Abstract
This study aimed to design methyprednisolone (MP)-loaded poly(d,l lactide-co-glycolide) (PLGA) microspheres (MS) intended for intra-articular administration. MP was encapsulated in four different types of PLGA by using an S/O/W technique. The effects of β-irradiation at the dose of 25 kGy were evaluated on the chemical and physicochemical properties of MS and the drug release profiles. The S/O/W technique with hydroxypropylmethylcellulose (HPMC) as surfactant allowed obtaining MS in the tolerability size (7–50 µm) for intra-articular administration. The MP encapsulation efficiency ranged 56–60%. HPMC traces were evidenced in the loaded and placebo MS by attenuated total reflectance Fourier transform infrared spectroscopy. MS made of the capped PLGA DL5050 2M (MS 2M) and uncapped PLGA DL5050 3A (MS 3A) prolonged the release of MP over a 2- to 3-month period with a triphasic (burst release–dormant period–second release pulse) and biphasic release pattern, respectively. The β-irradiation did not significantly alter the morphology, chemical, and physicochemical properties of MS. The only variation was evidenced in the drug release for MS 2M in term of shorting of the dormant period. The minimal variations in the properties of irradiated PLGA MS, which are in disagreement with literature data, may be attributed to a radioprotecting effect exerted by HPMC.
Key words: β-irradiation, HPMC, intra-articular administration, methylprednisolone, PLGA microspheres
INTRODUCTION
Corticosteroids locally administered by intra-articular injections have represented a major breakthrough in the treatment of arthritis, osteoarthritis, or musculoskeletal disorders to reduce pain and inflammation and facilitate motion and function (1). Nevertheless, due to the physiology of joints, drugs are cleared from synovial fluids by lymph drainage, which is largely dependent on the size and solubility of the molecules (2). Therefore, side effects of intra-articular corticosteroid injections have been attributed to their systemic availability (3–4). This clinical outcome weakens the rational on the distinction established by the World Anti-doping Agency among routes of administration (5). Indeed, all glucocorticosteroids are prohibited in competition when administered orally, rectally, intravenously, or intramuscularly. Their use requires a Therapeutic Use Exemption (TUE) approval, namely a permission to use for therapeutic purposes a drug or drugs, which are otherwise prohibited in sporting competition. No-systemic routes of administration, such as intra-articular, require an Abbreviated TUE (5).
Hence, from both a clinical and anti-doping perspective, there is a need for a delivery system that localizes corticosteroids in the joint cavity avoiding systemic concentration. Poly(d,l lactide-co-glycolide) (PLGA) microspheres are being proposed as delivery systems for intra-articular drug administration since no effects of microsphere dose on inflammation responses were observed (2,6). Nevertheless, it has been recognized that microsphere size (2) and sterility are the most critical issues.
For the terminal sterilization of PLGA formulations, γ-irradiation, which is considered the method of choice on the bases of the European Guideline 3AQ4a, cannot be applied due to the detrimental effects on the polymer. Indeed, γ-irradiation causes substantial degradation of the polyester chains because of the relative stable radicals, which propagate the chain scission over a prolonged period of time (7,8). Therefore, the production process is currently carried out under aseptic condition or in aseptic units (9). To control the bioburden in the raw materials, PLGA is often irradiated and held in quarantine until radical decay takes place. Few authors suggested that the radical yields could be limited irradiating the samples at low temperature (10,11).
In the recent years, e-beam radiation was exploited to sterilize PLGA drug delivery systems since alterations of polymer physical properties are limited, occurring in a predictable and fairly accurate manner (12,13). Similar types of paramagnetic radiation species are formed after exposure to both γ- and β-irradiations, but approximately twice as many radical species are generated in γ-irradiated PLGA microspheres compared to β-irradiated PLGA microspheres, regardless of the lactide/glycolide ratio and using approximately similar doses (14). After β-irradiation, long-term stability problems are unlikely to occur since the radicals persist for a shorter period of time (13,15). Nevertheless, variations in biopharmaceutical properties of drug-loaded microspheres after irradiation could be ascribed to the radiolysis and the presence of tertiary amine drug substances, namely, bupivacaine (13) and gentamicin (15), which may catalyze the hydrolytic cleavage of the ester bonds in polymer chains and accelerate polymer degradation (16). Therefore, the investigation of the effects of β-irradiations on PLGA microspheres loaded by a not-ionizable drug appears to be worth of investigation.
