Abstract
The present study was designed to investigate the effect of two plasticizers, i.e., triethyl citrate (TEC) and polyethylene glycol 6000 (PEG 6000) on the in vitro release kinetics of diclofenac sodium from sustained-release pellets. Ammonio methacrylate copolymer type B (Eudragit RS 30 D) is used as the release-retarding polymer. Both plasticizers were used at 10% and 15% (w/w) of Eudragit RS 30 D. Pellets were prepared by powder layering technology and coated with Eudragit RS 30 D by air suspension technique. Thermal properties of drug and drug-loaded beads were studied using differential scanning calorimeter (DSC). DSC thermogram represented the identity of raw materials and exhibited no interaction or complexation between the active and excipients used in the pelletization process. Dissolution study was performed by using USP apparatus 1. No significant difference was observed among the physical properties of the coated pellets of different batches. When dissolution was performed as pure drug, about 8.22% and 90% drug was dissolved at 2 h in 0.1 N HCl and at 30 min in buffer (pH 6.8), respectively. From all formulations, the release of drug in acid media was very negligible (maximum 1.8 ± 0.08% at 2 h) but in buffer only 12% and 30% drug was released at 10 h from coated pellets containing TEC and PEG 6000, respectively, indicating that Eudragit RS 30 D significantly retards the drug release rate and that drug release was varied according to the type and amount of plasticizers used. The amount of TEC in coating formulation significantly effected drug release (p < 0.001), but the effect of PEG 6000 was not significant. Formulations containing PEG 6000 released more drug (98.35 ± 2.35%) than TEC (68.01 ± 1.04%) after 24 h. Different kinetic models like zero order, first order, and Higuchi were used for fitting drug release pattern. Zero order model fitted best for diclofenac release in all formulations. Drug release mechanism was derived with Korsmeyer equation.
Key words: aqueous coating, diclofenac sodium, Eudragit RS 30 D, plasticizer, thermal analysis
INTRODUCTION
The pellet type of sustained-release preparation is often referred to as multiparticulate, bead, or spansule. In general, the beads (diameter 1–3 mm) are prepared by coating drug powder onto cores called nonpareil seeds (NPS). The drug-coated beads generally provide a rapid-release carrier for the drug depending on the coating solution used in coating the drug. Once the drug beads are prepared, they may be further coated with a protective coating to allow a sustained or prolonged release of the drug (1). A major advantage of pellet dosage form is that the pellets are less sensitive to the effect of stomach emptying. Because there are numerous pellets within a capsule, some pellets will gradually reach the small intestine and deliver the drug, whereas a single tablet may be delayed in the stomach for a long time due to erratic stomach emptying (1). The fluctuating drug concentrations in blood and tissues caused by conventional dosage forms lead to an insufficient influence on the mechanisms of disease and are related to the excessive use of drug. Various oral dosage forms able to control the rate and extent of drug delivery into systemic circulation have been prepared and studied (2–5).
Aqueous film-coating dispersions generally consist of polymeric colloidal particles, a plasticizer, a pigment, and an anti-adherent agent. Most polymers employed for the film-coating of pellets and tablets are brittle at room temperature and require the use of plasticizers to improve their handling and processing (6). The most widely used aqueous polymer dispersions for sustained-release coating applications are either ethyl cellulose-based (Aquacoat ECD, Surelease) or acrylate-based, i.e., polymethacrylates (Eudragit RL 30 D, Eudragit RS 30 D, Eudragit NE 30 D, and others), products. Because of ethyl cellulose’s relatively high glass transition temperature (Tg) and pseudolatex nature, ethyl cellulose aqueous dispersions require adequate plasticization with the end product needing further curing steps. Although Eudragit products are true latex with low Tg’s, particle coalescence at room temperature is still slow and incomplete, necessitating accelerated curing conditions and/or the incorporation of water-soluble additives (7). Polymethacrylates are primarily used in oral capsule and tablet formulations as film-coating agents (8,9). Depending on the type of polymer used, films of different solubility characteristics can be produced. Eudragit RL 30 D and Eudragit RS 30 D are 30% aqueous dispersion of copolymers of acrylic acid and methacrylic acid esters with a low content of quaternary ammonium groups (10,11). The quaternary groups occur as salts and are responsible for the permeability of films made from these polymers. Films prepared from Eudragit RL 30 D are readily permeable to water and to dissolve active substances, whereas films prepared from Eudragit RS 30 D are less permeable to water and release the active substances through diffusion (11,12). Film-coatings prepared from both polymers give pH-independent release of active substance. Plasticizers are usually added to render the film more pliable, to augment film elongation properties, and to reduce moisture or gas permeability (10). Triethyl citrate (TEC) effectively lowers the glass transition temperatures of acrylic films (13). The citrate esters promote coalescence of the latex and pseudolatex particles and cause a homogeneous and continuous polymeric film to form (14). TEC from Morflex is one of the most widely used plasticizers in aqueous and organic film-coatings. This plasticizer has an official monograph in the current USP/NF (15).
