete release | Denaturation, aggregation | (7) |
| Poor loading capacity, loading efficiency, and reproducibility | Formulation, complicated procedures, high cost | (48) |
| Formulation complexity | Denaturation, aggregation | (11) |
RECENT APPROACHES IN DEVELOPING SUSTAINED-RELEASE DOSAGE FORMS FOR PROTEIN DRUGS
To prevent protein denaturing by microencapsulation processes, most recent studies in developing sustained-release system for protein drugs have involved efforts to avoid exposing dissolved protein molecules to the interface of water and organic solvents. Reported formulation strategies may be classified into three categories: (1) to formulate proteins into solid particles or some other stabilized form to gain resistance to organic solvents prior to microencapsulation processes, (2) to microencapsulate proteins with polymeric materials soluble in water, and (3) to form sustained-release depots by an in vivo gelling process. To address protein aggregation and on-polymer adsorption during the prolonged course of sustained-release, some researchers suggested blending hydrophilic polymers or basic inorganic salts into polylactide-co-glycolide (PLG) systems to reduce hydrophobicity of the protein-loading matrix (64,65). Some have demonstrated strategies of using new polymeric materials, as well as PLG conjugated with a hydrophilic block (66,67). Studies representative for each of these strategies will be reviewed in the following paragraphs.
Chemical Basis of Protein Denaturing
In the processes of formulation and sustained release, proteins are subjected to a series of instability mechanisms such as hydrolysis, deamidation, acid- or enzyme-catalyzed degradation, and irreversible conformation changes including unfolding, aggregation, and adsorption on polymers (11,17). Among these mechanisms, irreversible conformation change is the mechanism lacking in peptides, and responsible for the deferred success to commercialize sustained-release protein dosage forms despite a number of sustained-release peptide drugs already being on the market. We will therefore focus our discussion of protein instability on noncovalent conformation changes.
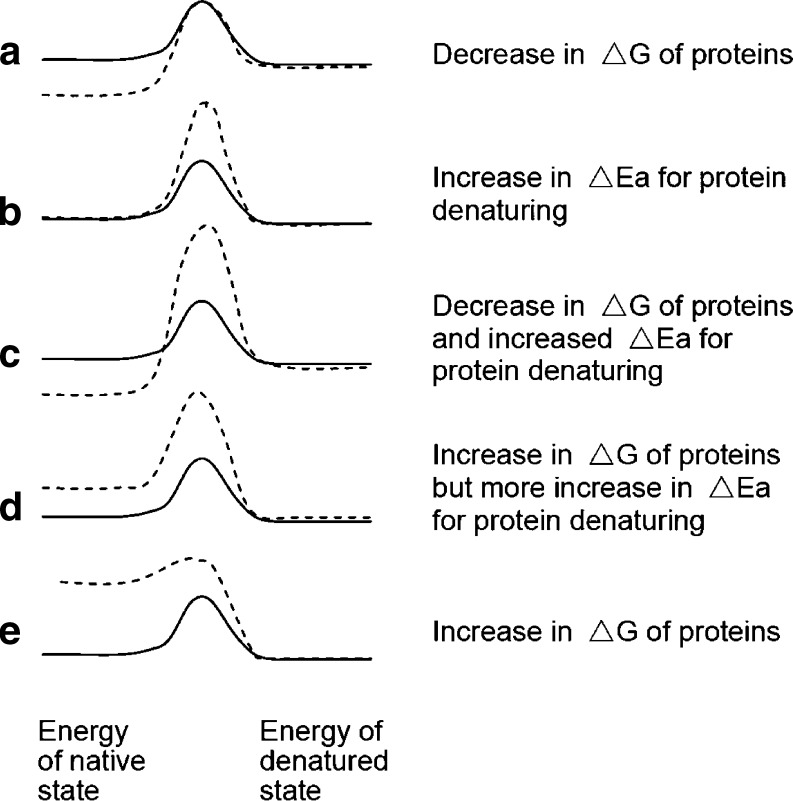
As discussed above, the energy barrier for protein conformation changes in solution is in the range of 5–20 kcal/mol, which is much lower than covalent changes (>100 kcal/mol) but similar to those of water–oil interfacial tension and hydrophobic interactions (16). To prevent proteins from denaturing (i.e., irreversible conformation changes), a delivery system should provide a microenvironment that reduces the chemical potential (free energy) of protein molecules loaded in it or to increase the energy barrier for a protein molecule to transfer from its native state to a denatured state (see Fig. 2a,b). For example, loading highly aqueous proteins in a hydrophilic matrix with abundant hydroxyls may significantly reduce the proteins’ free energy as compared with an environment lacking hydroxyls, while immobilizing proteins into a solid matrix or dispersing proteins in a viscous medium may increase the energy barrier for intramolecular movement and intermolecular contacts of proteins. The most favorable systems are, of course, those that can stabilize proteins thermodynamically and, at the same time, increase the kinetic barriers for proteins to denature (Fig. 2c). Carpenter reported that while loading proteins in a PEG solution of low concentration may cause an increase in the protein’s ΔG due to the unfavorable PEG environment, the PEG solution prevented proteins from aggregation by resulting in a shrinkage of protein molecules that raised the barrier for the protein molecules to extend their hydrophobic domains to each other (68). The same author also found that increasing PEG concentration, on the contrary, resulted in protein precipitation out of the solution and facilitated protein aggregation. This phenomenon may be explained in that low concentration PEG only slightly raised the protein’s free energy (ΔG) but significantly prohibited the protein–protein interaction, while for high PEG concentration, the ΔG increase was substantial so that protein molecules could no longer exist in the PEG solution (see Fig. 2d,e). Phase separation of a protein out of a cosolution with PEG as a function of temperature and PEG concentration was reported by Morita et al. (69).
Fig. 2.
Energy barriers for protein denaturing
Stabilizing Proteins Prior to Microencapsulation Involving Organic Solvents
A well-known method to convert proteins to fine solid particles for sustained-release microencapsulation is protein precipitation by bivalent metal ions, the technique used in Nutropin Depot (70). Protein complexation with bivalent metal ions in an aqueous phase was found as an effective way to form stabilized particles for some proteins, such as human growth hormone (hGH; 70), while the method was also reported to facilitate aggregation when applied to some other proteins such as erythropoietin (71). It was reported that hGH forms complexes with zinc ions in its native form in the body (70). This protein dependency in stabilization effect of bivalent metal ion may be explained in that the ionic complexation leads to a thermodynamically favored state for some proteins but raises structural constraints for others (Fig. 2).
To avoid zinc-induced aggregation, Zale et al. used a salting out method to prepare EPO particles (71). EPO was precipitated to particles by adding ammonium sulfate into its aqueous solution and collected for sustained-release microencapsulation using PLG. EPO released from the composite PLG microspheres prepared with the salting out particles showed significantly reduced dimers or oligomers as compared with that from the microspheres loaded with the EPO–zinc complex. However, this system showed severe initial burst release (71). Ammonium sulfate, like other salting-out reagents, is highly water soluble and cannot be easily removed from the precipitates of water-soluble proteins (due to its insolubility in organic solvents) prior to microencapsulation. The salt may easily dissolve inside hydrated microspheres after administration and result in a high osmotic pressure to drive burst release.
Morita et al. introduced another protein particle technique for sustained-release microencapsulation, precipitating proteins with PEG (69). The bovine serum albumin (BSA) particles were formed by phase separation of a protein–PEG cosolution during a freezing process. Since PEG is soluble in organic solvents, pure fine BSA particles were obtained by freeze drying its cosolution with PEG, followed by washing away the PEG phase. The protein particles were then added to a PLG/organic solvent solution to form a protein-in-PLG suspension and then emulsified into an aqueous continuous phase containing 1% polyvinyl alcohol to form composite microspheres. This process is widely known as solid-in-oil-in-water (S/O/W) microencapsulation. The composite PLG microspheres loaded with the pure BSA particles showed a release profile with minimal initial burst (69). However, the pure protein particles are directly surrounded by hydrophobic polymers (PLG for example) matrix in microspheres. Whether a delicate and bioactive protein can maintain its integrity and activity in this surrounding has not been discussed.