In this work, we aimed to design methyprednisolone (MP)-loaded PLGA microspheres intended for intra-articular administration. The placebo and MP-loaded microspheres were irradiated by e-beam at the dose of 25 kGy and characterized with respect to changes in the chemical and physicochemical properties and the drug release.
MATERIALS AND METHODS
Materials
Four different grades of PLGA were purchased by Alkermes (Cambridge, MA, USA): uncapped Medisorb® 4555 DL 2A (DL 4555 2A) [batch characteristics: lactide/glycolide mole ratio, 46:54; Mw = 15.9 kDa; PI = 1.3; Tg = 40.56 ± 0.04°C]; uncapped Medisorb® 5050 DL 2A (DL 5050 2A) [batch characteristics: lactide/glycolide mole ratio, 53:47; Mw = 14.7 kDa; PI = 1.4; Tg = 42.26 ± 0.01°C]; capped Medisorb® 5050 DL 2M (DL 5050 2M) [batch characteristics: lactide/glycolide mole ratio, 53:47; Mw = 17.2 kDa; PI = 1.8; Tg = 42.52 ± 0.05°C]; and uncapped Medisorb® 5050 DL 3A (DL 5050 3A) [batch characteristics: lactide/glycolide mole ratio, 54:46; Mw = 38.8 kDa; PI = 1.6; Tg = 47.73 ± 0.04°C].
Methylprednisolone (MP) was obtained by Farmalabor (Milan, Italy). Methocel® K100 LV (hydroxypropylmethylcellulose, HPMC) was kindly gifted by Colorcon (Gallarate, Italy). All solvents, unless specified, were of analytical grade.
Preparation of MP-Loaded and Placebo Microspheres
MP-loaded microspheres were prepared by a solid-in-oil-in-water (S/O/W) emulsion/solvent evaporation method using HPMC as emulsifier (17,18). Briefly, MP (30% w/w theoretical drug loading) was dispersed in a 20% w/w PLGA solution in dichloromethane. After mixing by vortex and sonicating, the S/O suspension was slowly injected into 25 mL of 2.5% HPMC solution under stirring at 600 rpm at 5 ± 3°C. The resulting S/O/W system was then poured into 200 mL water and stirred at 500 rpm and 40°C for 3 h for microsphere hardening. The resulting microspheres were filtered by a 1.2-μm nitrocellulose filter (Millipore, Milan, Italy), washed with ultrapure water and freeze-dried (freeze-drying system Alpha 1, Martin Christ, Osterode, Germany). After preparation, the microspheres were stored at 5 ± 3°C until use.
β-Irradiation of Placebo and MP-Loaded Microspheres
Placebo and MP-loaded microspheres were irradiated by using an electron beam accelerator (Bioster, Seriate, Italy). Irradiation was performed in the presence of air, calorimetry dose of 25.1 kGy, energy of 10 MeV, and irradiation temperature of 25°C.
Particle Size and Morphology
The mean particle size distribution of the microspheres was determined using a Malvern 2600 laser sizer (Worcestershire, UK). The lyophilized particles were suspended into ultrapure water, and a 0.01% polysorbate 80 solution was used as surfactant to prevent microspheres aggregation.
Particle size was expressed as undersize cumulative percentages, and the population dispersity was referred as span and calculated as reported in the Eq. 1:
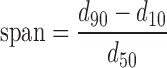 |
1 |
where d90, d10, and d50 are the mean diameters at the 90%, 10%, and 50% of the population distribution, respectively.
The microspheres morphology was investigated by scanning electron microscopy (SEM) (JEOL JSM 6380, Pieve Emanuele, Italy). Samples were prepared by placing an amount of dried microspheres powder onto an aluminum specimen stub. The samples were sputter coated with gold before taking the image.