Diclofenac sodium is a widely used nonsteroidal anti-inflammatory drug that exhibits antirheumatic, analgesic, osteoarthritis, and antipyretic activities. It has a short half-life in plasma (1–2 h). The daily dose varies between 75 and 200 mg/person, given in three or four divided portions depending on the route of administration. The most common adverse effects of the drug are gastritis, peptic ulceration, and depression of renal functions (16–18). Due to the short biological half-life and associated adverse effects, it is considered an ideal model drug for controlled drug delivery. This study was fabricated to observe the effect of two different types of plasticizers (polyethylene glycol 6000 [PEG 6000] and TEC) on the release behavior of Eudragit RS 30 D when using Eudragit RS 30 D for the coating of diclofenac sodium-loaded beads.
MATERIALS AND METHODS
Materials
Materials used throughout the experiment were diclofenac sodium (Square Pharmaceuticals, Bangladesh), sucrose (Cerestar, The Netherlands), lactose (The Lactose Co. of New Zealand Ltd. New Zealand), maize starch (Cerestar, The Netherlands), purified talc (Asian Mineral, Thailand), titanium dioxide (Warner Jenkinson, Italy), TEC (Morflex, USA), Kollidon 30 (BASF, Germany), Eudragit RS 30 D (Rohm, Germany), and PEG 6000 (Clariant, Germany). All the other chemicals used were of analytical grade.
Preparation of Drug-Loaded Beads
Powder layering method was chosen to prepare the diclofenac sodium beads. At first, the required amount of polyvinylpyrrolidone (Kollidon 30) was dissolved in isopropyl alcohol according to Table I to prepare binding solution. Then, the desired size (30/36) of NPS was loaded onto conventional coating pan (Ganson, India). The required amount of diclofenac sodium powder, lactose, and maize starch (according to Table I) was sieved through 80 mesh and mixed properly to prepare the powder blend. The powder blend was loaded manually on NPS with simultaneous spraying of binding solution. After completion of the process, drug-loaded pellets was dried at 60°C for 5.5 h in hot air oven (nonperforated tray drier) and then sieved through 20 mesh and 24 mesh, respectively, to get the desired size (20/24).
Table I.
Core and Coating Formulation of Diclofenac Sodium Sustained-Release Pellets (Weights are in Grams)
| Formulation code | |||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| Core materials | |||||
| Diclofenac sodium | 61.05 | 61.05 | 61.05 | 61.05 | 61.05 |
| NPS | 66.60 | 66.60 | 66.60 | 66.60 | 66.60 |
| Lactose | 41.63 | 41.63 | 41.63 | 41.63 | 41.63 |
| Maize starch | 19.62 | 19.62 | 19.62 | 19.62 | 19.62 |
| Polyvinylpyrrolidone (K-30) | 11.10 | 11.10 | 11.10 | 11.10 | 11.10 |
| Isopropyl alcohola up to | 100 | 100 | 100 | 100 | 100 |
| Coating materials | |||||
| Eudragit RS 30 D | 66.67 | 66.67 | 66.67 | 66.67 | 66.67 |
| Purified talc | 1 | 1 | 1 | 1 | 1 |
| Titanium dioxide | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| TEC | – | 2 | 3 | – | – |
| PEG 6000 | – | – | – | 2 | 3 |
| Water up to | 100 | 100 | 100 | 100 | 100 |
aWill not be present in final product
The coating suspensions were prepared by using Eudragit RS 30 D, purified talc, titanium dioxide, and water according to Table I. Drug-loaded pellets were taken in the fluid bed coater and coating suspension was sprayed. After completion of spraying, the coated pellets were dried at 60°C for 5 h in hot air oven and sieved through 18 and 24 mesh to get the desired size (18/24) of the diclofenac sodium sustained-release pellets. The prepared batch was termed as F1. The same process was followed by adding 10% TEC, 15% TEC, 10% PEG 6000, and 15% PEG 6000 individually (w/w of Eudragit RS 30 D on a dry basis) and the batches were termed as F2, F3, F4, and F5, respectively (Table I).
Moisture Content
The moisture content (percent loss on drying [%LOD]) of the dried and sieved pellets (18/24) was determined by using Mettler Toledo Halogen Moisture Analyzer (Model HB43, USA) where the working temperature was 105°C.
Friability
Friability test of the drug-loaded beads (18/24) was performed for 10 min at 24 rpm by using Electrolab EF-2 Friabilator (India).
Bulk Density
Bulk density of the dried and sieved pellets (18/24) was determined by using Stampfvolumeter bulk density detector (Model Stav 2003, Germany) after performing 100 strokes to measure the cylinder containing 10 g sample.