To protect delicate proteins from organic solvents and hydrophobic polymer matrix, packing these molecules into particles of sugars, polysaccharides, or other water soluble polymers prior to microencapsulation is a well-reported strategy (22–27). Among these sugar-based protein stabilizers, cyclodextrin and heparin were suggested by Rosa et al. and Wang, respectively, for their energy-favored interaction with proteins (72,73). The former authors suggested that cyclodextrin derivative associated with proteins to form a combined solid cake (72), and they lyophilized lysozyme together with cyclodextrin derivative and PEG, followed by milling the lyophilized powders to fine particles for microencapsulation. Heparin was selected by the latter authors for their natural existence in extracellular matrix (73). In general, direct lyophilization of proteins with polysaccharide results in larger, irregular, or fibrous particles that need to be further broken down to fine particles suitable for microencapsulation. The process of milling larger particles to small sizes may be hazardous to delicate proteins (21). Packing proteins into particles of small molecular sugars may encounter the similar osmotic pressure problem as inorganic salts.
To load proteins into polysaccharide fine particles without hydrophilic/hydrophobic interfacial tension, Jin et al. and Stenekes et al. demonstrated their method based on aqueous two-phase separation (24,74–76). Aqueous two-phase systems consisting of polysaccharide and PEG phases are documented in a number of materials for protein purification (77). The partition coefficient for proteins ranges from 10 to 100 (78).
To prevent polysaccharide-dispersed phases from fusing with each other and forming a block phase, Jin et al. introduced the third component, an anionic polysaccharide, sodium alginate, to a dextran–PEG aqueous two-phase system (24). The anionic alginate formed a diffuse electric double layer around the dispersed dextran droplets to keep them apart from each other so that the two-phase system became a stable “aqueous–aqueous emulsion”. Delicate proteins partitioned preferentially in the dextran-dispersed phase were converted to dense glassy particles highly resistant to organic solvents by means of lyophilization procedure (79). For some proteins which are unstable during the dehydration process of lyophilization, addition of a small portion of small molecular sugars (such as trehalose) made significant improvement (80,81). The dextran particles were harvested by rinsing the lyophilized powder with organic solvent to remove the PEG continuous phase and further microencapsulated into composite PLG microspheres through a S/O/W process. The researchers examined their method by microencapsulating an enzyme, β-galactosidase, in poly(lactic/glycolic acid) (PLGA) microspheres and assaying its catalytic activity after each formulation step. Bioactivity of β-galactosidase was well preserved (79). The sizes of the protein-loaded dextran particles, 1–2 μm in diameter, are in the appropriate range for being loaded into composite microspheres for human injection according to the suggestion that the inner particles should be less than 5% of the microspheres in diameter (82).
Nils et al. reported a method to load proteins into amylopectin particles via an aqueous phase separation process (26,27,83). Proteins were added into a 20% amylopectin solution and then dispersed into a PEG solution, followed by amylopectin gelling below 55°C and lyophilization. After the PEG continuous phase was removed using organic solvent, protein-loaded amylopectin particles, 20–80 μm in diameter, were harvested. For sustained-release purposes, the amylopectin particles were coated with a PLG shell using an air suspension technique. Due to the sizes of the amylopectin core (20–80 μm), however, the release-controlled PLG shell should be thin enough to ensure that the final microspheres are not too large for injection. To coat a thin and uniform polymer layer over the surface of microspheres may require some practice. In addition, if multiple amylopectin particles are enclosed in one PLG shell to form composite microspheres, the large amylopectin inner particles may result in burst release (82). Since phase separation and gelling of amylopectin occur around 55°C (26,27,83), preparation of smaller amylopectin particles may require rapid cooling to prevent fusion of separated dispersed phase.
Byung et al. demonstrated another technology to prepare small particles for loading proteins into composite PLG microspheres (22). Hydroxyethyl starch was grafted with acrylic acid through an ester bond, followed by an emulsion polymerization reaction through the C = C double bonds of the grafted acrylic groups. For protein microencapsulation, the internally cross-linked starch particles, around 140 nm in diameter, were first added in the protein solution to soak the solution into the particle matrix. The dry particles can soak protein solutions roughly ten times their original mass when impregnated in such a solution and can swell up to as much as 11-fold in diameter (22). The protein-carrying starch particles were added in a PLG/dichloromethane solution to form a starch-in-PLG suspension and then subjected to S/O/W process for microencapsulation. While this process still involved a water/oil interface, the authors found that by preloading horseradish peroxidase into starch particles, the activity retention after microencapsulated increased to 80.9% as compared with microencapsulation through the “water-in-oil-in-water” (W/O/W) process (61.5%; 22).
For preparation of protein particle-loaded composite microspheres, the spray freeze-drying or air suspension methods involve some special formulation conditions such as the use of liquid nitrogen, high temperature, and organic solvent ventilation system. These may complicate the formulation process especially for sterilized products. The emulsification based methods, such as S/O/W, need only mixers and conventional lyophilizers for manufacturing. However, since oil/water interfaces still exist, chances for the encapsulated protein particles to be hydrated and dissolved by penetrated water, contact with the oil/water interfaces, have to be taken into account. These issues may result in aggregation of some delicate proteins and low protein encapsulation efficiency. To avoid protein hydration in the microencapsulation process, a solid-in-oil-in-oil process involving an oil continuous phase was used (84). However, choices of the oil are limited to a few PLG solution-immiscible ones such as silicone oil, which need large amount hydrocarbon to clean up in postmicroencapsulation process. A continuous phase that does not dissolve the hydrophilic protein particles, immiscible with PLG solution, and soluble in water is demanded.
Stabilizing Proteins in Microsphere Matrix During Sustained Release
Proteins survived from microencapsulation processes are subjected to a number of stability constraints after injection to the body. The major hazardous conditions inside sustained-release microspheres are the hydrophobic environment of the polymer matrix and acid microenvironment generated from polymer degradation (most of them are polyesters). For microspheres with small molecular sugars or salts encapsulated as protein stabilizers, these reagents are normally highly soluble and rapidly diffuse out of the microspheres, leaving proteins unprotected in hydrophobic polymer matrix (42,85). Partially degraded polymers (such as PLG), on the other hand, form acidic oligomers that are entrapped inside the microspheres causing a substantial pH drop (as low as 1.5) within the microspheres (36). This acidic condition may result in protein instability.
To neutralize the localized acidity, Zhu et al. blended basic Mg(OH)2 or MgCO3 into PLGA microspheres (35). Since these magnesium compounds only slightly dissolve in water but become highly soluble in acidic media, they may dissolve in PLGA microspheres in response to acid generation and neutralize the entrapped acidity. More reports demonstrated another approach, blending hydrophilic polymers into PLGA matrix. Dispersing hydrophilic polymers in PLG microspheres will increase permeability of the PLG matrix during protein release and help release or alleviate the acids generated by PLG degradation (35). It has been reported that blending PEG into PLGA microspheres helped reduce entrapped acidity (64).
Moreover, since hydrophilic polymers are not released from PLGA microspheres as rapidly as small molecular sugars, they are retained in the PLGA matrix during the course of prolonged release and protect proteins from adsorption onto PLGA. Jiang and Schwendeman studied the effect of blending PEG into PLGA microspheres on incomplete release by codissolving PEG with PLGA in methylene chloride (64). When 10% PEG was blended in the microspheres, 45% of BSA loadings were released during 4 weeks, and 25% of the BSA loadings were identified from the remaining mass as insoluble aggregates (64). Increase in PEG contents to 20% lead to an increase in cumulative BSA release to 75% and disappearance of the insoluble aggregates (64). On the other hand, however, increase in PEG content from 10% to 20% also resulted in an increase in the first day release from 20% to 30% (64). After deducting this initial burst, about 45% of the total BSA loadings were subjected to slow release.
Kim et al. mixed a basic diblock copolymer, poly(ethylene glycol)-poly(l-histidine) into a BSA solution, then encapsulated the cosolution into PLGA microspheres (86,87). The basic copolymer played a number of roles in PLGA microspheres such as neutralizing acidity from PLGA degradation and associating with negatively charged BSA to shield the protein and to control its release rate (86,87). While many positively charged polymers are toxic and may cause protein deactivation in general, the author found that the 7-kDa cationic polymer did not result in secondary and tertiary structure changes of BSA. This is probably because the histidine group is a weak base with pKa around 7.0. Safety examination for using this copolymer to pharmaceutically active proteins is needed.
