Abstract
A number of synthesized chemical molecules suffer from low aqueous solubility problems. Enhancement of aqueous solubility, dissolution rate, and bioavailability of drug is a very challenging task in drug development. In the present study, solubility and dissolution of poorly aqueous soluble drug simvastatin (SIM) was enhanced using hydrophilic, low viscosity grade polymer hydroxypropyl methylcellulose (HPMC K3LV). The co-solvent evaporation method was developed for efficient encapsulation of hydrophobic drug in polymer micelles of HPMC K3LV. Spray drying and rotaevaporation method were applied for solvent evaporation. Co-solvent-evaporated mixture in solid state was determined by differential scanning calorimetry (DSC), X-ray diffraction studies (XRD), scanning electron microscopy, and Fourier-transform infrared spectroscopy. In vitro–in vivo studies were performed on co-solvent-evaporated mixture and compared with SIM. In vivo study was conducted on healthy albino rats (Wister strain), and formulations were administered by oral route. Results of the study show the conversion of crystalline form of SIM into amorphous form. The dissolution rate was remarkably increased in co-solvent-evaporated mixtures compared to SIM. co-solvent-evaporated mixtures showed better reduction in total cholesterol and triglyceride levels than the SIM. The low-viscosity grade HPMC acts as a surfactant, which enhances the wetting of drug and thus improves the solubility of drug. The co-solvent evaporation method provides good encapsulation efficiency and produces amorphous form of SIM, which gave better solubility and dissolution than the crystalline SIM.
Key words: co-solvent evaporation, hydroxypropyl methylcellulose, rotaevaporation, simvastatin, spray drying
INTRODUCTION
A number of synthesized chemical molecules suffer from low aqueous solubility problems. Although these molecules have potential pharmacodynemic property, they show low bioavailability due to poor aqueous solubility, and these molecules become unsuccessful to reach the market. Thus, enhancement of aqueous solubility, dissolution rate, and thereby the bioavailability of drug is a very challenging task in drug development.
Simvastatin (SIM) is a cholesterol lowering agent, which is a white, nonhygroscopic, crystalline powder having poor aqueous solubility and bioavailability. SIM is a potential inhibitor of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase. It catalyzes the conversion of HMG-CoA to mevalonate; this conversion is an early and rate-limiting step in the biosynthesis of cholesterol (1). The drug is poorly absorbed from the gastrointestinal (GI) tract; therefore, it is important to enhance the aqueous solubility, dissolution rate, and bioavailability from its oral solid dosage forms.
Many methods were reported for solubility and dissolution enhancement of poorly soluble drug such as micronization, complexation, solid dispersion, etc. However, all this methods have limitations like micronized powder having high energetic surface, which shows poor flow property (2), and particles often agglomerated. Complexation with cyclodextrin (3) shows low drug load and limitations for drug selection. Solid dispersion shows good improvement in dissolution rate and bioavailability (4,5) with water-soluble rate enhancing polymer such as polyethylene glycol, mannitol, and polyvinylpyrrolidone (PVP) (6), but high amount of this polymer is required (7) and scale up is difficult. The methods used for fabrication of solid dispersion include solid solution (8–10) and melt method (9–12), but these techniques has limitations (13). Many polymers were reported for solubility enhancement of SIM such as HP-β-cyclodextrin, arosil 200, PVP K30, self-microemulsifying agent, etc.
In the present study, low viscosity grade of hydroxypropyl methylcellulose (HPMC K3LV) having surfactant and wetting property leads to the enhancement of solubility and dissolution of drug and thus bioavailability. The co-solvent evaporation method provides advantage to use a lipophilic drug (SIM) with hydrophilic polymer (HPMC K3LV). Solvent evaporation method shows good encapsulation efficiency of hydrophilic polymers (14). Co-solvent evaporation was carried out by using spray drying and rotaevaporation method. The solid mixtures were characterized by differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), and Fourier-transform infrared spectroscopy (FTIR). Solubility and dissolution of co-solvent-evaporated mixture were compared with SIM, and in vivo study was performed on albino rats (Wistar strain).
MATERIALS AND METHODS
Materials
Simvastatin (SIM) (100 μm) was obtained as a gift sample (Artimis Biotech, IDA, Jeedimetla, Hyderabad, India), as well as HPMC K3LV (Colorcon Asia Limited, Verna, Goa, India), monobasic sodium phosphate (S.D. Fine Chemicals, Mumbai, India), sodium lauryl sulfate (Qualigens Fine Chemicals, Mumbai). Methanol and all other buffering agent of analytical grades were obtained and used for projected work.