Assay
The quantity of diclofenac sodium in the prepared pellets (18/24) was determined by spectrophotometric analysis and the absorbance was measured at 276 nm by using UV-visible spectrophotometer (Shimadzu, Japan).
In Vitro Dissolution Study
The dissolution of diclofenac sodium sustained-release pellets was studied by Erweka (Germany) dissolution tester using USP apparatus 1 (basket method). Diclofenac sodium sustained-release pellets equivalent to 100 mg of diclofenac sodium was poured in 900 mL of 0.1 N hydrochloric acid medium at 37 ± 0.5°C with a rotation of 100 rpm for 2 h. At the end of 2 h, the media was removed and drug content was determined spectrophotometrically at 276 nm using 0.1 N hydrochloric acid as blank. Then, 900 mL of phosphate buffer (Na3PO4) of pH 6.8 was placed in each vessel and rotated at 100 rpm at 37 ± 0.5°C for 24 h. Samples (10 mL) were drawn every 2 h and replaced by fresh medium to maintain the volume constant, and drug content was determined spectrophotometrically at 276 nm using phosphate buffer (pH 6.8) as blank. Dissolution of free diclofenac sodium incorporated into capsule shell was also performed simultaneously following the same method.
Drug Release Kinetics
To study the release kinetics, data obtained from in vitro drug release studies were plotted in zero order, first order, and Higuchi kinetic models. Zero order (Eq. 1) is plotted as cumulative amount of drug released versus time, first order (Eq. 2) as log cumulative percentage of drug remaining versus time, and Higuchi’s model (Eq. 3) as cumulative percentage of drug released versus square root of time (19). In Eqs. 1, 2, and 3, K0 is the zero order rate constant expressed in units of concentration over time, C0 is the initial concentration of drug, k is the first order constant, K is the Higuchi rate constant, and t is the time in hours:
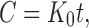 |
1 |
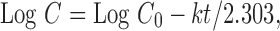 |
2 |
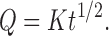 |
3 |
Mechanism of Drug Release
Drug release were plotted in the equation of Korsmeyer et al. (Eq. 4) as the log cumulative percentage of drug released versus log time, and the exponent n was calculated through the slope of the straight line:
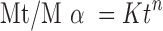 |
4 |
where Mt/Mα is the fractional solute release, t is the release time, K is the kinetic constant characteristic of the drug/polymer system, and n is the exponent that characterizes the mechanism of the release of tracers. For cylindrical matrix tablets, if the exponent n = 0.45, then the drug release mechanism is Fickian diffusion, and if 0.45 < n < 0.89, then it is non-Fickian or anomalous diffusion. An exponent value of 0.89 is indicative of case II transport or typical zero order release (20).
Differential Scanning Calorimetry
Thermograms of diclofenac SR beads were obtained using the differential scanning calorimeter (DSC) Q100 (TA Instruments, New Castle, USA) instrument equipped with an intracooler, a refrigerated cooling system. Indium standard was used to calibrate the DSC temperature and enthalpy scale. The powdered sample of beads was hermetically sealed in an aluminum pan and heated at a constant rate of 10°C/min, over a temperature range of 40°C to 340°C. Inert atmosphere was maintained by purging nitrogen at the flow rate of 50 mL/min. An empty pan was used as a reference.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was used to study the morphology of the prepared pellets as such without any coating around the pellets during analysis. SEM was performed using the Hitachi (Model S-3400 N, Japan) scanning electron microscope at 5 kV having different magnifications.
RESULTS AND DISCUSSION
Diclofenac sodium was loaded on NPS by powder layering method and finally coated with Eudragit RS 30 D using fluid bed coater. The drug release was studied by in vitro dissolution using the USP basket method.
