INTRODUCTION
Nanoemulsions are thermodynamically stable, transparent (or translucent) dispersions of oil and water stabilized by an interfacial film of surfactant and co-surfactant molecules having the droplet size less than 100 nm (1–3). Thermodynamic stability of nanoemulsions offer advantages over unstable dispersions, such as emulsions and suspensions, because they can be manufactured with little energy input (heat or mixing) and have a long shelf life (4). Ramipril, a potent antihypertensive drug, is almost completely converted to its active metabolite ramiprilat by hydrolytic cleavage of the ester group in the liver which has about six times angiotensin-converting enzyme (ACE) inhibitor activity of ramipril (1). Ramipril and ramiprilat inhibit ACE and lowers blood pressure effectively (5,6). Formulation of ramipril dosage form leads to a decrease in the assay of ramipril due to mechanical stress, compression, manufacturing processes, excipients, storage conditions, heat, moisture, and alkaline pH (7–9). Since the drug is highly lipophilic (log p 3.32), it was presumed that keeping it in lipophilic environment might increase its stability. In our experiments, when nanoemulsion formulation of ramipril was prepared using distilled water as an aqueous phase, ramipril was found to be degraded as its concentration decreased rapidly. A stable formulation of ramipril was claimed by a patent in which the drug was mixed with a physiologically tolerated buffer that ensures that a pH in the weakly acidic to weakly alkaline range is set up in a pharmaceutical formulation in the presence of moisture (10). Therefore, the aim of the present study was to perform pH degradation studies using different standard buffer solutions official in Indian Pharmacopoeia (I.P. 1996) as an aqueous phase in order to improve stability of ramipril in nanoemulsion formulation. The dose of ramipril varies between 2.5 and 20 mg, and the frequently prescribed dose is 5 mg for the adult. Therefore, for the present study, 5 mg/ml dose was selected for the development of nanoemulsion formulation.
MATERIALS AND METHODS
Materials
Ramipril base was a kind gift sample from Ranbaxy Research Laboratory (Haryana, India). Propylene glycol monocaprylic ester (Sefsol 218) was a kind gift sample from Nikko Chemicals (Tokyo, Japan). Diethylene glycol monoethyl ether (Carbitol), sodium perchlorate AG, and acetonitrile (HPLC grade) were purchased from E-Merck (Schuchardh, Hokenbrunn, Germany). Polyoxy-35-castor oil (Cremophor-EL) was purchased from Sigma Aldrich (St. Louis, MO, USA).Water was obtained from Milli-Q water purification system (Millipore, MA, USA). All other chemicals used were of analytical reagent grade.
Preparation of Ramipril Nanoemulsion
Nanoemulsion formulation of ramipril was prepared by aqueous phase titration method. Detail of its preparation and optimization is given in our previously published article (1). Twenty-five milligrams of ramipril (5 mg/ml) was dissolved in 20% w/w of Sefsol 218; 27% w/w mixture of Cremophor-EL and carbitol (1:1) were added slowly and vortex-mixed. Then, volume was made 100% w/w by slow addition of double distilled water or buffers of different pH which were used as aqueous phase.
Optimization of Aqueous Phase
Five milliliters of formulation was prepared using different standard buffers as the aqueous phase as per Table I. Three batches of each nanoemulsion formulation were prepared. Formulations were kept at 25 ± 2°C (room temperature) at ambient humidity. Samples were withdrawn periodically at predetermined time intervals (0, 15, 30, 45, 90, and 180 days) and evaluated for any physical change in the formulation and drug content. Zero time samples were used as controls. Analysis was carried out at each time interval by taking 50 μl of each formulation and diluting it to 5 ml with acetonitrile and injecting into the high-performance liquid chromatography (HPLC) system at 210 nm (11). HPLC system consisted of Shimadzu LC-10A VP with a UV detector (Shimadzu, Japan). In this method, acetonitrile/sodium perchlorate buffer (43:57) optimized as a mobile phase and a 25 × 4.6 mm RP C18 column having a 5-μm packing as a stationary phase was used. The mobile phase was delivered at flow rate of 1.5 ml/min with injection volume of 20 μl. This method was validated in our laboratory.
Table I.