Methods
Co-Solvent Evaporation Method
Rotaevaporation
The solvent evaporation of SIM and HPMC K3LV solution in ratio (1:1, w/w) was carried out by using Buchi Rota evaporator (Buchi Rota Vapor, R215, Buchi, Switzerland). The solutions were prepare by dissolving 2 g of SIM in 100 ml of methanol and 2 g of HPMC (K3LV) in 60 ml of distilled water and mixed both solutions, which produces clear solution. The clear solution evaporated at 253 tore pressure and 60°C for half hr in rotaevaporator. The dried rotaevaporated mixture of drug with HPMC K3LV is denoted as RHPD.
Spray Drying
The solvent evaporation of SIM and HPMC (K3LV) solution in ratio (1:1) was carried out by using spray dryer (LU-222, Advanced, Labultima, India). The solutions were prepared by dissolving 2 g of drug in 70 ml of methanol and 2 g of HPMC (K3LV) in 30 ml of distilled water and mixing both solutions, which produces a clear solution. The solvent evaporated at inlet 110°C and outlet 60°C, feed pump speed 10 ml per minute and aspiration 45%. The spray-dried mixture of drug with HPMC K3LV was obtained in 20–30 min, and it is denoted as SHPD.
Solid Mixture Characterization
Differential Scanning Calorimetry
Analyses of samples were carried out on DSC (Universal V2. 4F TA Inc., USA) instrument. Samples weighing between 1 and 10 mg were loaded into open aluminum pan and placed into the DSC cell. The cell had a nitrogen purge flowing at approximately 40 cm3/min. The DSC was used to analyze the samples from 10–350°C with a 10°C/min heating rate. An indium pan served as reference, and all scans were performed in triplicate. The instrument was calibrated before sample analysis, using an indium standard.
Powder X-Ray Diffraction Studies
PXRD patterns of samples were obtained using Philips diffractometer (PW3710, Almelo, The Netherland) and Cu-Kα line as a source of radiation, which was operated at the voltage 40 kV and the current 30 mA. All samples were measured in the 2θ angle range between 0° and 60° with a scanning rate of 3°/min and a step size of 0.02°.
Scanning Electron Microscopy
The morphology of samples were determined using a scanning electron microscope (SEM) (Jeol model 6390 LV, USA) operated at an accelerating voltage of 3 kV. Samples were prepared by mounting powder on to a brass stub using graphite glue and coated with gold under vacuum before use.
Fourier-Transform Infrared Spectroscopy
The pure drug, polymer, and co-solvent-evaporated mixture was mixed separately with IR grade KBr in the ratio of 100:1, and corresponding pellets were prepared by applying 10 metric ton of pressure in hydraulic press. The pellets were then scanned over a wave range of 4,000–400 cm−1 in FTIR instrument (8400 S Shimadzu, Japan).
Solubility Study
The solubility of SIM, RHPD, and SHPD was determined in distilled water, pH 1.2 HCl buffer, and pH 7 buffer (solution containing 0.5% SLS in 0.01 M sodium phosphate) according to USP dissolution profile of SIM. The solubility of SIM and co-solvent-evaporated mixtures were determined by using the method, in which SIM in an excess amount of 30 mg and the mixture equivalent to 30 mg of SIM were added in 10 ml of above the solvents, in Teflon facing screw-capped vial and kept at equilibrium for a period of 48 h on orbital shaking incubator at 37 ± 0.5°C and 50 rpm. The content of vials were filtered through 0.2-μm filter and analyzed on UV-Visible spectrophotometer (UV 1601, Shimadzu, Japan) at 238 nm.
Evaluation of Formulation
In vitro Study
Dissolution Test
Co-solvent-evaporated mixtures and SIM were filled in capsules and dissolution was performed using pH 1.2 HCl buffer and pH 7 (SLS, 0.5%) buffer with USP dissolution apparatus I at 50 rpm and 37 ± 0.5°C. Test samples (5 ml) were withdrawn at a particular time interval (5, 10, 15, 20, 30, 45, and 60 min) and replaced with fresh dissolution media maintained at 37 ± 0.5°C. The test samples were filtered (membrane filter, 0.45 μm) and the concentration of dissolved drug was determined using UV spectrophotometer at λmax 238 nm. The test was performed on three capsules and mean ± SD calculated.