ATR-FTIR Spectroscopy
About 15.0 mg sample was place on a diamond crystal mounted in attenuated total reflectance (ATR) cell (Perkin Elmer, Monza, Italy). Fourier transform infrared spectroscopy (FTIR) measurements were performed with Spectrum™ One spectrophotometer (Perkin Elmer). The spectra were recorded at 2 cm−1 resolution, and 32 scans were collected over the wavenumber region 4,000−650 cm−1.
Thermal Analysis
DSC data were recorded by using a DSC 2010 TA (TA Instruments, New Castle, USA). The samples of 5 mg exactly weighted (±0.01 mg) were sealed in aluminum pans and heated in inert atmosphere (70 mL/min of N2). The reference was an empty pan. The equipment was calibrated with an indium sample. Placebo and drug-loaded microspheres were scanned at 10 K/min from 30°C to 60°C in order to erase polymer thermal history, then cooled down from 60°C to 0°C at 20 K/min and re-heated up to 130°C at 10 K/min. All the determinations were performed in duplicate.
MP Content
The amount of MP encapsulated was evaluated by the high-performance liquid chromatography (HPLC) method described below. MP was extracted from the microspheres by incubation in 1 mL of tetrahydrofurane (THF) solution overnight at room temperature. After complete dissolution of PLGA matrices, the clear solution was diluted in the mobile phase (water/acetonitrile 50:50%, v/v). All measurements were carried out in triplicate. The drug content (%, w/w) and encapsulation efficiency were calculated.
In Vitro Drug Release
In vitro drug release tests were carried out in bottles closed by screwed stoppers and stirred in a shaker incubator (50 strokes/min) at 37 ± 2°C. Samples were exactly weighed (±0.01 mg) in order to get 2.0 mg MP. The microspheres were suspended in 20 mL of dissolution medium consisting in pH 7.4 phosphate buffer saline containing 0.02% w/v of sodium dodecyl sulfate (SDS). SDS allowed to assure the sink conditions throughout the experiment and avoid particle aggregation. At determined time points, 4 mL dissolution medium were withdrawn and replaced with fresh buffer. The amount of MP released was tested by the HPLC method described below.
HPLC Analysis
The following HPLC assay was developed: an Agilent 1100 HPLC system was used to determine drug concentration (1100 autosampler, 1100 quaternary pump with degasser, 1100 thermostated column compartment, and 1100 diode array detector) (Agilent, Palo Alto, CA). A Waters Spherisorb® ODS2 was used as the stationary phase (150 × 4.6 mm, 3 μm, Vimodrone, Italy), and a combination of acetonitrile with water at the ratio of 50 to 50 was used as the mobile phase. The flow rate was controlled at 1.0 mL/min. The effluent was monitored at 238 nm for 10 min with the drug retention time 6.0 min. The injection volume was 10 μL. The drug concentration was determined from two standard curves in the range of 0.05–5 μg/mL (r2 = 0.99992) and 1–50 (r2 = 0.99999) μg/mL.
In Vitro Degradation Study
Blank and loaded microspheres were incubated in pH 7.4 phosphate buffer saline containing 0.02% w/v of SDS at 37°C. At specified time points microspheres were collected by filtration, rinsed with distilled water, and dried for 24 h under vacuum. The microspheres were dissolved in THF and the molecular weight was determined by the gel permeation chromatography (GPC) method described below.
GPC Analysis
Polymer molecular weights (Mw) were determined by using a HP1100 Chemstation (Hewlett Packard, USA) equipped with a combination of two columns: μStyragel™ Toluene 104 Å 7.8 × 300 mm and μStyragel™ Toluene 103 Å 7.8 × 300 mm (Waters, Vimodrone, Italy).
Chromatographic conditions
Chromatographic conditions were as follows: mobile phase, THF; flow rate, 1.0 mL/min; detector, refractive index signal; and injection volume, 20 μl. The molecular weight (Mw) of each sample was calculated using a calibration curve made with monodisperse polystyrene standards, Mw ranging from 1,000 to 90,000 Da.