Volume and Description of Components Used for the Preparation of Cremophor EL Nanoemulsion Formulation for the Aqueous Phase Studies
| Cremophor EL nanoemulsion formulation | |||||
|---|---|---|---|---|---|
| Oil (ml) | S mix (ml) | Aqueous phase (ml) | Ramipril (mg) | Description of formulation | Code |
| 1 (20%) | 1.35 (27%) | 2.65 (53%) | 25 | The formulation was prepared using pH 1.2 hydrochloric acid buffer as an aqueous phase in formulation | pH 1.2 |
| 1 (20%) | 1.35 (27%) | 2.65 (53%) | 25 | The formulation was prepared using pH 2.2 hydrochloric acid buffer as an aqueous phase in formulation | pH 2.2 |
| 1 (20%) | 1.35 (27%) | 2.65 (53%) | 25 | The formulation was prepared using pH 3.0 acid phthalate buffer as an aqueous phase in formulation | pH 3.0 |
| 1 (20%) | 1.35 (27%) | 2.65 (53%) | 25 | The formulation was prepared using pH 4.0 acid phthalate buffer as an aqueous phase in formulation | pH 4.0 |
| 1 (20%) | 1.35 (27%) | 2.65 (53%) | 25 | The formulation was prepared using pH 5.0 neutralized phthalate buffer as an aqueous phase in formulation | pH 5.0 |
| 1 (20%) | 1.35 (27%) | 2.65 (53%) | 25 | The formulation was prepared using pH 6.0 phosphate buffer as an aqueous phase in formulation | pH 6.0 |
| 1 (20%) | 1.35 (27%) | 2.65 (53%) | 25 | The formulation was prepared using double distilled water as an aqueous phase in formulation. | pH B |
In addition, samples of pure oil (Sefsol 218), pure surfactant, and co-surfactant (Smix) were run separately to check that there is no interference of the excipients used in the formulations. The procedure of the sample preparation for analysis was:
Sefsol 218 pure: 50 μl oil was taken and diluted to 1 ml by acetonitrile.
Cremophor-EL and carbitol (Smix): 50 μl of the mixture was taken and diluted to 1 ml by acetonitrile.
Fifty microliters of formulation was taken and diluted with acetonitrile to 5 ml, giving a concentration of 50 μg/ml.
All the samples were filtered through 0.22-μm membrane filter and injected into the system for area calculation at 210 nm.
Stability Studies with Respect to the Assay of Ramipril in Nanoemulsion Formulations
Stability of a dosage form refers to the chemical and physical integrity of the dosage unit and when appropriate, the ability of the dosage unit to maintain protection against microbiological contamination. Ramipril is a very sensitive and unstable molecule. Formulation of ramipril dosage form leads to a decrease in its assay value (7–9). Therefore, it was necessary to check the stability of the ramipril nanoemulsion formulation. Stability was checked when the formulation were kept at different temperatures (40 ± 2°C, 25 ± 2°C, and 5 ± 2°C).
Nine batches of each nanoemulsion formulation were prepared, out of which, three batches were kept at three different temperatures 40 ± 2°C (stability chamber), 25 ± 2°C (room temperature), and 5 ± 2°C (refrigerator) at ambient humidity.
Samples were withdrawn periodically at predetermined time intervals (0, 15, 30, 45, 90, and 180 days) and evaluated for any physical change in the formulation and drug content. Zero time samples were used as controls. Analysis was carried out at each time interval by taking 50 μl of each formulation and diluting it to 5 ml with acetonitrile and injecting into the HPLC system at 210 nm. All the samples were filtered through a 0.22-μm membrane filter and injected into the system for area calculation at 210 nm.