In vivo Study
The hypolipidemic activity of RHPD and SHPD were determined in comparison with SIM in healthy albino rats (Wistar strain) weighing between 150 and 280 g. The rats were housed in a cage at room temperature and relative humidity of 55 ± 10%. Environmental conditions were monitored strictly. All experiments were performed according to protocol, submitted and approved by Institutional Animal Ethical Committee of R. C. Patel College of pharmacy, Shirpur. The animals were divided into four groups of three animals each. The treatment was given for 14 days. Each group daily received 2 ml of coconut oil orally using gavage feeding needles. After the feeding of coconut oil, reference and test (2) groups were administered orally 1 ml of 2% w/v gum acacia aqueous suspensions with RHPD and SHPD (equivalent to 10 mg/kg body weight), respectively. Control group also received daily 1 ml of 2% w/v gum acacia solution via oral administration. Blood samples were collected by using light ether anesthesia by retroorbital puncture: Initially, after 7 and 14 days. The serum samples were analyzed for total cholesterol, triglycerides (TG) by the in vitro diagnostic kit (15).
RESULTS
Solubility Study
Solubility data for SIM, RHPD, and SHPD in different solvents are given in Table I. Analysis of variance (ANOVA; P < 0.001) performed on solubility parameters and demonstrated significant difference between solubility of SIM and co-solvent-evaporated mixtures.
Table I.
Solubility of SIM* and Co-solvent Evaporation Mixture in Different Solvent at 37 ± 0.5°C After 48 h
| Sample | Medium | ||
|---|---|---|---|
| Water (mg/ml) | pH 1.2 buffer (mg/ml) | pH 7 buffer (mg/ml) | |
| SIM | 0.067 ± 0.005 | 0.042 ± 0.029 | 0.46 ± 0.023 |
| SHPD | 1.25 ± 0.038 | 1.35 ± 0.026 | 1.74 ± 0.062 |
| RHPD | 0.85 ± 0.21 | 0.91 ± 0.166 | 1.59 ± 0.21 |
All results were calculated as mean ± 3 SD
SIM simvastatin, SHPD spray-dried product, RHPD rotaevaporated product, D drug
Differential Scanning Calorimetry
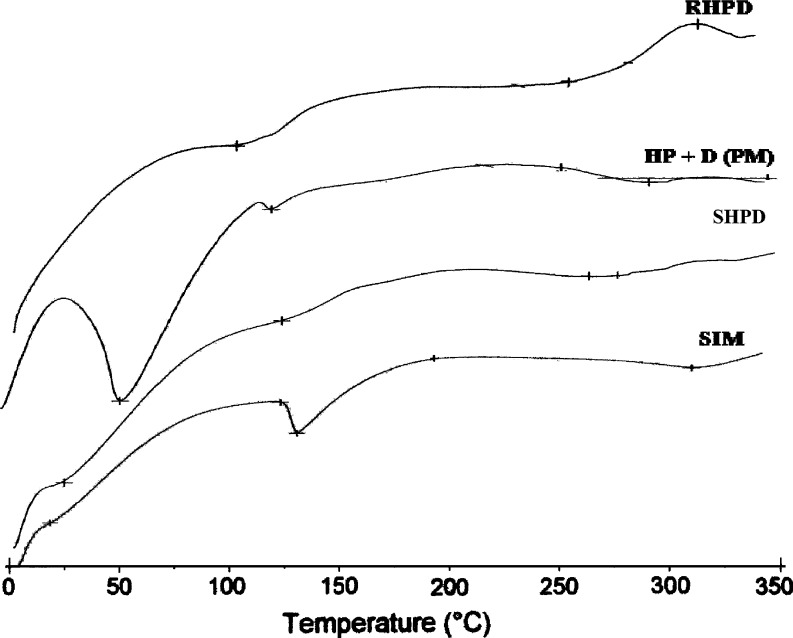
The DSC thermograms of SIM, SIM, and HPMC K3LV physical mixture (PM), RHPD, and SHPD are given in Fig. 1. SIM was characterized by sharp melting endothermic peak at 140.63°C during DSC analysis. The DSC thermograms of SHPD shows endothermic peak at less temperature 28.72°C than SIM due to conversion of SIM crystalline to amorphous form. The co-solvent-evaporated mixture of RHPD and SHPD shows the decrease in crystallinity and increase in amorphous form (Fig. 2).
Fig. 1.