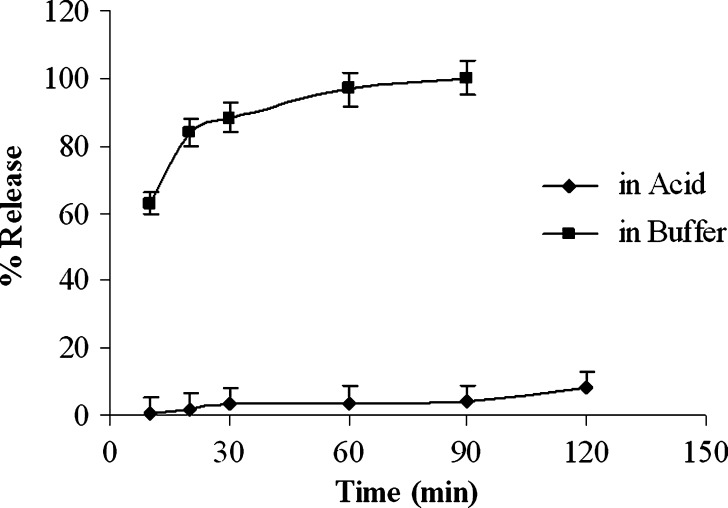
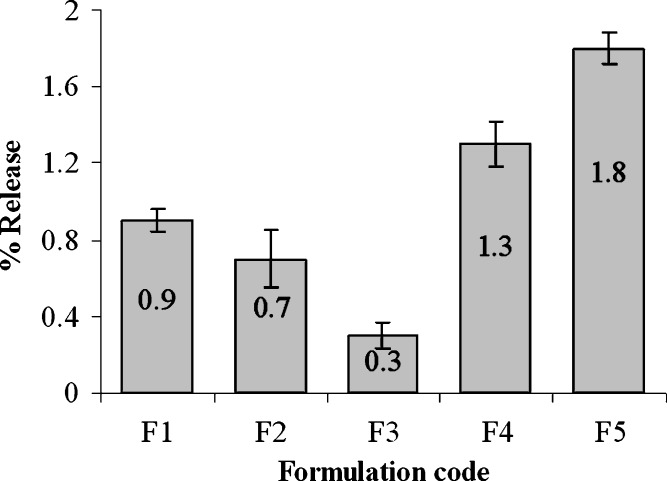
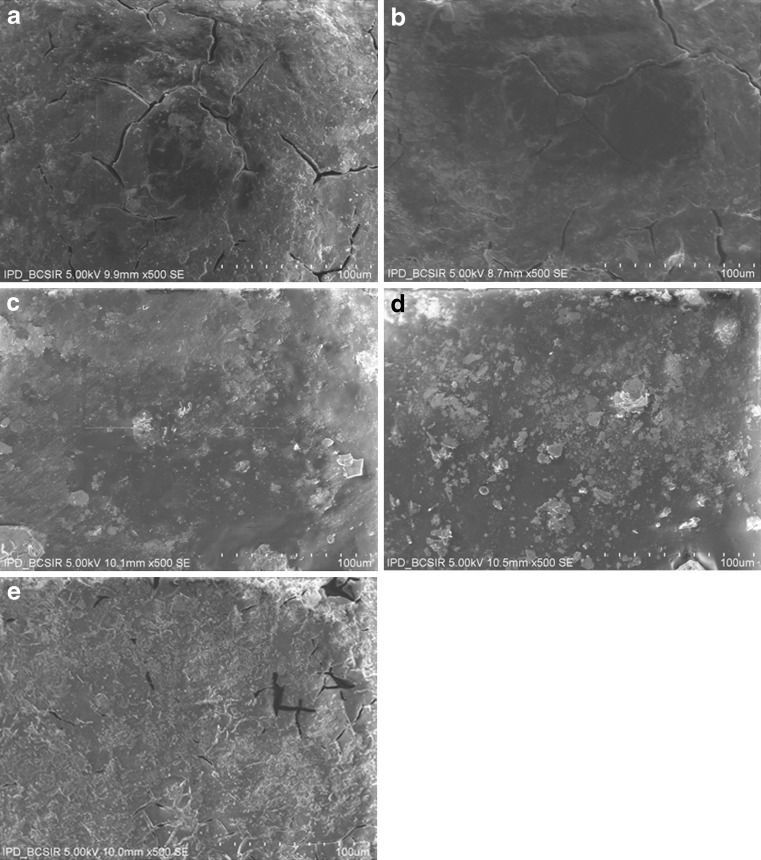
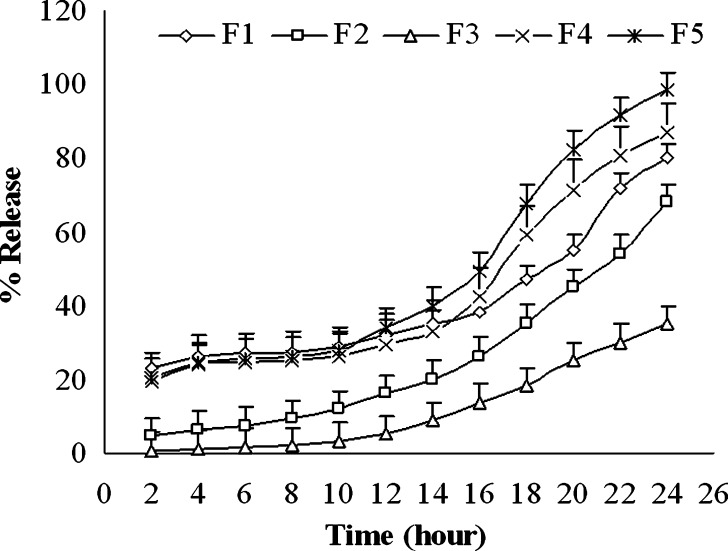
The weakly acidic drug, diclofenac sodium (pKa = 4.0), is almost insoluble at acidic pH of the stomach (3). When dissolution of diclofenac sodium powder incorporated in capsule was carried out in 0.1 N HCl, only 3.47 ± 0.4% and 8.22 ± 0.6% drug was released after 30 and 120 min, respectively (Fig. 1). Similarly, diclofenac sodium released very poorly (0.3–1.8%) from pellets in acidic environment at 2 h (Fig. 2). In acid media, 0.9% drug was released from unplasticized coated pellets. Minimum amount of drug was released (0.3–0.7%) from the pellets coated with TEC and it was revealed that drug release decreased along with increase in TEC load (Fig. 2). But a reverse phenomena was depicted in the case of PEG 6000. When PEG 6000 was increased from 10% to 15%, drug release also increased from 1.3% to 1.8%. It may be due to the solubility of PEG 6000 in water and presence of cracks on the surface of pellets containing PEG 6000 as shown in Fig. 3a,b. On the other hand, 62.88 ± 1.56%, 88.13 ± 1.93%, and 96.74 ± 0.45% drug was released within 10, 30, and 60 min, respectively, in buffer media (pH 6.8) when given as raw powder (Fig. 1) which expressed the prompt solubility of diclofenac at pH 6.8. Dissolution of coated pellets containing TEC as plasticizer showed dependence of plasticizer amount on drug release in buffer media. With pellets coated with Eudragit RS 30 D having 10% TEC in coating (formulation F2), about 5%, 12%, and 70% drug was released at 2, 10, and 24 h, respectively (Fig. 4). On the contrary, only 0.58%, 3.4%, and 35.12% drug was released at the same time when coating contains 15% TEC (formulation F3). This indicates that the flexibility of acrylic polymer film is increased along with increase in TEC that retard the drug release by coalescence of the polymer more with TEC around the pellets surface as well as reduces the chance of cracking drastically (Fig. 3c,d). The amount of TEC in coating formulation significantly affected drug release (p < 0.001). According to the Korsmeyer equation, both formulations F2 and F3 showed zero order drug release (Table II). The plasticizing effect of TEC is shown in SEM (Fig. 3c,d). The unplasticized coating (Fig. 3e) showed cracking which is responsible for higher drug release which indicates that a plasticizer is necessary for flexible film around the pellets. Eudragit RS particles are coalesced by TEC (21) and cracks were absent in formulations F2 and F3 (Fig. 3c,d). Due to this nonporous coating, the drug release from these formulations was negligible even up to 16 h. It was also observed that 10% TEC is enough to cause this plasticization; therefore, increasing the plasticizer level decreases the drug release (Fig. 4).