RESULTS AND DISCUSSION
Optimization of Aqueous Phase
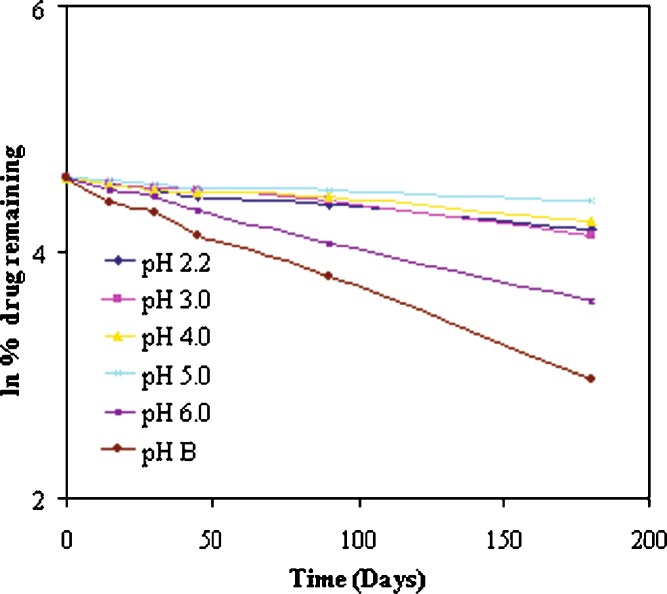
Formulation of ramipril dosage form leads to very rapid decrease in the assay of ramipril due to increase in diastereomers as related substances (7). Even though ramipril is without question one of the most important ACE inhibitors available today, current ramipril formulations show a considerable degree of instability. The degradation of ramipril is believed to occur mainly via two pathways: (a) hydrolysis to ramipril–diacid and (b) cyclization or condensation to ramipril–diketopiperazine (ramipril-DKP). These ramipril–diacid and ramipril–DKP compounds form, as indicated above, as a result of cyclization, condensation, and/or breakdown arising from exposure to heat, air, moisture, alkali addition, stress, compaction, manufacturing processes excipients, storage conditions, thermal oxidation, or other interactions or events (7–9). It is thus generally difficult to select the excipients that enable dosage forms with adequate stability. Therefore, pH degradation studies were performed using different standard buffer solutions (I.P. 1996) as an aqueous phase in the formulation (12). It was observed that least degradation was observed when standard buffer pH 5 was used as an aqueous phase. Therefore, standard buffer pH 5 was selected as an aqueous phase for the preparation of ramipril nanoemulsion. It is well known that ramipril degrades in the presence of moisture and in alkaline pH (7,8). In our experiments, when nanoemulsion formulation of ramipril was prepared using distilled water as an aqueous phase, ramipril was found to be degraded as its concentration decreased rapidly.
It was observed that the pH of the nanoemulsion formulation was 7 when prepared using double distilled water. It is also well reported in the literature that ramipril degrades in alkaline pH (7,8). Degradation of the ramipril in acidic pH is not reported; thus, formulations were prepared using different standard pH buffer solutions (I.P. 1996) as aqueous phase in the pH range of 1.2 to 6, and formulation prepared using distilled water as an aqueous phase was used as a control. It was observed that nanoemulsion formulation with pH 1.2 converted to emulsion after 1 month of the storage. Thus, it was concluded that at this pH, the nanoemulsion formulation itself did not remain stable and affected the phase behavior of the formulation, which is also reported in literature that the formation and stability of nanoemulsions consisting of nonionic components is not affected by the pH and ionic strength of aqueous phase in the pH range between 3 and 10 (13). Therefore, these formulations were dropped from the stability studies.
Drug concentration remaining was quantified at predetermined time intervals (0, 15, 30, 45, 90, and 180 days) where zero time samples were taken as initial concentration (100%). Formulations prepared in different standard buffer solutions of different pH (1.2, 2.2, 3, 4, 5, 6) and in distilled water kept at room temperature were analyzed individually. The percentage of undecomposed ramipril remaining after 180 days of storage was 82.53% in nanoemulsion formulation when standard buffer pH 5.0 was used as the aqueous phase as compared to other formulations. This value of ramipril was significant when compared with other formulations (p < 0.05). Although rapid decrease was observed in the assay of ramipril, the decrease was significantly slow when buffers were used instead of distilled water as an aqueous phase of the nanoemulsion formulation (p < 0.05).
To check the order of degradation of ramipril, natural log (ln) of percent drug remaining when plotted against time gave the straight line, which signified that the degradation of ramipril in nanoemulsion formulation followed first-order kinetics (Fig. 1). Correlation coefficient values for first-order degradation plots are given in Table II. From degradation constant (k) values obtained (Table II), it was observed that least degradation was seen when standard buffer pH 5 was used as an aqueous phase. This was in confirmation with the results revealed by a patent in which the patent describes that weakly acidic or weakly alkaline pH range should be obtained in the presence of moisture (10). This indicated that standard buffer pH 5 is the most suitable buffer for ramipril nanoemulsion formulation, as minimum degradation of ramipril was observed using this standard buffer solution. Therefore, phase diagrams and nanoemulsion formulation were prepared using standard buffer pH 5 and not distilled water or any other standard buffer solution (1). There was no change found in the phase behavior of the pseudo-ternary phase diagram when standard buffer pH 5 was used as an aqueous phase instead of distilled water, which is also well reported in the literature that the formation and stability of nanoemulsions consisting of nonionic surfactants is not affected by the change in pH or ionic strength (1,4,14–18).