DSC thermograms of SIM, HPMC, and drug (PM), SHPD, and RHPD. SIM simvastatin, HPMC hydroxypropyl methylcellulose, SHP D spray-dried product, RHP D rotaevaporated product, PM physical mixture, D drug. All results were calculated as mean ± 3 SD
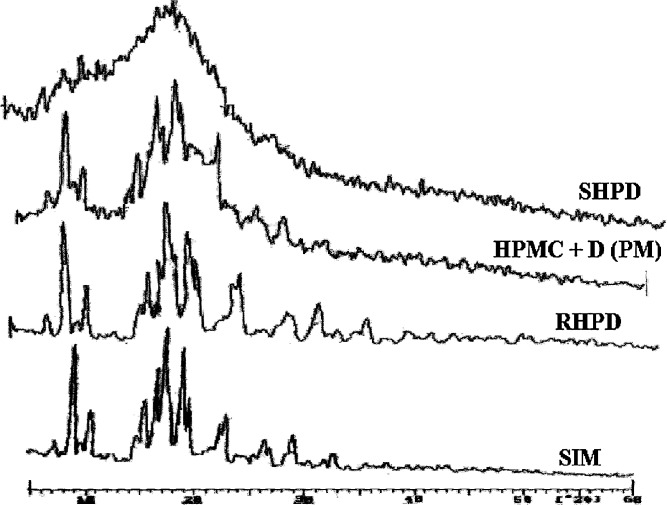
Fig. 2.
Powder X-ray diffraction patterns of SIM, RHPD, Physical mixture of HPMC and drug, SHPD. SIM simvastatin, HPMC hydroxypropyl methylcellulose, SHP D spray-dried product, RHP D rotaevaporated product, PM physical mixture, D drug. All results were calculated as mean ± 3 SD
Powder X-Ray Diffraction studies
XRD characteristics of SIM, SIM, and HPMC K3LV PM, RHPD, and SHPD are shown in Fig. 3. The characteristic peaks appeared in the XRD of SIM at different angles of 5.84°, 8.97°, 12.73°, 16.26°, 17.34°, 18.60°, 22.33°, 25.66°, and 26.23°. It was observed that the XRD of SHPD shows absence of characteristic peaks of SIM, and intensity of peaks in SHPD was also reduced. X-RD pattern of RHPD and PM shows some distinct characteristic peaks of SIM.
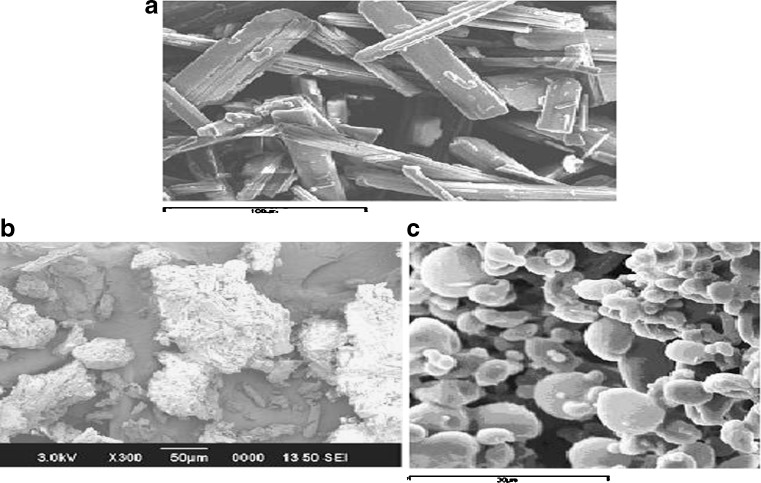
Fig. 3.
SEM of a SIM, b RHPD, and c SHPD. SIM simvastatin, HPMC hydroxypropyl methylcellulose, SHP D spray-dried product, RHP D rotaevaporated product, D drug. All results were calculated as mean ± 3 SD
Scanning Electron Microscopy
The SEM of SIM, RHPD, and SHPD is shown in Fig. 3. SIM particles appeared as plate-like crystals (100 μm) with smooth surfaces. SIM was co-solvent-evaporated with HPMC K3LV using spry drying and rotaevaporation techniques. It seemed that the morphology of SIM was changed in co-solvent-evaporated mixtures.