RESULTS AND DISCUSSION
Formulation of MP-Loaded Microspheres
In the formulation of microspheres intended for intra-articular administration, the particle size requires particular attention because this factor can elicit various levels of inflammation (4) and, consequently, can compromise the efficacy of the therapy. Recent studies individuated two ranges of tolerability size. Submicron particles are rapidly taken up by phagocytic cells of the synovium, while microspheres ranging 3–60 µm remain dispersed in the joint cavity or adhered on the surface of the synovial membrane and are well tolerated. Meanwhile, in the smaller size range, microspheres produce a great inflammation response causing joint swelling and proteoglycan loss (6). The experimental setup permitted to obtain particles sizing from 7 to 50 μm (Table I). Size dispersity can be considered low for MS 2A compared to the other formulations, which had similar values (Table I). The preparation method presented a yield of about 55%, and the MP encapsulation efficiency was about 60% (Table I).
Table I.
Particle Size Distribution and MP Loading of PLGA Microspheres
| Formulation code | PLGA | Size distribution (μm) | Span | MP content (%, w/w) | E.E. (%) | ||
|---|---|---|---|---|---|---|---|
| d 10 a | d 50 b | d 90 c | |||||
| MS 4555 2A | DL 4555 2A | 7.8 | 25.1 | 68.7 | 2.4 | 17.2 ± 2.1 | 57.4 ± 7.0 |
| MS 2A | DL 5050 2A | 10.4 | 22.5 | 49 | 1.7 | 16.7 ± 2.5 | 55.8 ± 8.2 |
| MS 3A | DL 5050 3A | 5.3 | 15.6 | 45.6 | 2.6 | 17.7 ± 1.2 | 58.9 ± 4.1 |
| MS 2M | DL 5050 2M | 7 | 18.2 | 46.5 | 2.2 | 17.3 ± 2.0 | 57.8 ± 6.7 |
aTen percent of particles were smaller than that number
bFifty percent of particles were smaller than that number
cNinety percent of particles were smaller than that number
The drug-loaded microspheres were spherical and regular, with a smooth surface; no drug crystals appeared on their surface. However, a number of pores were present, contrary to placebo microspheres indicating a discontinuity of the PLGA coating around the MP crystals. As an example, the morphology of MS 2M is illustrated in Fig. 1a.
Fig. 1.
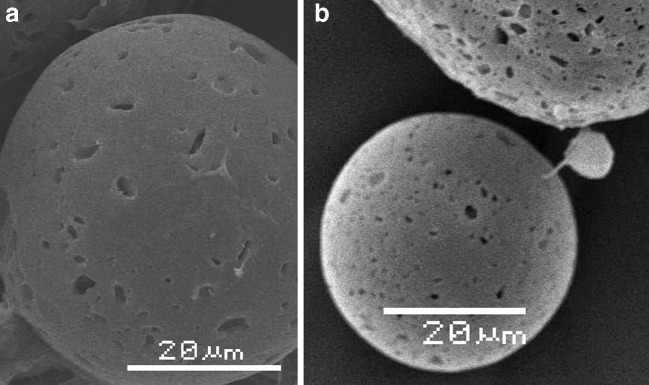
Scanning electron microphotography of MS 2M a before and b after irradiation. The bar corresponds to 20 μm
The molecular weights of PLGAs were not affected by the preparation process (p > 0.05).