Fig. 1.
Dissolution profile of diclofenac sodium powder in 0.1 N HCl and pH 6.8 phosphate buffer
Fig. 2.
Release of diclofenac sodium from different formulations of coated pellets at 2 h in 0.1 N HCl. F1 coated pellets without plasticizer, F2 coated pellets with 10% TEC, F3 coated pellets with 15% TEC, F4 coated pellets with 10% PEG 6000, F5 coated pellets with 15% PEG 6000
Fig. 3.
Scanning electron micrograph of diclofenac sodium coated pellets: a coated pellets with 10% PEG 6000, b coated pellets with 15% PEG 6000, c coated pellets with 10% TEC, d coated pellets with 15% TEC, and e coated pellets without plasticizer
Fig. 4.
Zero order release kinetics of diclofenac sodium from coated pellets in phosphate buffer. F1 coated pellets without plasticizer, F2 coated pellets with 10% TEC, F3 coated pellets with 15% TEC, F4 coated pellets with 10% PEG 6000, F5 coated pellets with 15% PEG 6000
Table II.
Regression Coefficients and Rate Constants for Diclofenac Release from Coated Pellets
| Formulation code | Zero order | First order | Higuchi | Korsmeyer | ||||
|---|---|---|---|---|---|---|---|---|
| k 0 | r 2 | k 1 | r 2 | k H | r 2 | n | r 2 | |
| F1 | 2.38 | 0.83 | 0.02 | 0.76 | 14.51 | 0.72 | 0.46 | 0.71 |
| F2 | 2.74 | 0.89 | 0.02 | 0.8 | 16.81 | 0.79 | 1.11 | 0.89 |
| F3 | 1.6 | 0.89 | 0.01 | 0.84 | 9.81 | 0.78 | 1.76 | 0.93 |
| F4 | 3.15 | 0.87 | 0.03 | 0.78 | 19.26 | 0.76 | 0.60 | 0.74 |
| F5 | 3.73 | 0.89 | 0.05 | 0.67 | 22.94 | 0.79 | 0.65 | 0.77 |
F1 coated pellets without plasticizer, F2 coated pellets with 10% TEC, F3 coated pellets with 15% TEC, F4 coated pellets with 10% PEG 6000, F5 coated pellets with 15% PEG 6000, k 0 zero order rate constant, k 1 first order rate constant, k H Higuchi rate constant, n diffusion coefficient, r 2 regression coefficient
Pellets containing PEG 6000 as plasticizer released drug in buffer media indifferently. There was no significant difference in drug release up to 12 h in buffer media from pellets coated with 10% PEG 6000 (formulation F4) and 15% PEG 6000 (formulation F5) as well as from the unplasticized coated pellets (formulation F1) which might be due to the low permeability properties of Eudragit RS 30 D (12). But from 14 h, the drug release showed terminal increase exponentially (Fig. 4) which might be the terminal release-enhancing properties of Eudragit RS 30 D (22). It was also evident that, at the end of the dissolution study, 98.35% drug was released from formulation F5 compared to 87.03% drug release from formulation F4 due to the more porous surface created for the presence of aqueous soluble PEG 6000. On the other hand, about 80% drug was released terminally from formulation F1 which contains no plasticizer.