Fourier-transform infrared spectroscopy
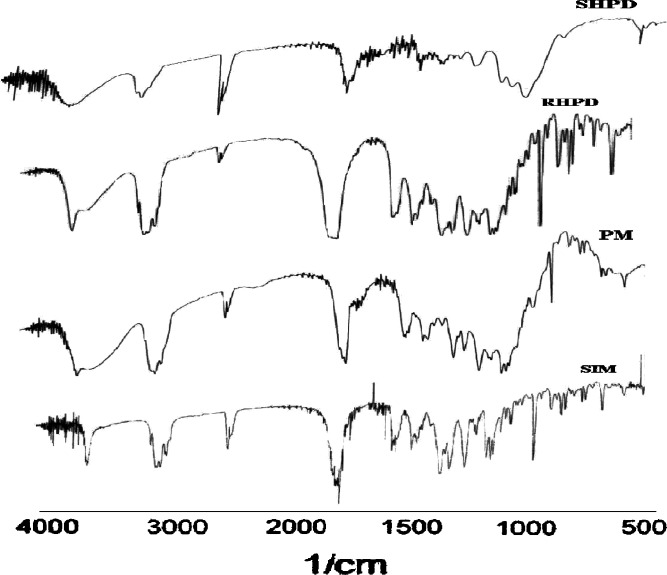
FTIR spectra of SIM, SIM, and HPMC K3LV PM, RHPD, and SHPD are shown in Fig. 4. The characteristic absorption peaks of SIM was found at 3,545 cm−1 (free O–H stretch), 2,970 cm−1 (methyl C–H asymmetric stretch), 1,695 cm−1 (ester C=O stretch, associated), 1,265 cm−1 (lactone –C–O–C stretch). In co-solvent-evaporated mixture (SHPD), the peaks of SIM were not prone and intensity also reduced. No change was observed in case of RHPD and PM.
Fig. 4.
FTIR spectra of SIM, HPMC, and drug (PM), SHPD, and RHPD. SIM simvastatin, HPMC hydroxypropyl methylcellulose, SHP D spray-dried product, RHP D rotaevaporated product, PM physical mixture, D drug. All results were calculated as mean ± 3 SD
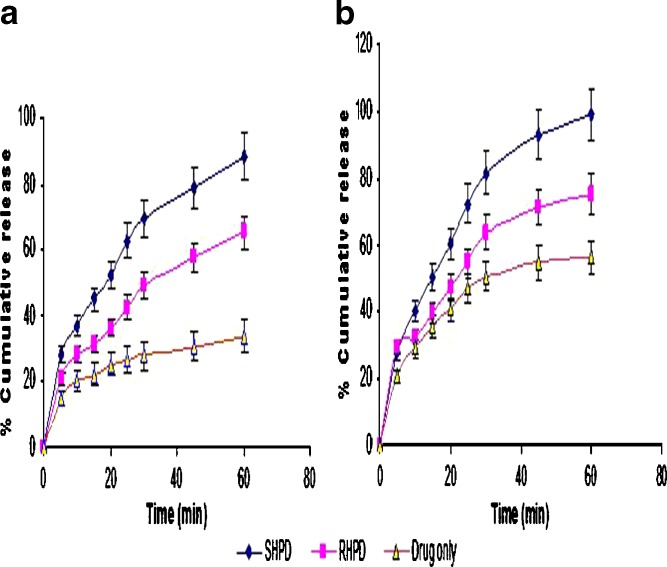
Dissolution Test
The dissolution study of SIM, RHPD, and SHPD was carried out in pH 1.2 HCl and pH 7 buffers. The dissolution efficiency data (DE10 and DE30) of SIM, RHPD, and SHPD are given in Table II. The dissolution profiles of SIM, RHPD, and SHPD in pH 1.2 HCl and pH 7 buffers are shown in Fig. 5. ANOVA performed on the dissolution efficiency (DE) of DE30 parameter of SIM, RHPD, and SHPD shows significant difference between the SIM with co-solvent-evaporated mixtures. Co-solvent-evaporated mixtures of SHPD have shown better solubility and dissolution enhancement than the RHPD.
Table II.
Dissolution Efficiency (DE) of SIM and Various Co-solvent-Evaporated Mixtures
| Product | pH | |||
|---|---|---|---|---|
| pH 1.2 HCL buffer | pH 7 buffer | |||
| DE10 | DE30 | DE10 | DE30 | |
| SIM | 21.12 ± 0.92 | 27.95 ± 0.38 | 28.30 ± 0.90 | 53.66 ± 0.69 |
| SHPD | 36.60 ± 4.39 | 88 ± 1.60 | 40.33 ± 0.43 | 95 ± 0.55 |
| RHPD | 28.10 ± 4.36 | 73.33 ± 0.76 | 32.60 ± 4.36 | 78.21 ± 1.62 |
All results were calculated as mean ± 3 SD
SIM Simvastatin, SHPD spray-dried product, RHPD rotaevaporated product, D drug
Fig. 5.