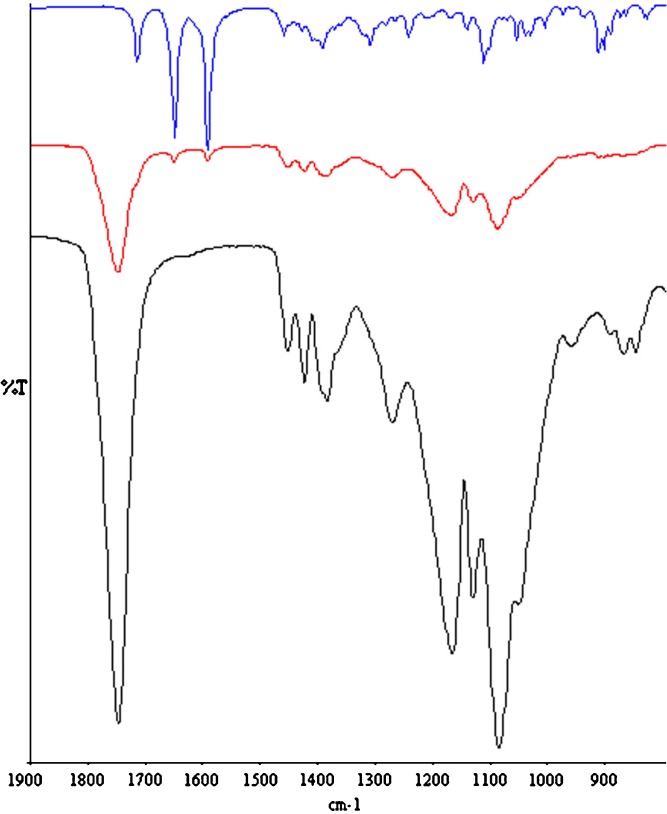
Some variation in the CHx region of the ATR-FTIR spectra of microspheres were detected with respect to the raw PLGA. In particular, a shoulder at about 2,840 cm−1 was detected only in the placebo and MP-loaded microspheres (Fig. 2). The spectra of the second-derivative permitted to better resolve the band at 2,834 cm−1. Independently of the type of copolymers, this band was absent in the raw PLGA spectra and their second derivatives, while in the spectra of the second-derivative of the placebo microspheres, it was recorded as a maximum in the range 2,833–2,828 cm−1. These evidences are compatible with the presence of HPMC traces in the microspheres. Furthermore, the υ(CO) of the PLGA ester groups slight shifted toward higher wavenumbers, and its intensity slightly decrease with respect to the raw PLGA (Fig. 3). Since these variations were noticeable both in the placebo and drug-loaded microspheres, independently of the type of copolymer, it can be assumed that PLGA weakly interacts with HPMC by means of H-bonds. Even if it was not possible to quantify the amount of HPMC within the microspheres by the common analytic techniques, the hypothesis of the entrapment of HPMC during the preparation process can also justified by the increase of Tg for all polymer of about 2°C which, in our opinion, cannot only be ascribed to the leaking of PLGA oligomers and/or monomers during the solvent evaporation step. Tg values of MS 2M, MS 3A, and the corresponding placebo microspheres are reported in Table II.
Fig. 2.
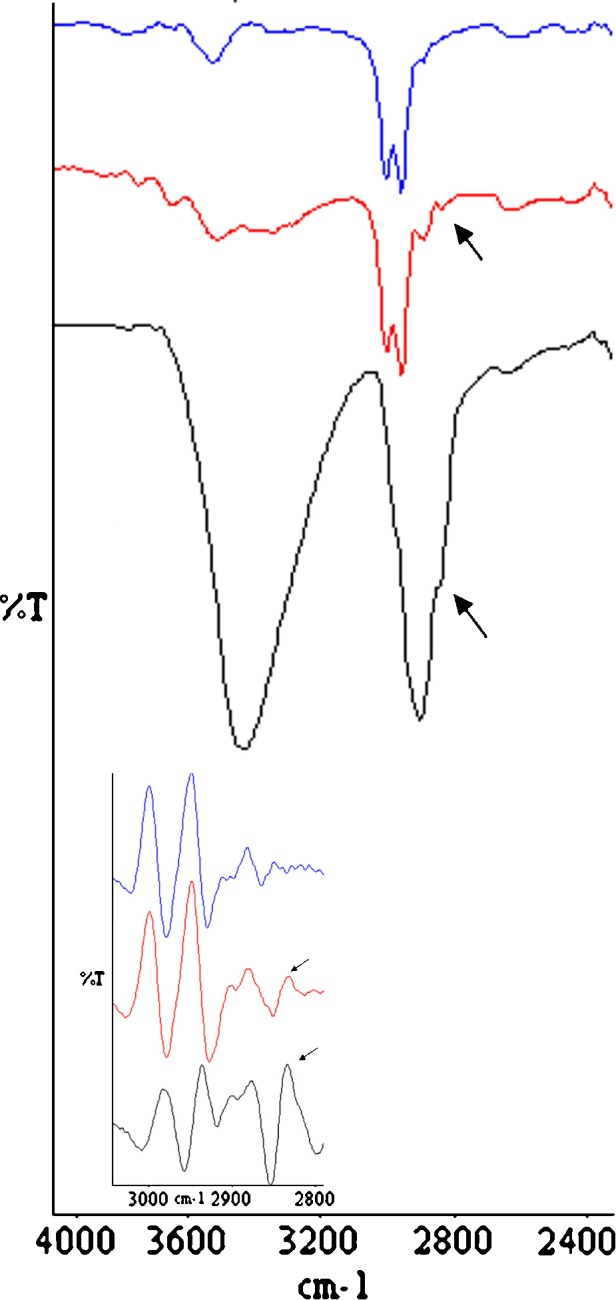
ATR-FTIR spectra of raw HPMC (black line), PLGA DL 5050 2M (blue line) and placebo microspheres made of PLGA DL 5050 2M (red line) and their second derivatives
Fig. 3.