Fig. 1.
First-order degradation kinetics of ramipril in Cremophor-EL nanoemulsion formulation using different pH standard buffer solutions as aqueous phase and stored at 25 ± 2°C temperature
Table II.
Influence of pH on Degradation of Ramipril in Cremophor-EL Nanoemulsion Formulations at 25 ± 2°C
| Formulation | pH | Cremophor-EL ka (day−1) | Correlation coefficients (R 2) |
|---|---|---|---|
| pH 2.2 | 2.2 | 0.0022 | 0.9800 |
| pH 3.0 | 3 | 0.0025 | 0.9866 |
| pH 4.0 | 4 | 0.0018 | 0.9703 |
| pH 5.0 | 5 | 0.001 | 0.9746 |
| pH 6.0 | 6 | 0.0056 | 0.9985 |
| pH B | 7 | 0.0089 | 0.9971 |
aDegradation constant (k) from slopes of the first-order degradation kinetic plots
Stability Studies with Respect to the Assay of Ramipril in Nanoemulsion Formulations
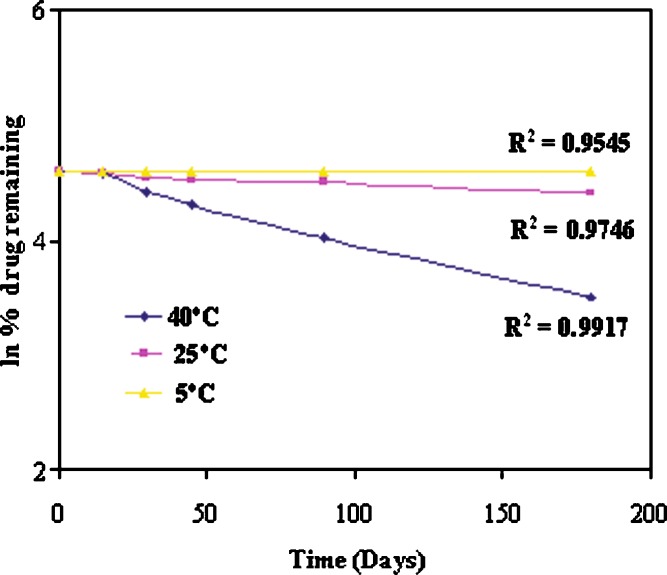
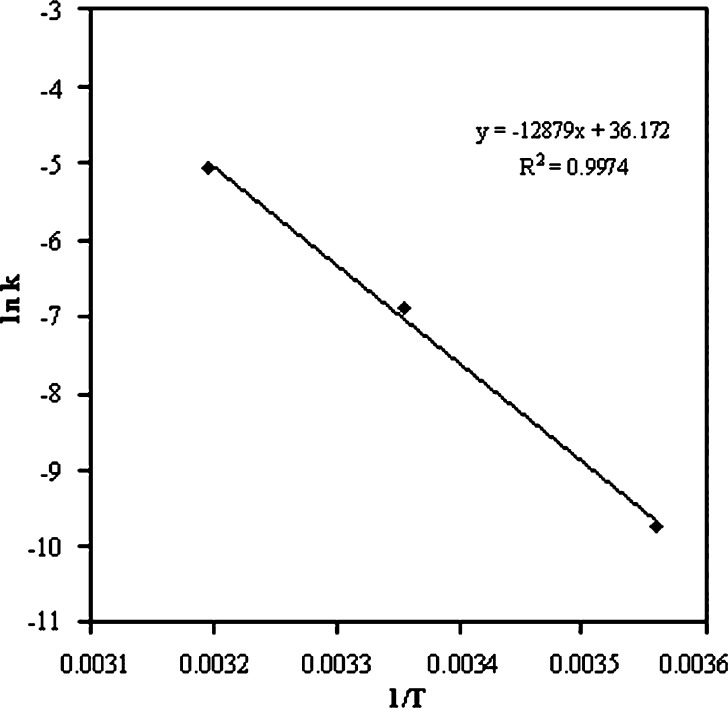
Formulations kept at 40°C, 25°C, and 5°C were analyzed individually (Table III). The percentage of undecomposed ramipril remaining in nanoemulsion formulations was 32.80%, 82.53%, and 98.87% at 40°C, 25°C, and 5°C, respectively, after 180 days of storage (Table III). The order of degradation of ramipril was found to be first order as indicated by a graph between the natural log (ln) of percent drug remaining against time which gave the straight line (Fig. 2). From the degradation constant (k) values obtained from the Fig. 2, shelf lives of the formulation were calculated as given in Table IV. The shelf lives of ramipril nanoemulsions at 40°C, 25°C, and 5°C were found to be 0.0449, 0.2877, and 4.7945 years, respectively (Table IV). It was found that the shelf life of optimized formulation was significantly higher (4.7945 years) when stored at refrigerator temperature (p < 0.05). Arrhenius plot was constructed by plotting ln k versus reciprocal of absolute temperature (1/T, Fig. 3). The slope obtained from plot gave the energy of activation (Ea) of ramipril in the nanoemulsion formulation. The activation energy of ramipril nanoemulsion formulation was found to be 12.879 kcal/mol.