Dissolution profile of SIM, RHPD, and SHPD in pH 1.2 HCl buffer and pH 7 buffer. SIM simvastatin, HPMC hydroxypropyl methylcellulose, SHP D spray-dried product, RHP D rotaevaporated product, D drug. All results were calculated as mean ± 3 SD
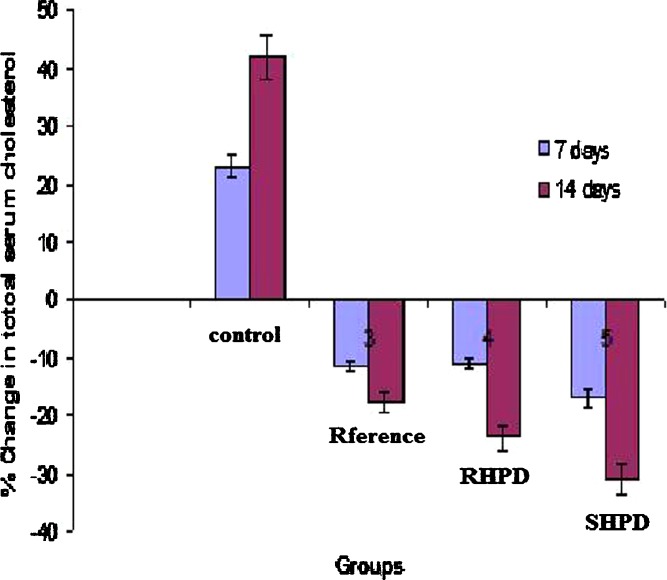
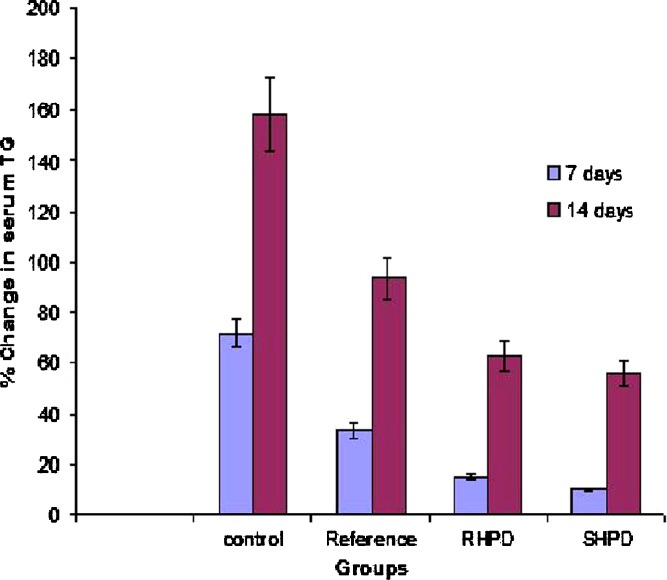
In vivo Study
The serum lipid profiles of all the experimental groups at initial, 7-, and 14-day time intervals are presented in Table III and the corresponding percent change in lipid profiles are shown in Figs. 6 and 7. As expected, after 7 days of treatment with excess coconut oil, control group showed significant increase in total cholesterol and TG. However, reference group showed approximately 4% decrease in total cholesterol and 40% increase in TG. It was noted that the test group presented 3.3-fold decrease in total cholesterol (SHPD), RHPD shows significant decrease in total cholesterol and 2.5-fold increase in TG as compared to the reference group. After 14 days of similar treatment, control group shows further increase in all the lipid levels. The reference group showed approximately 2.4-fold decrease in total cholesterol and 1.7-fold increase in TG. On the other hand the test group showed further approximately twofold decrease in total cholesterol and 1.7-fold increase in TG in comparison with reference group.
Table III.