Dispersing solvent-insoluble polysaccharide particles into PLGA microspheres is another method to blend hydrophilic polymers into hydrophobic matrix. Using BSA, myoglobin, and granulocyte-macrophase colony-stimulating factor as model proteins, Jin et al. found that the strategy of loading proteins in dextran glassy particles prior to microencapsulation protects proteins from deactivation, aggregation, and adsorption onto PLGA both in the formulation process and in the course of sustained release (79). Cumulative protein release from these dextran–particle-blending composite PLGA microspheres was therefore as high as more than 90% of the total protein loadings over the period of sustained release (79). Taking the fact that most of the water soluble proteins portioned preferentially in dextran phase into account (24,78), the dextran-dispersed phase may stabilize proteins by both reduced ΔG of proteins and increased kinetic barrier for protein conformation changes. Proteins released from amylopectin-composite PLGA microspheres showed a similar “complete” profile (26).
Contrary to the composite microsphere strategy, directly conjugating a PEG block to hydrophobic microsphere-forming polymers lead to uniform microsphere matrix with increased hydrophilicity (64). Bezemer et al. conjugated a PEG block to poly(butylene terephthalate) and used the resulting block copolymer to form microspheres encapsulating hGH, bone morphogenetic protein 2 (BMP-2), transfer growth factor (TGF-β) and immunoglobulin (88–90). The authors demonstrated that the mechanical strength, chemical degradation, as well as controlled release functions of the microspheres may be precisely adjusted by varying the length and ratio of each of the blocks. For example, polymers with more PEG contents degraded faster than those with less PEG. While direct experiment results were not presented, the authors suggested that the amphiphilic block copolymers exerted a surfactant function in microencapsulation; thus, proteins were protected from the interfacial tension between water and organic solvent in which the polymer was dissolved (89).
A completely different strategy to avoid protein denaturing during the period of sustained release is to form microspheres with highly degradable but water-impermeable polymers, for example, polyanhydrides (91). Degradation of the microspheres made of hydrophobic polyanhydrides undergoes a surface erosion mechanism, for which the core of the microspheres remained anhydrous. Proteins loaded in this anhydrous matrix are expected to maintain their immobilized dry state until being released (91). A question regarding this system is probably still related with protein protection during the microencapsulation process: How a delicate protein can survive from the interfacial tension between aqueous protein solution and organic polymer solution. If hydrophilic protein stabilizers are loaded together in the matrix of polyanhydrides, will the polymer degradation turn out to be a bulky process due to stabilizer-induced water permeability of the microsphere matrix?
Microencapsulation Using Water Soluble Materials
To completely circumvent the protein stability issue raised by organic solvents and hydrophobic polymers, various hydrophilic particulate systems have been developed for protein sustained release. These microsphere systems are in general formed from water soluble materials with their dissolution retarded by surface deposition of blocking materials or interior cross-linking through covalent, ionic, or hydrophobic (domain) interactions (www.altus.com/products/product-pipeline.cfm#theraclec Altus company website; www.octoplus.nl OctoPlus company website; 63,92–100).
For surface deposition, an immediate example is human growth hormone microcrystals deposited with an overlayer of polyarginine (ALTU-238; www.altus.com/products/product-pipeline.cfm#theraclec Altus company website; 92,93). While bare hGH microcrystals completely dissolve in 24 h, those with surface deposition of polyarginine extended their dissolution to 4–5 days (92). Result of clinical phase II trials showed that single administration of the weekly injection ALTU-238 induced constant insulin-like growth factor (IGF) response in blood (www.altus.com/products/product-pipeline.cfm#theraclec Altus company website). This result indicates that polyarginine coating may be an effective robust platform to extend dissolution time of protein crystals.
Hahn et al. demonstrated oil-coated hyaluronate microspheres for one-injection-per-week administration of hGH (94,95). The microspheres were prepared by spray drying an aqueous cosolution containing sodium hyaluronate, hGH, and lecithin. The microspheres were then suspended in medium chain triglycerides prior to injection. Release of hGH from the hyaluronate microspheres was extended to 50 h with 80% of hGH loads released in the first 24 h (94). A high performance liquid chromatography and sodium dodecyl sulfate polyacrylamide gel electrophoresis assay of hGH released from the hyaluronate microspheres showed no protein aggregation, and blood serum assay using beagle dogs exhibited an area under the curve featured with Cmax of 69.5 ± 8.0 ng/ml and Tmax between 10 and 12 h. A single injection induced elevation of serum IGF-I level until the 6th day (95). For some delicate proteins, especially those with net positive charges at native states, how the negative charges of hyaluronate affect protein stability was not discussed.
The examples above suggest that while microspheres made of water soluble materials are friendly to proteins, the dissolution retardation by the surface deposition is limited to 1 week. For longer periods of sustained release, different approaches are needed.
A dextran particulate system called OctoDEX represents a type of interiorly cross-linked microspheres of water soluble polymers (63,96,97; www.octoplus.nl OctoPlus company website). For cross-linking reaction, dextran was esterificated with methyl acrylate, a carboxylic acid with C = C double bond. The obtained methacrylated dextran (dex-MA) then was dissolved in water, added to proteins, and then emulsified into a solution of PEG to form a temporal “aqueous–aqueous emulsion” (due to the aqueous phase separation nature of the two water soluble polymers). Under continuous stirring, cross-linking initiator was added, and the dex-MA dispersed phase was solidified by an interior cross-linking through the C = C double bonds of methyl acrylate groups grafted on dextran (97). Since the acrylate groups are grafted onto dextran through ester bonds, hydrolysis of the ester bonds at physiological condition offers a bulky degradation-controlled release.
ProMaxx is another hydrophilic particulate system for protein sustained release which is formed of cross-linked human serum albumin (98). Formation of ProMaxx particles involved hetastarch (a polyanionic polysaccharide), divalent metal cations, a chemical cross-linking agent, and heat. The technical details regarding cross-linking reaction, cross-linking condition, and their effects on protein stability are not mentioned.
In Vivo Gelation Systems for Sustained-Release Delivery of Proteins
In vivo gelation systems, especially those formed of hydrophilic and biocompatible materials, are attractive for formulating sustained-release depots for protein drugs due to their formulation simplicity and organic solvent-free condition. For such a carrier system, protein to be delivered is added to its solution or fluid form prior to administration. This protein-carrying fluid converts to gel form immediately after injection into the body and encapsulates the proteins in a depot. The mechanisms for in vivo gelling include thermal gelling (with temperature decrease), reversed thermal gelling (with temperature increase), and covalent and ionic cross-lining (99–104). The cross-linking junctions of in vivo gelling systems are the same as those for various hydrogels, such as crystalline domains, hydrophobic domains, and covalent, ionic, and hydrogen-bonded domains.
Many naturally occurring polymers such as gelatin, agarose, starch (amylase and amylopectin), carrageenans, gellan, chitosan, and alginate possess thermal gelling property when being cooled from an aqueous solution. This property may be used to form sustained-release depots. For example, Yamamoto et al. and Park et al. reported using POE as in vivo gelling depots for sustained-release delivery of BMP-2 and TGF-β, respectively (105,106). The systems were heated with proteins to solution state above body temperature and injected into the body. By cooling to body temperature, the system gelled to form a sustained-release depot. These natural polymers are in general biocompatible, biodegradable, and friendly to delicate proteins. The need for heating prior to administration, however, is problematic for patient compliance and protein stability.
Among in vivo gelling depots, the most feasible one is probably a reversed thermal-gelling system as it meets a number of criteria simultaneously: free of organic solvent and reactive cross-linking reagents, relative rapid gelling at body temperature, and biocompatible and biodegradable nature of the forming materials (4,107,108). ReGel is an example in this category that is formed of a tri-block copolymer consisting of PEG and PLG (or PLA) blocks as PLG–PEG–PLG. It gels at elevated temperature due to removal of hydrogen-bonded water molecules from the polymer so that the system becomes hydrophobic and water insoluble and gelation (109). This system can be designed to be a liquid at room temperature and a gel at body temperature by adjusting the chain length of the PEG and PLGA blocks and overall concentration (6). This nature imparts the system a great convenience as a sustained-release depot; thus, water-soluble biologic can easily be added in its fluid form and encapsulated in its depot form after injection. The drawbacks of this system might include that the gelation process associates with a hydrophobic shrinkage, by which the protein-carrying liquid may be squeezed out of the depot and result in initial burst. Kissel et al. compared the stability of erythropoietin loaded in ABA copolymers and in PLG microspheres prepared using a W/O/W double emulsion method and found even more aggregated proteins in ABA copolymers than in PLG microspheres (110,111). The authors attributed EPO aggregation to the protein unfriendly nature of the PEG domain in polymer matrix (111). This argument is consistent with the observation that concentrated PEG solution caused delicate proteins to become thermodynamically unstable (69).