The amount of drug release from pellets containing PEG 6000 (formulations F4 and F5) was much higher than that of TEC-containing pellets (formulations F2 and F3). In the case of 10% PEG 6000 (formulation F4), about 20%, 60%, and 80% drug was released at 2, 18, and 22 h, respectively (Fig. 4), whereas only 5%, 35%, and 55% drug comes to dissolution at the same time while the pellets contain 10% TEC (formulation F2). It was also evident that from 15% PEG 6000-containing pellets (formulation F5) about 20%, 50%, and 90% drug was released at 2, 16, and 22 h, respectively (Fig. 4), but about 1%, 15%, and 30% drug was released at the mentioned times from 15% TEC-containing beads (formulation F3). These phenomena could be attributed to higher water solubility of polyethylene glycol than TEC, which helps to create channels through sustained-release coating. This finding was also supported by SEM, as shown in Fig. 3a–d. There were large cracks and channels over the coated surface of pellets containing PEG 6000 which helped drug release. Increased level of PEG 6000 can reduce these channels (Fig. 3b) by coalescing Eudragit particles. From both formulations F4 and F5, release of drug followed non-Fickian or anomalous diffusion where n = 0.60 and 0.65, respectively (Table II).
The incorporation of a plasticizer in the system was found to be an important factor, without which a much faster dissolution rate was found (Table II; Fig. 4). It was revealed that when the percentage of TEC was increased then the drug release rate constant was decreased (Table II) but when the percentage of PEG 6000 was increased the release rate constant was increased rhythmically. In most cases, the release of drug followed zero order release kinetics (Table II; Fig. 4). It is also observed that the specific type of plasticizer played an important role on drug release, as shown in Figs. 2 and 4. At the concentrations chosen in this study, TEC was found to be most effective in attenuating drug release, followed by polyethylene glycol. So the type and amount of plasticizer played a vital role in drug release which might be due to alter membrane permeability, probably by affecting particle coalescence, modifying film hydrophilicity, or the combination thereof (23). From the overall dissolution study, it was evident that pellets coated with Eudragit RS 30 D can sustain drug release for a long period, but to meet optimum plasma drug concentration, it would be better to use the combination of acrylic polymers having high permeability properties (such as Eudragit RL) or the incorporation of channel formers. The physical appearance of the prepared pellets was found almost white in all formulations. Maximum friability was found to be 0.31%, indicating that pellets were stable enough to withstand the mechanical force (Table III). Among the physical parameters of the pellets, no significant variation was observed between the unplasticized (F1) and plasticized pellets (F2–F5).
Table III.