Serum Total Cholesterol and TG* of Experimental Group at Initial, 7-days, 14-days Time Intervals Respectively
| Experimental groups | Time intervals | Total cholesterol (mg/dl) | TG (mg/dl) |
|---|---|---|---|
| Control | Initial | 65.29 ± 6.67 | 70.35 ± 5.02 |
| 7 days | 80.34 ± 7.62 | 120.94 ± 5.05 | |
| 14 days | 92.64 ± 10.67 | 181.57 ± 5.53 | |
| Reference | Initial | 69.95 ± 5.42 | 75.37 ± 5.31 |
| 7 days | 61.88 ± 5.64 | 100.83 ± 5.05 | |
| 14 days | 57.43 ± 4.10 | 146.06 ± 5.53 | |
| Test (SHPD) | Initial | 64.74 ± 11.29 | 78.05 ± 9.76 |
| 7 days | 57.48 ± 8.2 | 86.12 ± 12.96 | |
| 14 days | 48.99 ± 5.83 | 121.60 ± 4.02 | |
| Test (RHPD) | Initial | 66.74 ± 9.23 | 77.05 ± 8.54 |
| 7 days | 58.80 ± 4.84 | 89.11 ± 4.53 | |
| 14 days | 50.01 ± 2.13 | 125.62 ± 5.02 |
All results were calculated as mean ± SD, n = 3
TG triglyceride, SHPD spray-dried product, RHPD rotaevaporated product, D drug
Fig. 6.
Percent changes in serum total cholesterol of experimental groups at 7 and 14 days. SIM simvastatin, HPMC hydroxypropyl methylcellulose, SHP D spray-dried product, RHP D rotaevaporated product, D drug. All results were calculated as mean ± 3 SD
Fig. 7.
Percent increase in serum TG of experimental groups at 7- and 14-days time intervals (mean ± S.D), n = 3. SIM simvastatin, HPMC hydroxypropyl methylcellulose, SHP D spray-dried product, RHP D rotaevaporated product, PM physical mixture, D drug. All results were calculated as mean ± 3 SD
DISCUSSION
Solubility data demonstrate that, solubility of SIM increases with HPMC K3LV, which acts as surfactant and enhance the wetting of drug particles. Solubility of SIM was higher in spray-dried product compared to the rotaevaporated product.
DSC thermogram of SHPD shows sharp and broad endothermic peak compared to RHPD, which indicates more amorphous nature of SHPD than RHPD. Results of DSC study indicate the conversion of SIM crystalline nature to amorphous one.
XRD of SIM shows characteristic peaks at different angles and indicates that the SIM is present as a crystalline form. Results of XRD indicate conversion of SIM crystalline to amorphous form. These results of PM and RHPD X-ray indicates that SHPD is having more amorphous nature than that of RHPD.
Morphology of SIM completely changed in SHPD; the crystalline form of SIM was completely changed in SHPD mixture when compared with RHPD. A SEM photograph shown in Fig. 3 indicates the reduction in the particle size after spray drying and rotaevaporation.
In co-solvent-evaporated mixture, SHPD, the peaks of SIM was not prone and the intensity of all peaks was reduced, which shows complexation in SHPD. RHPD and PM show the same absorption peaks as SIM, which indicates no drug polymer interaction.
Results of dissolution study indicate that the dissolution rate of SIM improves in the presence of HPMC K3LV. Results of dissolution data demonstrates that SHPD is optimized in comparison to RHPD. Spray drying often is used as an encapsulation technique; a substance to be encapsulated (the load) and the carrier are homogenized as a clear solution or suspension in water or other solvents (the slurry). The slurry is then fed into a spray drier, usually a tower heated to temperatures well over the boiling point of water. As the slurry enters the tower, it is atomized. Partly because of the high surface tension of water and partly because of the hydrophobic/hydrophilic interactions between the amphipathic carrier, the water, and the load, the atomized slurry forms micelles. The small size of the drops results in a relatively large surface area that dries quickly. As the water dries, the carrier forms a hardened shell around the load. In case of rotaevaporation, atomization step is absent and possibly causes incomplete encapsulation.
Hypolipidemic drug like SIM (HMG-CoA reductase inhibitors) are known to reduce elevated total cholesterol and TG levels in blood, which promote the removal of cholesterol from peripheral cells and facilitate its delivery back to the liver (15). This pharmacodynamic effect is reported to be dose-dependent; hence, it was used as basis for the comparison of in vivo performance of SIM and co-solvent-evaporated mixtures. Administration of excess coconut oil, which is a rich source of saturated fatty acids, promotes biosynthesis of cholesterol in liver and leads to hypercholesterolemia. The results of the in vivo study at the end of 14 days indicates that co-solvent-evaporated mixtures (SHPD and RHPD) performed better than the SIM in reduction of total cholesterol and TG levels.