A poly(ethylene glycol)-based copolymer that contains multiple thiol (–SH) groups along the polymer backbone was reported to form a polymer hydrogel under mild preparation conditions. The proteins are entrapped in it via physical binding (112). While this system showed a sustained-release function for bovine serum albumin for 2–4 weeks and preserved activity of erythropoietin (112), it is nondegradable in the body and requires surgical removal.
Biodegradable hydrophobic polymers dissolved in water-miscible (yet Food and Drug Administration approved) organic solvents are another type of in vivo gelling systems for sustained-release delivery of peptide drugs (113). Alzamer® from Alza/Johnson & Johnson, Atrigel® from Atrix Labs/QLT Therapeutics, and the SABER™ from Durect are in this category. After injection to body, the water-miscible solvents are drained to the surrounding tissues and the water-insoluble polymers precipitate as a depot. Drugs are encapsulated in the gelling depots and released in a sustained profile along degradation of the precipitated polymers. This type of systems has two major drawbacks: the need for organic solution and relatively slow gelling process. The former system may denature proteins (if applied to protein delivery), while the latter associates with burst release. Griebenow and Klibanov examined stability of proteins incubated in water–organic solvent mixtures of various water/solvent ratio and found that mixtures are remarkably more hazardous to protein stability than pure solvent and pure water (114). For these in vivo gelling systems, solvent–water mixing is unavoidable during the relatively slow solvent-draining process. While SABER™, a solution containing sucrose acetate isobutyrate, PLA, and benzyl alcohol, was reported for sustained-release delivery of proteins, studies regarding protein stability have not been disclosed (113).
Implantable Devices for Protein Delivery
The research effort on implantable devices for protein delivery has grown exponentially in recent years, in parallel to the rapid development of new protein therapeutics. There have been great progresses in implant devices for protein delivery (115–120). Mohl and Winter developed a triglyceride implant aiming for sustained protein (115,116). Tristearin implants containing lyophilized rh-interferon α (IFN)-2a and varying amounts of PEG 6000 were prepared by compression. Release studies exhibited that more than 90% of the incorporated IFNα-2a can be liberated in a continuous way over 1 month. Integrating hydroxypropyl-β-cyclodextrin into the matrices proved to stabilize IFNα-2a and led to a higher and faster protein release due to solubilizing effects. Surini et al. designed an implantable controlled-release system based on a polyion complex device composed of chitosan and sodium hyaluronate for protein delivery (117). Insulin release from chitosan–hyaluronate pellets was markedly influenced by both the change in the polymer mixing ration and the total pellet weight, whereas the compression pressure did not affect the release profile significantly. However, this system only may remain 12 h release. Liao et al. introduced a protein-loaded fibers from interfacial polyelectrolyte complexation to sustain proteins (118). Chitosan–alginate fibers were produced by pulling from the interface between two polyelectrolyte solutions at room temperature. Depending on the component properties, the release time of encapsulated protein from these fibers can range from hours to weeks. Electrostatic interaction between the fiber components and the charged encapsulated proteins controls the release kinetics. The fibers were able to release platelet-derived growth factor-bb with native state in a steady fashion for over 3 weeks without an initial burst. Yamagata et al. developed a delivery system for proteins based on polyglycerol esters of fatty acids (PGEFs; 119). The cylindrical matrix was prepared by a heat extrusion technique using a lyophilized powder of the protein and 11 types of synthetic PGEFs which varied in degree of glycerol polymerization, chain length of fatty acids, and degree of fatty acid esterification. Both in vitro and in vivo release studies present that IFN-α release from matrices prepared from monoglycerides and diglycerol esters was initially high. On the contrary, the initial burst from matrices prepared using the tetraglycerol esters of palmitate and stearate was significantly reduced and was followed by a constant rate of release. Ryu et al. constructed a planar shaped biodegradable micro-osmotic pumps based on microelectromechanical system technology for long-term controlled release of basic fibroblast growth factor (bFGF; 120). The implantable devices were constructed by micromolding and thermal assembly of 85/15 poly(l-lactide-co-glycolide) sheets. Moreover, it can be further miniaturized and used for the delivery of multiple proteins at the individual releasing schedules. Different from the conventional delivery systems made of biodegradable polymers, the release mechanism of the biodegradable osmotic pumps was decoupled from the degradation of polymers. The release profile was controlled only by the microgeometries shaped in the devices and the permeability of a polymer. Degradation of devices occurred after the bFGF was released completely, and the release of bFGF was modulated at 40 ng/day for 4 weeks. This decoupling of the release from degradation of devices offers great advantages over degradation-based delivery systems. The advantages include more accurate and easier modulation of release, avoidance protein exposure to an acidic environment from degradation, reduction of adverse inflammatory response during the release, and minimization of any possible reaction between proteins and degraded polymers. The exceptional characteristics of these implantable devices above mentioned suggest their potential for protein delivery.
OTHER APPLICATIONS OF SUSTAINED-RELEASE DELIVERY OF PROTEINS
Stimulating Homing and Differentiation of Stem Cells at Targeted Tissues
In addition to treatment of chronic conditions for which reduction of injection frequency is demanded for improving patient’s compliance, sustained-release delivery of proteins is found useful in tissue regeneration therapy and in medical devices. For tissue regeneration, the homing, differentiation, and proliferation of stem cells at the site of the tissue to be repaired rely on therapeutic level of administrated cell growth factors within the targeting tissue for sufficient period of time, normally several weeks (121). However, in vivo life of these proteins range from several hours to several days, far from therapeutic needs. While tissue regeneration by mobilized or administrated steam cells is not a chronic process, many tissues, such as cardiac muscles, can only be injected for very limited times. Thus, having therapeutic level of cell growth factors extended for weeks after a single injection is essential.
Reported examples in this area of applications are the collagen-based matrix system for extended release of bone morphogenetic proteins 2 for bone regeneration (122,123). Collagens, however, are usually derived from animal sources, which can be a source of pathogen transmission. In addition, collagen-based carriers can only retain soluble proteins in their matrix for 1–2 weeks (with half life of 2 days; 124), substantially less than therapeutically preferred duration (>6 weeks; 125). The protein retention time will be even shorter when an injectable form of collagen is used (to some tissues that require small volume of injection).
Partially, due to the unavailability of appropriate system to deliver proteins for tissue regeneration, methods to deliver genes to express cell growth factors in targeted tissues actively studied. However, gene delivery, by either viral or nonviral systems, also encounters a series of technical challenges, such as immunogenicity, chemotoxicity, and control of gene expression (126,127). Sustained-release systems that deliver cell growth factors to targeted tissues for sufficient period of time will offer a direct, effective, and safe solution to regeneration therapy of tissue and organs.
Protein Drug Eluting Cardiovascular Stents
Protein-eluting cardiovascular stents represent another potential application of protein sustained-release technology. The chemical drugs used on current drug eluting stents, although prevent after-stenting restenosis, inhibit healing of the blood vessel endothelium damaged by stent installation. The delayed endothelium recovery causes incident bleeding and thrombus forming (128). Several proteins have been found effective to suppress vascular smooth muscle proliferation and to stimulate vessel endothelium recovery when directly introduced to the stenting site (129). However, loading these proteins onto stents resulted in ineffectiveness (130). In these work, stents precoated with a layer of hydrophobic polymer was impregnated in a protein solution to adsorb proteins on the polymer surface (131). However, adsorbing proteins on hydrophobic polymer surfaces is a known cause for protein denaturing (132). In addition, only limited amount of proteins can be adsorbed on a stent surface (<20 μg/stent; 133). A recent work reported by Jin et al. suggests that mixing protein-loaded polysaccharide glassy particles into the polymer solution for stent coating is an effective yet simple method to improve loading capacity, stability, and release kinetics of proteins (134).