ATR-FTIR spectra of raw MP (blue line), PLGA DL 5050 2A (black line), and MS 2A (red line)
Table II.
Molecular Weight (M w) and Glass Transition Temperature (T g) of Drug Loaded and Placebo Microspheres Before (Not irr) and After β-irradiation (Irr)
| Formulation code | Mw (kDa) | T g (°C) | ||
|---|---|---|---|---|
| Not irr | Irr | Not irr | Irr | |
| MS 2M | 16.7 ± 0.2 | 15.8 ± 0.0 | 45.39 ± 0.15 | 45.17 ± 0.21 |
| MS 3A | 38.7 ± 0.0 | 35.3 ± 0.2 | 49.82 ± 0.13 | 49.80 ± 0.10 |
| Placebo 2M | 15.7 ± 0.6 | 15.8 ± 0.3 | 45.18 ± 0.59 | 44.43 ± 0.06 |
| Placebo 3A | 34.8 ± 0.8 | 33.2 ± 1.0 | 49.47 ± 0.11 | 49.18 ± 0.01 |
As expected, MP was incorporated in the microspheres mainly in the crystalline state, and the evaporation solvent process did not significantly modify its solid state. Indeed, the three strong absorption peaks in the region 1,580–1,750 cm−1 in the ATR-FTIR spectrum of raw MP, attributed to the Form I (19), were clearly evident in the spectra of the MP-loaded microspheres (Fig. 3).
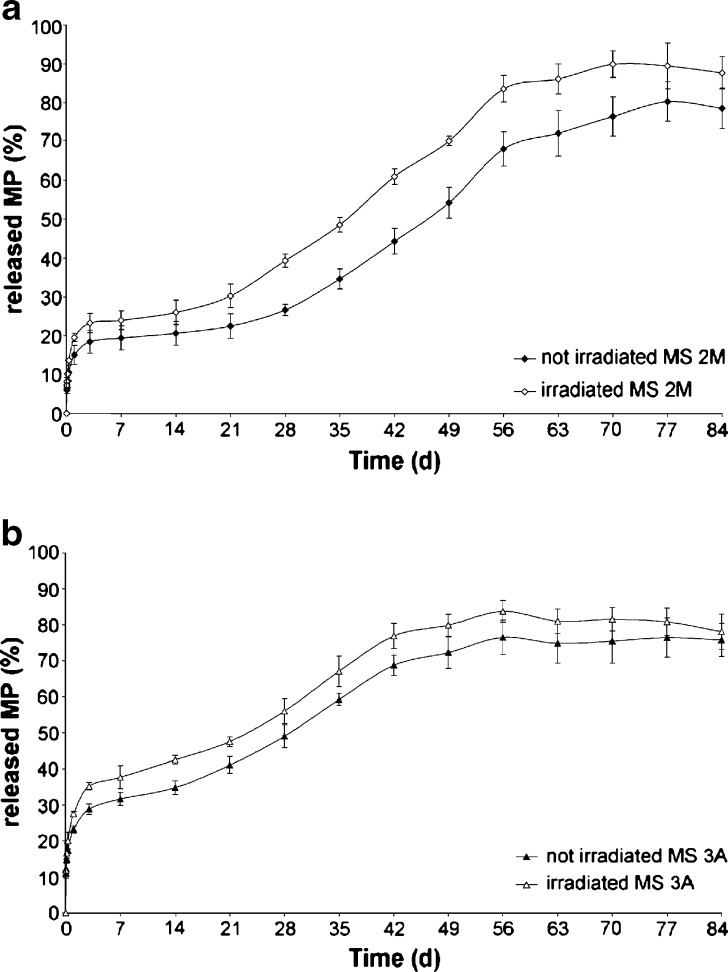
The profiles outlining MP release remarked long-term sustained release characteristics only for MS 3A and 2M (Fig. 4a,b). As a matter of fact, when PLGA DL5050 2A and PLGA DL4555 2A were used, the drug release can be considered completed within a week for both formulations and the experimental data fitted the Higuchi model (K2A = 0.787 ± 0.046, r2 = 0.97; K4555 2A = 0.473 ± 0.043, r2 = 0.97), indicating that the MP release was governed only by diffusion.