CONCLUSION
HPMC K3LV has a potential to be used for enhancement of solubility and dissolution rate, thereby bioavailability of SIM. The co-solvent evaporation methods with HPMC K3LV enhances the solubility of SIM by converting it in to amorphous form by reducing the particle size and increasing wettability. SHPD and RHPD show higher dissolution as compared to that of SIM, and this complex shows better reduction in total cholesterol and TG level. Low molecular weight and low viscosity grade hydrophilic HPMC gave better wetting characteristic to drug particles, and spray-drying method produced efficient encapsulation of hydrophobic drug in polymer micelles of HPMC and enhanced the solubility and dissolution of SIM very effectively.
Acknowledgments
The authors are very thankful to Artimis Biotech, IDA, Jeedimetla, Hyderabad, India and hydroxylpropyl methylcellulose K3LV (HPMC K3LV) Colorcon Asia Limited, Verna, Goa, India, for providing gift samples of Simvastatin and HPMC K3LV, respectively.
References
- 1.H. G. Brittain. Analytical Profiles of Drug Substances and Excipients, vol. 22, Academic, New York, 2003, p. 36.
- 2.Feeley J. C., York P., Sumby B. S., Dicks H. Determination of surface properties and flow characteristics of salbutamol sulphate, before and after micronisation. Int. J. Pharm. 1998;172:89–96. doi: 10.1016/S0378-5173(98)00179-3. [DOI] [Google Scholar]
- 3.Szejtli J. Cyclodextrin Technology. Amsterdam: Kluwer; 1993. [Google Scholar]
- 4.Chiou W. L., Riegelman S. Pharmaceutical application of solid dispersion system. J. Pharm. Sci. 1971;60:1281–1302. doi: 10.1002/jps.2600600902. [DOI] [PubMed] [Google Scholar]
- 5.Serajuddin A. T. M. Solid dispersion of poorly water-soluble drugs: early promises, subsequent problems, and recent breakthroughs. J. Pharma. Sci. 1999;159:85–93. doi: 10.1021/js980403l. [DOI] [PubMed] [Google Scholar]
- 6.El-Zein H., Riad L., El-Bary A. A. Enhancement of carbamazepine dissolution: in vitro and in vivo evaluation. Int. J. Pharm. 1998;168:209–220. doi: 10.1016/S0378-5173(98)00093-3. [DOI] [Google Scholar]
- 7.Narang A. S., Srivastava A. K. Evaluation of solid dispersions of clofazimine. Drug Dev. Ind. Pharm. 2002;28:1001–1013. doi: 10.1081/DDC-120006431. [DOI] [PubMed] [Google Scholar]
- 8.Sumnu M. Increasing dissolution rate and gastrointestinal absorption of nifidipine via solid dispersion. STP Pharma. 1986;2:214–220. [Google Scholar]
- 9.Mura P., Faucci M. T., Manderioli A., Bramanti G., Ceccarelli L. Properties of solid dispersion of naproxen in various polyethylene glycols. Drug Del. Ind. Pharm. 1996;22:909–916. doi: 10.3109/03639049609065920. [DOI] [Google Scholar]
- 10.Doshi D. H., Ravis W. R., Betageri G. V. Carbamazepine and polyethylene glycol solid dispersion: preparation’ in vitro dissolution, and characterization. Drug Del. Ind. Pharm. 1997;23:1167–1176. doi: 10.3109/03639049709146154. [DOI] [Google Scholar]
- 11.Mura P., Faucci M. T., Manderioli A., Bramanti G., Parrini P. Thermal behaviour and dissolution properties of naproxen from binary and ternary solid dispersions. Drug Del. Ind. Pharm. 1999;25:257–264. doi: 10.1081/DDC-100102169. [DOI] [PubMed] [Google Scholar]
- 12.Yan G., Li H., Zhang R., Ding D. Preparation and evaluation of a sustained-release formulation of nifedipine HPMC tablets. Drug Del. Ind. Pharm. 2000;26:681–686. doi: 10.1081/DDC-100101284. [DOI] [PubMed] [Google Scholar]
- 13.Broman E., Khoo C., Taylor L. S. A comparison of alternative polymer excipients and processing methods for making solid dispersion of poorly water soluble drug. Int. J. Pharm. 2001;222:139–151. doi: 10.1016/S0378-5173(01)00709-8. [DOI] [PubMed] [Google Scholar]
- 14.Aliabadi H. M., Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin. Drug Del. 2006;3:139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- 15.Jun S. W., Kim M. S., Kim J. S., Hwang S. J. Preparation and characterization of Simvastatin/hydroxypropyl- β -cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2007;66:413–421. doi: 10.1016/j.ejpb.2006.11.013. [DOI] [PubMed] [Google Scholar]