CONCLUSION
Although there has yet to be a single pharmaceutical dosage form for sustained release of native proteins commercialized to date, the concentrated research efforts have built up comprehensive knowledge basis to pave the way to industrial success. Among various recent formulation strategies, the methods to preload proteins into polysaccharide fine particles prior to microencapsulation and those to load proteins into hydrophilic in vivo gelling systems seem to be comprehensive. Since protein sustained-release depot systems can be formulated with all injectable excipients, as more and more proteins sustained-release dosage forms have entered clinic trials, commercial products in this category should be soon to come to sight.
Acknowledgement
In preparation of this review article, data base search and other information collecting are financially supported by the National Natural Science Foundation of China (Grant No.30472096).
References
- 1.Frokjaer S., Otzen D. E. Protein drugs stability: a formulation challenge. Nat. Rev. Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 2.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000;203:1–60. doi: 10.1016/s0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 3.Marc S., Juergen S., Hennink W. E., Wim J. Recombinant gelatin hydrogels for the sustained release of proteins. J. Control Release. 2007;119:301–312. doi: 10.1016/j.jconrel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Jeong B., Bae Y. H., Lee D. S., Kim S. W. Biodegradable copolymers as injectable drug-delivery systems. Nature. 1997;388(28):860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 5.Jostel A., Mukherjee A., Alenfall J., Smethurst L., Shalet S. M. A new sustained-release preparation of human growth hormone and its pharmacokinetic, pharmacodynamic and safety profile. Clin. Endocrinol. 2005;62:623–627. doi: 10.1111/j.1365-2265.2005.02271.x. [DOI] [PubMed] [Google Scholar]
- 6.Langer R., Folkman J. Polymers for sustained release of proteins and other macromolecules. Nature. 1976;263:793–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 7.Berkland C., Pollauf E., Raman C., Silverman R., Kim K., Pack D. W. Macromolecule release from monodisperse PLG microspheres: control of release rates and investigation of release mechanism. J. Pharm. Sci. 2007;96(5):1176–1191. doi: 10.1002/jps.20948. [DOI] [PubMed] [Google Scholar]
- 8.Sinha V. R., Trehan A. Biodegradable microspheres for protein delivery. J. Control Release. 2003;90:261–280. doi: 10.1016/s0168-3659(03)00194-9. [DOI] [PubMed] [Google Scholar]
- 9.Schwendeman S. P. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2002;19(1):73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 10.Bilati U., Allemanm E., Doelker E. Strategic approaches for overcoming peptide and protein instability within biodegradable nano- and microparticles. Eur. J. Pharm. Biopharm. 2005;59:375–388. doi: 10.1016/j.ejpb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen L., Moeller E. H., Nielsen H. M., Frokjaer S. Preparing and evaluating delivery systems for proteins. Eur. J. Pharm Sci. 2006;29:174–182. doi: 10.1016/j.ejps.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein A. V. Proteins: structural, thermodynamic and kinetic aspects. EDP Sci. 2003;77:651–692. [Google Scholar]
- 13.Rosenberg A. S. Effects of protein aggregates: an immunologic perspective. AAPS J. 2006;8(3):E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marco V. W., Hennink W. E., Wim J. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 2000;17(10):1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 15.Eva Y. C., Sampathkumar K., Theodore W. R., Carpenter J. F. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm. Res. 2003;20(9):1325–1336. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 16.Dill K. A. Dominant forces in protein folding. Biochemistry. 1990;29:7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 17.Volkin D. B., Klibanov A. M. Minimizing protein inactivation. In: Creighton T. E., editor. Protein Function A Practical Approach. Oxford, UK: Information; 1989. pp. 1–24. [Google Scholar]
- 18.Lai M. C., Topp E. M. Solid-state chemical stability of proteins and peptides. J. Pharm. Sci. 1999;88(5):489–500. doi: 10.1021/js980374e. [DOI] [PubMed] [Google Scholar]
- 19.Shire S. J., Shahrokh Z., Liu J. Challenges in the development of high protein concentration formulations. J. Pharm. Sci. 2004;93(6):1390–1402. doi: 10.1002/jps.20079. [DOI] [PubMed] [Google Scholar]
- 20.Huub S. Immunogenicity of therapeutic proteins. Nephrol. Dial. Transpl. 2003;18:1257–1259. doi: 10.1093/ndt/gfg164. [DOI] [PubMed] [Google Scholar]
- 21.Cleland J. L., Andrew J. S. Stable formulations of recombinant human growth hormone and interferon-r for microencapsulation in biodegradable microspheres. Pharm. Res. 1996;13(10):1464–1475. doi: 10.1023/a:1016063109373. [DOI] [PubMed] [Google Scholar]
- 22.Byung H. W., Ge J., Yeong W. J., DeLuca P. P. Preparation and characterization of a composite PLGA and poly(acryloyl hydroxyethyl starch) microsphere system for protein delivery. Pharm. Res. 2001;8(11):600–606. doi: 10.1023/a:1013090700443. [DOI] [PubMed] [Google Scholar]
- 23.Stenekes R. J. H., Franssen O., Bommel E. M., Crommelin D. J. A., Hennink W. E. The preparation of dextran microspheres in an all-aqueous system: effect of the formulation parameters on particle characteristics. Pharm. Res. 1998;15(4):557–561. doi: 10.1023/a:1011925709873. [DOI] [PubMed] [Google Scholar]
- 24.T. Jin, L. Chen, H. Zhu, and inventors. Stable polymer aqueous/aqueous emulsion system and uses thereof. US patent 6 805 879. October 19, 2004.
- 25.T. Jin, H. Zhu, J. Zhu, and inventors. Aquespheres, their preparation and uses thereof. US patent 6 998 393. Feb 14, 2006.
- 26.O. G. Nils, and R. Mats. Starch microparticles. US patent 6 692 770 B2. Feb 17, 2004.
- 27.L. Timo, and R. Mats. Encapsulation method. US patent 6 861 064 B1. Mar 1, 2005.