Fig. 4.
MP release profiles from a MS 2M and b MS 3A before and after irradiation
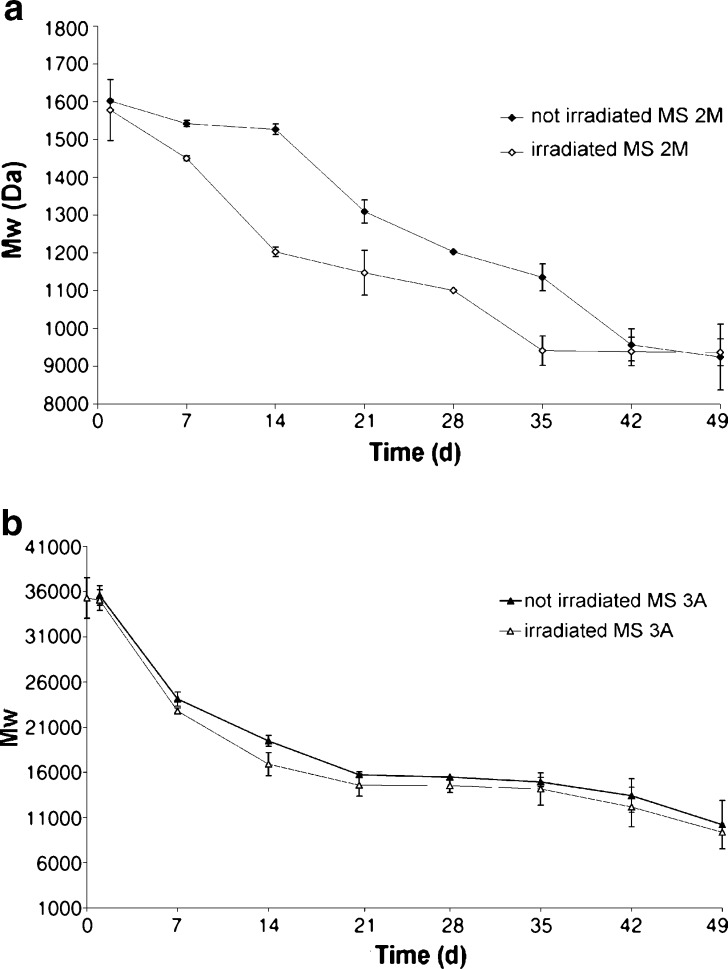
MS 2M presented a triphasic release pattern (burst release–dormant period–second release pulse). In the first 3 days of incubation, the MP release was governed by the diffusion thought the PLGA matrix (K2M = 0.081 ± 0.01, r2 = 0.9882). The diffusion was followed by a lag phase of about a 2-week period; afterwards, the release pattern exhibited a near zero-order kinetic (r2 > 0.99) up to day 56. The analysis of Mw showed a pattern in agreement with the release data. Indeed, a significant reduction of Mw occurred up to 2 weeks of incubation (Fig. 5a), with the concurrent increase from 1.3 to 1.7 in the polydispersity index (PI). Afterwards, the hydrolytic degradation followed a zero-order kinetics (r2 = 0.9559) until day 42 where the decrease in Mw seemed to slow down. A possible explanation for this stabilization is that monomers and low-molecular weight oligomers should diffuse through the matrix and dissolve in the incubation medium and, as a result, are not more available for the calculation of GPC data (10).