Physical Parameters of Dried and Sieved (18/24) Coated Pellets
| Parameters | Formulation code | ||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| Appearance | White to off-white spherical pellets | White to off-white spherical pellets | White to off-white spherical pellets | White to off-white spherical pellets | White to off-white spherical pellets |
| Product yield (after coating) (%) | 95.68 | 96.77 | 98.31 | 95.94 | 97.44 |
| Loss on drying (%) | 1.72 ± 0.09 | 1.66 ± 0.12 | 1.83 ± 0.14 | 1.75 ± 0.38 | 1.92 ± 0.51 |
| Potency (%) | 24.89 ± 1.56 | 24.14 ± 2.45 | 23.01 ± 1.77 | 24.50 ± 1.03 | 23.88 ± 2.82 |
| Friability (%) | 0.27 ± 0.03 | 0.31 ± 0.01 | 0.12 ± 0.02 | 0.26 ± 0.01 | 0.18 ± 0.01 |
| Bulk density (g/cc) | 0.91 ± 0.01 | 0.90 ± 0.02 | 0.89 ± 0.01 | 0.94 ± 0.02 | 0.92 ± 0.01 |
F1 coated pellets without plasticizer, F2 coated pellets with 10% TEC, F3 coated pellets with 15% TEC, F4 coated pellets with 10% PEG 6000, F5 coated pellets with 15% PEG 6000
N = 3 for each trial except percent yield
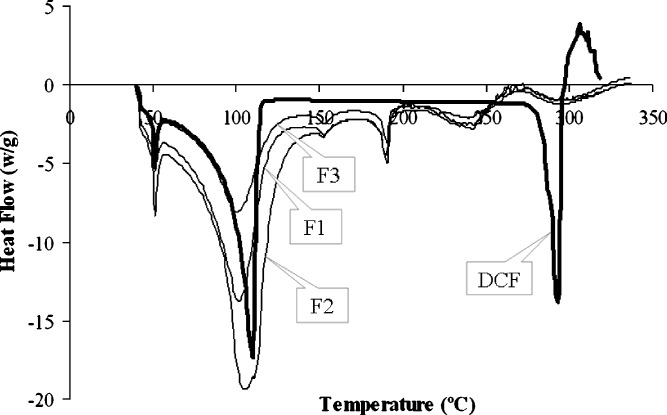
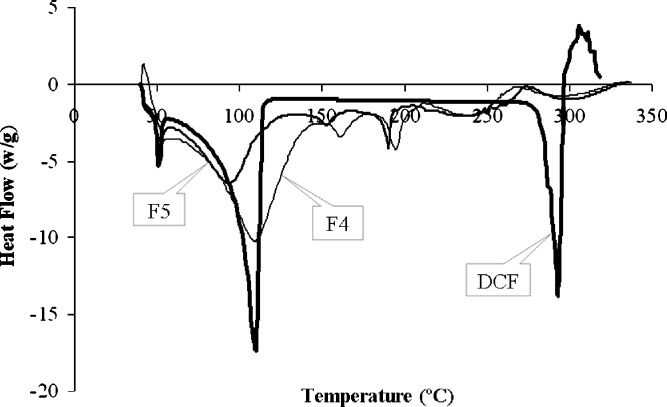
The DSC thermogram of drug and drug-loaded beads are shown in Figs. 5 and 6. Diclofenac showed sharp melting endotherm at 285.74°C which corresponded to its melting with normalized energy of 83.19 J/g. The thermogram of beads of formulation F1 to F5 showed the endotherm at 278.35°C, 281.53°C, 278.70°C, 275.95°C, and 280.88°C, respectively, with energies of 13.67, 17.14, 18.63, 9.92, and 16.96 J/g, respectively. The melting endotherm at 52.67°C, 65.42°C, 149.54°C, and 224.44°C was represented by PEG 6000, moisture, povidone (K-30), and lactose anhydrous, respectively (Figs. 5 and 6), which were present in the formulations. Also, the acrylic polymer showed the burning end point at 185.79°C. The corresponding qualitative composition of the drug formulations represented the identity of each of the components and indicated the absence of interaction or complexation throughout the process of pelletization and coating.
Fig. 5.
DSC thermograms of pure diclofenac sodium as well as TEC-containing coated pellets. DCF free diclofenac sodium, F1 coated pellets without plasticizer, F2 coated pellets with 10% TEC, F3 coated pellets with 15% TEC
Fig. 6.
DSC thermograms of pure diclofenac sodium as well as polyethylene glycol-containing coated pellets. DCF free diclofenac sodium, F4 coated pellets with 10% PEG 6000, F5 coated pellets with 15% PEG 6000
CONCLUSION
Different types of plasticizers can influence diclofenac sodium release from coated pellets. It is possible to modify the release of drug by changing the amount of TEC in coating suspension by keeping the amount of polymer constant. Polyethylene glycol will be suitable for higher rate of drug release than that of TEC.
Acknowledgements
The authors would like to thank Eskayef Bangladesh Ltd. (formerly, SmithKline & French, UK) for providing the raw materials as well as manufacturing facilities, Bangladesh Council of Scientific and Industrial Research (BCSIR), and Renata Ltd. Bangladesh (formerly, Pfizer, USA) for providing the scanning electron micrograph and the DSC thermogram, respectively.
Contributor Information
Golam Kibria, Phone: +880-1-816105604, FAX: +880-2-8615583, Email: gkibria123@yahoo.com.
Reza-ul Jalil, Phone: +880-2-9120325, FAX: +880-2-8615583, Email: raju1559@yahoo.com.