- 28.Cleland J. L., Olu F. L., Johnsonb S. P., Andrew J. S. Recombinant human growth hormone poly(lactic-co-glycolic acid) microsphere formulation development. Adv. Drug Delivery Rev. 1997;28:71–84. doi: 10.1016/s0169-409x(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 29.Kim H. K., Park T. G. Microencapsulation of human growth hormone within biodegradable polyester microspheres: Protein aggregation stability and incomplete release mechanism. Biotechnol. Bioeng. 1999;65:659–667. doi: 10.1002/(sici)1097-0290(19991220)65:6<659::aid-bit6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int. J. Pharm. 2005;289:1–30. doi: 10.1016/j.ijpharm.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Tillmann H. C., Kuhn B., Pill J. Efficacy and immunogenicity of novel erythropoietic agents and conventional rhEPO in rats with renal insufficiency. Kidney Int. 2006;9:60–67. doi: 10.1038/sj.ki.5000006. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y., Li L. C. Current advances in sustained release systems for parenteral drug delivery. Exp. Opin. Drug Deliv. 2005;6:1039–1058. doi: 10.1517/17425247.2.6.1039. [DOI] [PubMed] [Google Scholar]
- 33.Estey T., Kang J. C., Schwendeman S. P., Carpenter J. F. BSA degradation under acidic conditions: a model for protein instability during release from PLGA delivery systems. J. Pharm. Sci. 2006;95:1626–1639. doi: 10.1002/jps.20625. [DOI] [PubMed] [Google Scholar]
- 34.Li L., Schwendeman S. P. Mapping neutral microclimate pH in PLGA microspheres. J. Control Release. 2005;101:163–173. doi: 10.1016/j.jconrel.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Zhu G., Mallery S. R., Schwendeman S. P. Stabilization of proteins encapsulated in injectable poly (lactide-co-glycolide) Nat. Biotechnol. 2000;18:52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 36.Fu K., Pack D. W., Klibanov A. M., Langer R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) Microspheres. Pharm. Res. 2000;17(1):100–106. doi: 10.1023/a:1007582911958. [DOI] [PubMed] [Google Scholar]
- 37.Shenderova A., Burke T. G., Schwendeman S. P. The acidic microclimate in poly(lactide-co-glycolide) microspheres stabilizes camptothecins. Pharm. Res. 1999;16(2):241–248. doi: 10.1023/a:1018876308346. [DOI] [PubMed] [Google Scholar]
- 38.Zhu G., Schwendeman S. P. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: mechanism of stabilization by basic additives. Pharm. Res. 2000;17(3):351–357. doi: 10.1023/a:1007513425337. [DOI] [PubMed] [Google Scholar]
- 39.Marc H. V., Katia B., Regenmortel F. B. Immunogenicity of biopharmaceuticals: An example from erythropoietin. BioPharm. Int. 2005;18(8):36–47. [Google Scholar]
- 40.Vivek S., Purohit C., Russell M., Sathyamangalam V. Influence of aggregation on immunogenicity of recombinant human factor VIII in hemophilia a mice. J. Pharm. Sci. 2006;95:358–371. doi: 10.1002/jps.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X., Zhang Y., Yan R. Influence of process parameters on the protein stability encapsulated in poly-DL-lactide-poly(ethylene glycol) microspheres. J. Control Release. 2000;68:41–52. doi: 10.1016/s0168-3659(00)00235-2. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez A., Villamayor B., Guo Y., McIver J., Alonso M. J. Formulation strategies for the stabilization of tetanus toxoid in poly(lactide-co-glycolide) microspheres. Int. J. Pharm. 1999;185:255–266. doi: 10.1016/s0378-5173(99)00178-7. [DOI] [PubMed] [Google Scholar]
- 43.Gander B., Johansen P., Nam-Tran H., Merkle H. P. Thermodynamic approach to protein microencapsulation into poly(D,L-lactide) by spray drying. Int. J. Pharm. 1996;129:51–61. [Google Scholar]
- 44.Jiang G., Woo B. H., Kang F., Singh J., Deluca P. P. Assessment of protein release kinetics, stability and protein polymer interaction of lysozyme encapsulated poly(D,L-lactide-co-glycolide) microspheres. J. Control Release. 2002;79(1–3):137–145. doi: 10.1016/s0168-3659(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 45.Schwendeman S. P., Tobio M., Alonso M. J., Langer R. New strategies for the microencapsulation of tetanus vaccine. J. Microencapsul. 1998;15:299–318. doi: 10.3109/02652049809006859. [DOI] [PubMed] [Google Scholar]
- 46.Schwendeman S. P., Cardamone M., Brandon M. R., Klibanov A., Langer R. Stability of proteins and their delivery from biodegradable polymer microspheres. In: Bernstein S. C. H., editor. Microparticulate Systems for the Delivery of Proteins and Vaccines, vol. 77. New York: Mercel Dekker; 1996. pp. 1–49. [Google Scholar]
- 47.Weert M., Hof R. V., Heeren M. A., Posthuma G., Hennink W. E. Lysozyme distribution and conformation in a biodegradable polymer matrix as determined by FTIR techniques. J. Control Release. 2000;68(1):31–40. doi: 10.1016/s0168-3659(00)00227-3. [DOI] [PubMed] [Google Scholar]
- 48.Perez C., Castellanos I. J., Costantino H. R., Al-Azzam W. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J. Pharm. Pharmacol. 2002;54:301–313. doi: 10.1211/0022357021778448. [DOI] [PubMed] [Google Scholar]
- 49.Sah H. Protein behavior at the water/methylene chloride interface. J. Pharm. Sci. 1999;88:1320–1325. doi: 10.1021/js9900654. [DOI] [PubMed] [Google Scholar]
- 50.Reisz P. Free radical formation induced by ultrasound and its biological implications. Free Rad. Biol. Med. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 51.Suslick K. S., Hammerton D. A., Cline R. E. The sonochemical hot spot. J. Am. Chem. Soc. 1986;108:5641–5642. [Google Scholar]
- 52.Kim H. K., Park T. G. Microencapsulation of human growth hormone within biodegradable polyester microspheres: protein aggregation, stability and incomplete release mechanism. Biotechnol. Bioeng. 1999;65:659–667. doi: 10.1002/(sici)1097-0290(19991220)65:6<659::aid-bit6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 53.Alonso M. J., Gupta R. K., Min C., Siber G. R., Langer R. Biodegradable microspheres as controlled-release tetanus toxoid delivery systems. Vaccine. 1994;12:299–306. doi: 10.1016/0264-410x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 54.Cohen T., Yoshioka M., Lucarelli L. H., Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 1991;8:713–720. doi: 10.1023/a:1015841715384. [DOI] [PubMed] [Google Scholar]
- 55.Park T. G., Lu W. Importance of in vitro experimental conditions on protein release kinetics, stability and polymer degradation in protein encapsulated poly(D,L-lactic acid-coglycolic acid) microspheres. J. Control Release. 1995;33:211–222. [Google Scholar]
- 56.Johansen P., Men Y., Audran R., Corradin G., Merkle H. P., Gander B. Improving stability and release kinetics of microencapsulated tetanus toxoid by co-encapsulation of additives. Pharm. Res. 1998;15:1103–1110. doi: 10.1023/a:1011998615267. [DOI] [PubMed] [Google Scholar]
- 57.Mass C., Hermeling S., Bouma B., Jiskoot W., Martijin F. B. G. A role for protein misfolding in immunogenicity of biopharmaceuticals. J. Biol. Chem. 2007;282(4):2229–2236. doi: 10.1074/jbc.M605984200. [DOI] [PubMed] [Google Scholar]
- 58.Huub S., Nicole C. Immunogenicity of recombinant human proteins: causes and consequences. J. Neurol. 2004;251(Suppl 2):1114–1119. doi: 10.1007/s00415-004-1202-9. [DOI] [PubMed] [Google Scholar]
- 59.Thomas C. C. The drug development crisis: efficiency and safety. Annu. Rev Med. 2007;58:1–16. doi: 10.1146/annurev.med.58.042705.124037. [DOI] [PubMed] [Google Scholar]
- 60.Rossert J. Erythropoietin-induced, antibody-mediated pure red cell aplasia. Eur. J. Clin. Invest. 2005;35(Suppl. 3):95–99. doi: 10.1111/j.1365-2362.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 61.Braun A., Kwee L., Labow M. A., Alsenz J. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-alpha) in normal and transgenic mice. Pharm. Res. 1997;14:1472–1478. doi: 10.1023/a:1012193326789. [DOI] [PubMed] [Google Scholar]
- 62.Schernthaner G. Immunogenicity and allergenic potential of animal and human insulins. Diabetes Care. 1993;16(Suppl 3):155–165. doi: 10.2337/diacare.16.3.155. [DOI] [PubMed] [Google Scholar]
- 63.Cadee J. A., Jiskoot W., Hennink W. E. Release of recombinant human interleukin-2 from dextran-based hydrogels. J. Control Release. 2002;78:1–13. doi: 10.1016/s0168-3659(01)00483-7. [DOI] [PubMed] [Google Scholar]
- 64.Jiang W., Schwendeman S. P. Stabilization and controlled release of bovine serum albumin encapsulated in poly(D, L-lactide) and poly(ethylene glycol) microsphere blends. Pharm. Res. 2001;18(6):878–885. doi: 10.1023/a:1011009117586. [DOI] [PubMed] [Google Scholar]
- 65.Lavelle E. C., Yeh M. K., Davis S. S. The stability and immunogenicity of a protein antigen encapsulated in biodegradable microparticles based on blends of lactide polymers and polyethylene glycol. Vaccine. 1999;17:512–529. doi: 10.1016/s0264-410x(98)00229-1. [DOI] [PubMed] [Google Scholar]
- 66.Gao H., Wang Y. N., Fan Y. G., Ma J. B. Conjugates of poly(DL-lactide-co-glycolide) on amino cyclodextrins and their nanoparticles as protein delivery system. J. Biomed. Mater. Res—Part A. 2007;80(1):111–122. doi: 10.1002/jbm.a.30861. [DOI] [PubMed] [Google Scholar]
- 67.Akagi T., Baba M., Akashi M. Preparation of nanoparticles by the self-organization of polymers consisting of hydrophobic and hydrophilic segments: Potential applications. Polymer. 2007;48(23):6729–6747. [Google Scholar]
- 68.Carpenter J. F., Crowe J. H. The mechanism of cryoprotection of proteins by solutes. Cryobiology. 1988;25:244–255. doi: 10.1016/0011-2240(88)90032-6. [DOI] [PubMed] [Google Scholar]
- 69.Morita T., Horikiri Y., Yamahara H., Suzuki T., Yoshino H. Formation and isolation of spherical fine protein microparticles through lyophilization of protein–poly(ethylene glycol) aqueous mixture. Pharm. Res. 2000;17(11):1367–1373. doi: 10.1023/a:1007526301331. [DOI] [PubMed] [Google Scholar]
- 70.Brodbeck K. J., Pushpala S., McHugh A. J. Sustained release of human growth hormone from PLGA solution depots. Pharm. Res. 1999;16:1825–1829. doi: 10.1023/a:1018943107688. [DOI] [PubMed] [Google Scholar]
- 71.S. E. Zale, P. A. Burke, H. Berstein, A. Brickner, and inventors. Composition for sustained release of non-aggregated erythropoietin. US patent 5 716 644. Feb 10, 1998.