Table III.
Mean Concentration of Drug Remaining (±SD, n = 3) at Predetermined Time Intervals in Nanoemulsion Formulation (pH 5) Stored at 40 ± 2°C, 25 ± 2°C, and 5 ± 2°C Temperature
| Time (days) | Cremophor nanoemulsion formulation | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 40 ± 2°C | 25 ± 2°C | 05 ± 2°C | |||||||
| Dr. rea μg/mL (±S.D) | %b | ln %c | Dr. rea μg/mL (±S.D) | %b | ln %c | Dr. rea μg/mL (±S.D) | %b | ln %c | |
| 0 | 50.6 (±0.5) | 100.0 | 4.6051 | 50.8 (±1.4) | 100.0 | 4.6051 | 44.0 (±1.5) | 100.0 | 4.6051 |
| 15 | 49.1 (±1.0) | 96.96 | 4.5743 | 49.5 (±1.1) | 97.51 | 4.5800 | 44.0 (±0.8) | 99.90 | 4.6042 |
| 30 | 42.1 (±0.9) | 83.29 | 4.4223 | 48.3 (±0.6) | 95.05 | 4.5544 | 43.9 (±1.0) | 99.70 | 4.6022 |
| 45 | 37.4 (±0.3) | 73.92 | 4.3030 | 47.0 (±0.7) | 92.60 | 4.5283 | 43.8 (±0.9) | 99.50 | 4.6002 |
| 90 | 28.0 (±0.6) | 55.33 | 4.0134 | 45.9 (±1.1) | 90.40 | 4.5042 | 43.7 (±0.5) | 99.31 | 4.5982 |
| 180 | 16.6 (±0.3) | 32.80 | 3.4904 | 41.9 (±0.9) | 82.53 | 4.4132 | 43.5 (±0.8) | 98.87 | 4.5938 |
aMean concentration of drug remaining
bPercent drug remaining
cNatural log percent drug remaining
Fig. 2.
First-order degradation kinetics of ramipril in Cremophor-EL nanoemulsion formulation stored at 40 ± 2°C, 25 ± 2°C, and 5 ± 2°C temperature
Table IV.
Shelf Life of Nanoemulsion Formulation (pH 5) at 40 ± 2°C, 25 ± 2°C, and 5 ± 2°C Temperatures
| Formulation | Temp (°C) | T a (K) | 1/T (K−1) | k b (day−1) | ln k | t 90% c = 0.105/k (day) | t 90% c (year) |
|---|---|---|---|---|---|---|---|
| Cremophor-EL | 40 | 313 | 0.00319 | 0.0064 | −5.0515 | 16.41 | 0.0449 |
| 25 | 298 | 0.00336 | 0.001 | −6.9078 | 105.00 | 0.2877 | |
| 5 | 278 | 0.0036 | 0.00006 | −9.7212 | 1750.00 | 4.7945 |
aTemperature in degree Kelvin
bDegradation constant (k) from slopes of the first-order degradation kinetic plots
cShelf life of nanoemulsion formulations
Fig. 3.
Arrhenius plot of Cremophor-EL nanoemulsion formulation
SUMMARY AND CONCLUSION
The degradation of ramipril was slowest in a nanoemulsion formulation with an aqueous phase buffered at pH 5.0 as compared to the other formulations. The shelf life of nanoemulsion formulation was found to be highest at refrigerator temperature (4.7945 years). Activation energy for ramipril nanoemulsion formulation was found to be12.879 kcal/mol. These results indicated enhanced stability of ramipril in nanoemulsion formulation using standard buffer solution of pH 5.0 as an aqueous phase.