References
- 1.Andrew B. C., Shargel L. Applied Biopharmaceutics and Pharmacokinetics. Connecticut: Appleton & Lange; 1941. pp. 225–264. [Google Scholar]
- 2.Bidah D., Vergnaud J. M. Dosage forms with a polymer matrix and a swelling polymer. Int. J. Pharm. 1991;77:81–87. doi: 10.1016/0378-5173(91)90306-9. [DOI] [Google Scholar]
- 3.Bravo S. A., Lamas M. C., Salamon C. J. In-vitro studies of diclofenac sodium controlled-release from biopolymeric hydrophilic matrices. J. Pharm. Pharm. Sci. 2002;5(3):213–219. [PubMed] [Google Scholar]
- 4.Ford J. L., Rajabi-Siahboomi A. R., Rubinstein M. H., Sadeghi F. Comparative study of drug release from pellets coated with HPMC or Surelease. Drug Dev. Ind. Pharm. 2000;26(6):651–660. doi: 10.1081/DDC-100101280. [DOI] [PubMed] [Google Scholar]
- 5.Jayasagar G., Rao Y. M., Veni J. K. Formulation and evaluation of diclofenac sodium using hydrophilic matrices. Drug Dev. Ind. Pharm. 2001;27(8):759–766. doi: 10.1081/DDC-100107239. [DOI] [PubMed] [Google Scholar]
- 6.W. Chuanbin, and W. M. James. Influence of ibuprofen as a solid-state plasticizer in Eudragit® RS 30 D on the physicochemical properties of coated beads. AAPS PharmSciTech.2(4) article 24 (2001). [DOI] [PMC free article] [PubMed]
- 7.L. L. Augsburger, A. Lin, N. A. Muhammad, and D. Pope. Effect of curing and storage conditions on drug release from pellets coated with Eudragit NE30D. AAPS Annual Meeting Supplement2(4) (2000).
- 8.Dreher D., Lehmann K. The use of aqueous synthetic polymer dispersions for coating pharmaceutical dosage forms. Drugs Made Ger. 1973;16:126–136. [Google Scholar]
- 9.Obi C. E., Okor R. S. Drug release through aqueous-based film coatings of acrylate-methacrylate, a water-insoluble copolymer. Int. J. Pharm. 1990;58:89–91. doi: 10.1016/0378-5173(90)90291-B. [DOI] [Google Scholar]
- 10.G. Cole (eds.), Pharmaceutical Coating Technology, Taylor & Francis Ltd., London, 2001, pp. 27–31.
- 11.J. W. McGinity (eds.), Aqueous Polymeric Coating for Pharmaceutical Dosage Forms, Marcel Decker Inc., New York, 1996, pp. 101–174.
- 12.Lehmann K. Practical Course in Film Coating of Pharmaceutical Dosage Forms with Eudragit. Darmstadt: Pharma Polymers; 2001. pp. 8–15. [Google Scholar]
- 13.Pharmaceutical Coatings Bulletin. Influence of Citrate Ester Plasticizers on the Properties of Acrylic Resin Polymers, Morflex, Inc., 1993, p. 102.
- 14.Pharmaceutical Coatings Bulletin. Physical and Mechanical Properties of Acrylic and Cellulosic Polymers Plasticized with Citrate Esters. Morflex, Inc., 1994, p. 102.
- 15.USP 23/NF 18. Triethyl Citrate-Official Monograph, 1995, p. 2316.
- 16.Tapia C., Escobar Z., Costa E., et al. Comparative studies on polyelectrolyte complexes and mixture of chitosan-alginate and chitosan-carrageenan as prolonged diltiazem clorhydrate release system. Eur. J. Pharm. Biopharm. 2004;57:65–75. doi: 10.1016/S0939-6411(03)00153-X. [DOI] [PubMed] [Google Scholar]
- 17.Gilman A. G., Rail T. W., Nies A. S., Taylor P. The Pharmacological Basis of Therapeutics. New York: Pergamon Press; 1990. [Google Scholar]
- 18.Morkhade D. M., Fulzele S. V., Satturwar P. M., Joshi S. B. Gum copal and gum damar: novel matrix forming materials for sustained drug delivery. Ind. J. Pharm. Sci. 2006;68:53–58. doi: 10.4103/0250-474X.22964. [DOI] [Google Scholar]
- 19.Higuchi T. Mechanism of sustained action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 20.Korsmeyer R. W., Gurny R., Doelker E., Buri P., Peppas N. A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y., Mehta K. A., McGinity J. W. Influence of plasticizer level on the drug release from sustained release film coated and hot-melt extruded dosage forms. Pharm. Dev. Tech. 2006;11:285–294. doi: 10.1080/10409230600767551. [DOI] [PubMed] [Google Scholar]
- 22.Kibria G., Jalil R. The effect of the ratio of two acrylic polymers on the in-vitro release kinetics of ketoprofen from pellets prepared by extrusion and spheronisation technique. Pak. J. Pharm. Sci. 2008;21(2):92–97. [PubMed] [Google Scholar]
- 23.Saettone M. F., Perini G., Rijli P., Rodriguez L., Cini M. Effect of different polymer-plasticizer combinations on in vitro release of theophylline from coated pellets. Int. J. Pharm. 1995;126:83–88. doi: 10.1016/0378-5173(95)04099-4. [DOI] [Google Scholar]