- 72.Rosa D., Larobina D., Rotonda M. I. L., Musto P., Quaglia F., Ungaro F. How cyclodextrin incorporation affects the properties of protein-loaded PLGA-based microspheres: the case of insulin/hydroxypropyl-h-cyclodextrin system. J. Control Release. 2005;102:71–83. doi: 10.1016/j.jconrel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 73.Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int. J. Pharm. 1999;185:129–188. doi: 10.1016/s0378-5173(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 74.T. Jin, Y. Geng, F. Wu, and W. E. Yuan. Sustained-release system for EPO and GM-CSF, PCT/CN2007/002962.
- 75.Stenekes R. J. H., Franssen O., van Bommel E. M. G., Crommelin D. J. A., Hennink W. E. The preparation of dextran microspheres in an all-aqueous system: effect of the formulation parameters on particle. Pharm. Res. 1998;15(4):557–561. doi: 10.1023/a:1011925709873. [DOI] [PubMed] [Google Scholar]
- 76.Stenekes R. J. H., Franssen O., van Bommel E. M. G., Crommelin D. J. A., Hennink W. E. The use of aqueous PEG:dextran phase separation for the preparation of dextran microspheres. Int. J. Pharm. 1999;183:29–32. doi: 10.1016/s0378-5173(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 77.Edelman M. W., Linden E. V. D., Tromp R. H. Phase separation of aqueous mixtures of poly(ethylene oxide) and dextran. Macromolecules. 2003;36:7783–7790. [Google Scholar]
- 78.Gisela T., Bibiana N., Guillero P. Relationship between the protein surface hydrophobicity and its partitioning behaviour in aqueous two-aqueous systems of polyethyleneglycol–dextran. J. Chromatogr. B. 2004;799:293–301. doi: 10.1016/j.jchromb.2003.10.060. [DOI] [PubMed] [Google Scholar]
- 79.W. E. Yuan, and T. Jin. Aqueous–aqueous emulsion based sustained protein delivery system and its application in recombinant human growth hormone. Shanghai Jiao Tong University; 2007.
- 80.Arakawa T., Prestrelski S. J., Kenney W. C., Carpenter J. F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 1993;10:1–28. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 81.Carpenter J. F., Chang B. S., Randolph T. W. Rational design of stable lyophilized protein formulations: some practical advice. Pharm. Res. 1997;14:969–975. doi: 10.1023/a:1012180707283. [DOI] [PubMed] [Google Scholar]
- 82.Morita T., Yoshino H. Preparation of gelatin microparticles by co-lyophilization with poly(ethylene glycol): characterization and application to entrapment into biodegradable microspheres. Int. J. Pharm. 2001;219:127–137. doi: 10.1016/s0378-5173(01)00642-1. [DOI] [PubMed] [Google Scholar]
- 83.O. G. Nils, J. Monica, R. Mats, and inventors. Microparticle preparation. US patent 7 033 609 B2. April 25, 2006.
- 84.Carrasquillo K. G., Stanley A. M., Aponte C. J. C., De J. P., Costantino H. R., Bosques C. J., Griebenow K. Non-aqueous encapsulation of excipient-stabilized spray-freeze dried BSA into poly(lactide-co-glycolide) microspheres results in release of native protein. J. Control Release. 2001;76(3):199–208. doi: 10.1016/s0168-3659(01)00430-8. [DOI] [PubMed] [Google Scholar]
- 85.Morlock M., Koll H., Winter G., Kissel T. Microencapsulation of rh-erythropoietin using biodegradable poly(D,L-lactideco-glycolide): protein stability and the effects of stabilizing excipients. Eur. J. Pharm. Biopharm. 1997;43:29–36. [Google Scholar]
- 86.Kim J. H., Taluja A., Knutson K., Bae Y. H. Stability of bovine serum albumin complexed with PEG-poly(l-histidine) diblock copolymer in PLGA microspheres. J. Control Release. 2005;109:86–100. doi: 10.1016/j.jconrel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 87.Kim J. H., Taluja A., Knutson K., Bae Y. H. Role of a novel excipient poly(ethylene glycol)-b-poly(L-histidine) in retention of physical stability of insulin in aqueous solutions. Pharm. Res. 2007;24(8):1517–1526. doi: 10.1007/s11095-007-9270-z. [DOI] [PubMed] [Google Scholar]
- 88.Bezemer J. M., Dijkstra P. J., Feijena J. A controlled release system for proteins based on poly(ether ester) block-copolymers: polymer network characterization. J. Control Release. 1999;62:393–405. doi: 10.1016/s0168-3659(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 89.Bezemer J. M., Radersma R., Feijena J. Zero-order release of lysozyme from poly(ethylene glycol)/poly(butylene terephthalate) matrices. J. Control Release. 2000;64:179–192. doi: 10.1016/s0168-3659(99)00127-3. [DOI] [PubMed] [Google Scholar]
- 90.Bezemer J. M., Radersma R., Grijpma D. W., Dijkstra P. J., Feijena J. Microspheres for protein delivery prepared from amphiphilic multiblock copolymers 2. Modulation of release rate. J. Control Release. 2000;67:249–260. doi: 10.1016/s0168-3659(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 91.Kumar N., Langer R., Bomb A. J. Polyanhydrides: an overview. Adv. Drug Del. Rev. 2002;54:889–910. doi: 10.1016/s0169-409x(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 92.Govardhan C., Khalaf N., Jung C. W., Simeone B., Higbie A., Qu S., Chemmalil L., Pechenov S., Basu S. K., Margolin A. L. Novel long-acting crystal formulation of human growth hormone. Pharm Res. 2005;22(9):1461–1470. doi: 10.1007/s11095-005-6021-x. [DOI] [PubMed] [Google Scholar]
- 93.Pechenov S., Shenoy B., Yang M. X. Injectable controlled release formulations incorporating protein crystals. J. Control Release. 2004;96:149–158. doi: 10.1016/j.jconrel.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 94.Hahn S. K., Kim S. J., Kim M. J., Kim D. H. Characterization and in vivo study of sustained-release formulation of human growth hormone using sodium hyaluronate. Pharm. Res. 2004;21(8):1374–1381. doi: 10.1023/b:pham.0000036910.41224.de. [DOI] [PubMed] [Google Scholar]
- 95.Kim S. J., Hahn S. K., Kim M. J., Kim D. H., Lee Y. P. Development of a novel sustained release formulation of recombinant human growth hormone using sodium hyaluronate microparticles. J. Control Release. 2005;104:323–335. doi: 10.1016/j.jconrel.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 96.Cadee J., Brouwer L. A., Plantinga J. A., Hennink W. E. In vivo biocompatibility of dextran-based hydrogels. J. Biomed. Mat. Res. 2000;50:397–404. doi: 10.1002/(sici)1097-4636(20000605)50:3<397::aid-jbm14>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 97.Hoogeboom J. A. M., Hennink W. E. Degradation and release behavior of dextran-based hydrogels. Macromolecules. 1997;30:4639–4645. [Google Scholar]
- 98.T. L. Scott, L. R. Brown, F. J. Riske, C. D. Blizzard, S. J. Rashba, and inventors. Sustained release microspheres. US patent 6 458 387. October 1, 2002.