Acknowledgment
The authors are thankful to Nikko Chemicals, Japan and Ranbaxy Research Laboratory, India for providing the gift sample of Sefsol 218 and ramipril base, respectively.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1208/s12249-011-9596-z.
References
- 1.Shafiq S., Shakeel F., Talegaonkar S., Ahmad F. J., Khar R. K., Ali M. Design and development of oral oil in water ramipril nanoemulsion formulation: In vitro and in vivo assessment. J. Biomed. Nanotech. 2007;3:28–44. doi: 10.1166/jbn.2007.008. [DOI] [Google Scholar]
- 2.Shakeel F., Baboota S., Ahuja A., Ali J., Aqil M., Shafiq S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech. 2007;8:E104. doi: 10.1208/pt0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baboota S., Shakeel F., Ahuja A., Ali J., Shafiq S. Design development and evaluation of novel nanoemulsions formulations for transdermal potential of celecoxib. Acta Pharm. 2007;8:316–332. doi: 10.2478/v10007-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 4.Shafiq S., Shakeel F., Talegaonkar S., Ahmad F. J., Khar R. K., Ali M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007;66:227–242. doi: 10.1016/j.ejpb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S., Gerstein H., Hoogwerf B., Pogue J., Bosch J., Wolffenbuttel B. H. R., Zinman B. HOPE study investigators, ramipril and the development of diabetes. JAMA. 2001;286:1882–1885. doi: 10.1001/jama.286.15.1882. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S., Sleight P., Pogue J., Bosch J., Davies R., Dagenais G. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N. Engl. J. Med. 2000;34:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 7.Hanysova L., Václavková M., Dohnal J., Klimeš J. Stability of ramipril in the solvents of different pH. J. Pharm. Biomed. Anal. 2005;24:335–342. doi: 10.1016/j.jpba.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 8.Hogan B. L., Williams M., Idiculla A., Veysoglu T., Parente E. Development and validation of a liquid chromatographic method for the determination of the related substances of ramipril in Altace capsules. J. Pharm. Biomed. Anal. 2000;23:637–651. doi: 10.1016/S0731-7085(00)00342-3. [DOI] [PubMed] [Google Scholar]
- 9.Nitin et al. Stable pharmaceutical formulations. U S Patent 0,045,911 (2006).
- 10.R. Eyjolfsson. Formulations of Ramipril. W O Patent 2005067887 (2005).
- 11.Belal F., Al-Zaagi I. A., Gadkariem M. A., Abounassif M. A. A stability-indicating LC method for the simultaneous determination of ramipril and hydrochlorthiazide in dosage forms. J. Pharm. Biomed. Anal. 2001;24:335–342. doi: 10.1016/S0731-7085(00)00474-X. [DOI] [PubMed] [Google Scholar]
- 12.Indian Pharmacopoeia. Reagents and solutions. Appendix 13, pp. A144–A145 (1996).
- 13.Constantinides P. P. Lipid microemulsions for improving drug dissolution and oral absorption and biopharmaceutical aspects. Pharm. Res. 1995;12:1561–1572. doi: 10.1023/A:1016268311867. [DOI] [PubMed] [Google Scholar]
- 14.Constantinides P. P., Lancaster C. M., Marcello J., Chiossone D. C., Orner D., Hidalgo I., Smith P. L., Sarkahian A. B., Yiv S. H., Owen A. J. Enhanced intestinal absorption of an RGD peptide from water-in-oil microemulsions of different composition and particle size. J. Control. Release. 1995;34:109–116. doi: 10.1016/0168-3659(94)00129-I. [DOI] [Google Scholar]
- 15.Eccleston J. Microemulsions. In: Swarbrick J., Boylan J. C., editors. Encyclopedia of Pharmaceutical Technology. New York: Marcel Dekker; 1994. pp. 375–421. [Google Scholar]
- 16.Ghosh P. K., Murthy R. S. R. Microemulsions: A potential drug delivery systems. Curr. Drug. Del. 2006;3:167–180. doi: 10.2174/156720106776359168. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence M. J., Rees G. D. Microemulsion-based media as novel drug delivery systems. Adv. Drug. Deliv. Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 18.Shafiq S., Shakeel F., Talegaonkar S., Ali J., Baboota S., Ahuja A., Khar R. K., Ali M. Formulation development and optimization using nanoemulsion technique: A technical note. AAPS PharmSciTech. 2007;8:E28. doi: 10.1208/pt0803055. [DOI] [PMC free article] [PubMed] [Google Scholar]