- 99.Cai S. Y., Liu X., Zheng S., Prestwich G. D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 100.Gupta D., Tator C. H., Shoichet M. S. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 101.Hatefi A. Biodegradable injectable in situ forming drug delivery systems. J. Control Release. 2002;80:9–28. doi: 10.1016/s0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 102.Amsden B., Bravo-Grimaldo E. Development of biodegradable injectable thermoplastic oligomers. Biomacromolecules. 2004;5(2):637–642. doi: 10.1021/bm034457n. [DOI] [PubMed] [Google Scholar]
- 103.Chenite A., Chaput C., Wang D. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21(21):2155–2161. doi: 10.1016/s0142-9612(00)00116-2. [DOI] [PubMed] [Google Scholar]
- 104.Yong C. S., Choi J. S., Quan Q. Z. Effect of sodium chloride on the gelation temperature, gel strength and bioadhesive forces of poloxamer gels containing diclofenac sodium. Int. J. Pharm. 2001;226(1–2):195–205. doi: 10.1016/s0378-5173(01)00809-2. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto M., Takahashi Y., Tabata Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006;12:1305–1311. doi: 10.1089/ten.2006.12.1305. [DOI] [PubMed] [Google Scholar]
- 106.Park H., Temenoff J. S., Holland T. A., Tabata Y., Mikos A. G. Delivery of TGF-b1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 107.Gaylen M. Z., Ramesh R. C., Chung S. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control Release. 2001;72:203–221. doi: 10.1016/s0168-3659(01)00276-0. [DOI] [PubMed] [Google Scholar]
- 108.Choi S., Baudys M., Kim S. W. Control of blood glucose by novel GLP-1 delivery using biodegradable triblock copolymer of PLGA–PEG–PLGA in type 2 diabetic rats. Pharm. Res. 2004;21(5):827–831. doi: 10.1023/b:pham.0000026435.27086.94. [DOI] [PubMed] [Google Scholar]
- 109.Jeong B., Bae Y. H., Kim S. W. In situ gelation of PEG–PLGA–PEG triblock copolymer aqueous solutions and degradation thereof. J. Biomed. Mater. Res. 2000;50:171–177. doi: 10.1002/(sici)1097-4636(200005)50:2<171::aid-jbm11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 110.Kissel T., Li Y. X., Unger F. ABA-triblock copolymers from biodegradable polyester A-blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins. Adv. Drug Delivery Rev. 2002;54:99–134. doi: 10.1016/s0169-409x(01)00244-7. [DOI] [PubMed] [Google Scholar]
- 111.Harris J. M. Introduction to biotechnical and biomedical applications of poly (ethylene glycol) In: Harris J. M., editor. Poly(ethylene glycol) Chemistry. New York, USA: Plenum; 1992. pp. 1–14. [Google Scholar]
- 112.Qiu B., Stefanos S., Ma J., Lalloo A., Perry B. A., Leibowitz M. J., Sinko P. J., Stein S. A hydrogel prepared by in situ cross-linking of a thiol containing poly(ethylene glycol)-based copolymer: a new biomaterial for protein drug delivery. Biomaterials. 2002;24(1):11–18. doi: 10.1016/s0142-9612(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 113.Okumu F. W., Dao L. N., Fielderb P. J., Dybdal N., Brooksa D., Sane S., Cleland J. L. Sustained delivery of human growth hormone from a novel gel system: SABER™. Biomaterials. 2002;23:4353–4358. doi: 10.1016/s0142-9612(02)00174-6. [DOI] [PubMed] [Google Scholar]
- 114.Griebenow K., Klibanov A. M. On protein denaturation in aqueous–organic mixtures but not in pure organic solvents. JACS. 1996;118:11695–11700. [Google Scholar]
- 115.Mohl S., Winter G. Continuous release of rh-interferon α-2a from triglyceride matrices. J. Control Release. 2004;97:67–78. doi: 10.1016/j.jconrel.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 116.Mohl S., Winter G. Continuous release of rh-interferon α-2a from triglyceride implants: storage stability of the dosage forms. Pharm. Dev. Technol. 2006;11:103–110. doi: 10.1080/10837450500464230. [DOI] [PubMed] [Google Scholar]
- 117.Surini S., Akiyama H., Morishita M., Nagai T., Takayama K. Release phenomena of insulin from an implantable device composed of a polyion complex of chitosan and sodium hyaluronate. J. Control Release. 2003;90:291–301. doi: 10.1016/s0168-3659(03)00196-2. [DOI] [PubMed] [Google Scholar]
- 118.Liao I. C., Wan A. C. A., Yim E. K. F., Leong K. W. Controlled release from fibers of polyelectrolyte complexes. J. Control Release. 2005;104:347–358. doi: 10.1016/j.jconrel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 119.Yamagata Y., Iga K., Ogawa Y. Novel sustained-release dosage forms of proteins using polyglycerol esters of fatty acids. J. Control Release. 2000;63:319–329. doi: 10.1016/s0168-3659(99)00206-0. [DOI] [PubMed] [Google Scholar]
- 120.Ryu W., Huang Z., Prinz F. B., Goodman S. B., Fasching R. Biodegradable micro-osmotic pump for long-term and controlled release of basic fibroblast growth factor. J. Control Release. 2007;124:98–105. doi: 10.1016/j.jconrel.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 121.Chen R. R., Mooney D. J. Polymeric growth factor delivery strategies for tissue engineering. Pharm. Res. 2003;20(8):1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 122.Saitoa N., Murakamib N., Takahashib J., Horiuchib H., Otab H., Katob H., Takaokae K. Synthetic biodegradable polymers as drug delivery systems for bone morphogenetic proteins. Adv. Drug Delivery Rev. 2005;57:1037–1048. doi: 10.1016/j.addr.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 123.Luginbuehl V., Meinel L., Merkle H. P., Gander B. Localized delivery of growth factors for bone repair. Eur. J. Pharm. Biopharm. 2004;58:197–208. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Geigera M., Lib R. H., Friessc W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Delivery Rev. 2003;55:1613–1629. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 125.Einhorn T. A., Majeska R. J., Mohaideen A., Kagel E. M., Bouxsein M. L., Turek T. J., Wozney J. M. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J. Bone Joint Surg. Am. 2003;85-A:1425–1435. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 126.Mulligan R. C. The basic science of gene therapy. Science. 1993;260:926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- 127.Cutroneo K. R. Gene therapy for tissue regeneration. J. Cell Biochem. 2003;88:418–425. doi: 10.1002/jcb.10357. [DOI] [PubMed] [Google Scholar]
- 128.McKenna C. J., Holmes D. R., Schwartz R. S. Novel stents for the prevention of restenosis. Trends Cardiovasc. Med. 1997;7:245–249. doi: 10.1016/S1050-1738(97)00066-2. [DOI] [PubMed] [Google Scholar]
- 129.Kamol L., John G. W., Ronald G., Michael J. Y., Arthur D. Mechanisms, management, and outcome of failure of delivery of coronary stents. Am. J. Cardiol. 1999;83:779–781. doi: 10.1016/s0002-9149(98)00990-4. [DOI] [PubMed] [Google Scholar]
- 130.Aggarwal R. K., Ireland D. C., Azrin M. A., Ezekowitz M. D., Bono D. P., Gershlick A. H., Aggrawal R. K., Ireland M. A. Antithrombotic potential of polymer-coated stents eluting platelet glycoprotein IIb/IIIa receptor antibody. Circulation. 1996;94:3311. doi: 10.1161/01.cir.94.12.3311. [DOI] [PubMed] [Google Scholar]
- 131.Foo R. S., Gershlick A. H., Hogrefe K. Inhibition of platelet thrombosis using an activated protein C-loaded stent: in vitro and in vivo results. Thromb. Haemost. 2000;83(3):496. [PubMed] [Google Scholar]
- 132.Gombotz W. R., Pettit D. K. Biodegradable polymers for protein and peptide drug delivery. Bioconjugate. Chem. 1995;6:332–351. doi: 10.1021/bc00034a002. [DOI] [PubMed] [Google Scholar]
- 133.Belle E. V., Couffinhal T., Couffinhal T. Stent endothelialization, time course, impact of local catheter delivery, feasibility of recombinant protein administration, and response to cytokine expedition. Circulation. 1997;95(2):438. doi: 10.1161/01.cir.95.2.438. [DOI] [PubMed] [Google Scholar]
- 134.T. Jin, F. Wu, and W. E. Yuan, inventors. Polysaccharide microparticles containing biological agents: their preparation and applications. WO 2007001777. April 1, 2007.