Fig. 5.
Decrease in M w of a MS 2M and b MS 3A before and after irradiation
The MP release profile from MS 3A did not evidence a lag-phase after the initial diffusion (K3A = 0.115 ± 0.02, r2 = 0.9851, Fig. 4b) due to the polymer hydrolysis, which took place from the early stage of incubation. After 7 days, MP was released by following a near zero-order kinetics (r2 = 0.9803), reaching the plateau in about 65 days.
The degradation process of PLGA DL5050 3A can be divided into two phases. The hydrolytic cleavage started since the very early stage of incubation and proceeded subsequently with a pseudo-zero order kinetics (r2 = 0.9584) until day 21 (Fig. 5b). In the second phase, the Mw remained quite stable, with the concurrent decrease on PI from 1.77 ± 0.36 at day 21 to 1.49 ± 0.16 at day 56.
On the bases of the results of physico-chemical characterization and the release patterns, MS 3A and 2M appeared suitable to provide sustained release of MP over a period of several weeks and were subjected to further investigations.
β-Irradiation of PLGA Microspheres
After irradiation, the microspheres did not show any visual modification. SEM analysis revealed that the morphological characteristics of microspheres were not modified by irradiation. The particle sizes of the irradiated batches overlapped those of not-irradiated samples (Fig. 1b). All samples could easily be reconstituted in water, and there was no tendency for particle agglomeration or sedimentation. The exposure to e-beam did not alter the drug loading (MP content: MS 2M = 19.7 ± 1.3%; MS 3A = 17.0 ± 0.3%). This result is in agreement with the solid state of the drug within the polymer (microcrystals) and MP stability at γ-irradiation at the dose of 6 Mrad (20). As expected, no variation in drug solid state was recorded since the ATR-FTIR spectra of irradiated and irradiated microspheres overlapped (data not shown). After irradiation, a change in Mw related to the radiation chemical yields of chain scission occurred. The exposure to e-beam did not determine a significant reduction in Mw for both the type of microspheres (Table II). Indeed, the variation was in the 5–10% range, without being affected by the loaded drug. The GPC data were also in agreement with the negligible decrease of the Tg values of the microspheres (Table II).
These results are in contrast with the findings reported in literature for β-irradiated microspheres made of copolymers with similar characteristics (13,15). As an example, in a work by Friess et al., the molecular weights of uncapped and capped PLGA DL 5050 resulted halved after exposure to e-beam at the dose of 25 kGy (15). A possible explanation for these differences may be due to presence of HPMC traces within the PLGA matrices, as verified by ATR-FTIR spectroscopy. As a matter of fact, empty HPMC capsules underwent some structural modifications after both γ-irradiation and electron beam (21). Moreover, it has been demonstrated by Maggi et al. (22) that the exposure to γ-irradiation induced the dose-dependent decrease on the average molecular weight of HPMC due to the chain scission. After γ-irradiation the calculated radiolytic yields for HPMC (1.2 µm/J (22) resulted 4.5-fold higher than that reported for the capped PLGA DL5050 (0.26 µm/J, (23)), indicating that the former material is more sensitive to ionizing radiations than PLGA. Even if different exposure times are required during the sterilization process by using e-beam or γ-irradiation, it can be hypothesized that the HPMC chains were preferentially cleaved by e-beam treatment, acting as a radioprotecting ingredient for PLGA. Such hypothesis should be confirmed and fully understood by an electron paramagnetic resonance spectroscopy study that is beside the purpose of the present work.
As far as the drug release is concerned, the irradiation did not significantly increased both the extent and the rate of MP released. The only significant variation was registered in the case of MS 2M, in which the release profile did not further exhibit a pronounced triphasic pattern because of the shorting of the lag phase (Fig. 4a). This feature can be considered a consequence of a significant (p = 0.04) decrease in Mw starting at day 7 (Fig. 5a). The shortening of the lag phase implied that the MP release process resulted complete within 60 days for irradiated MS 2M in respect to ~80 days for the not-irradiated formulation.
When MS 3A were irradiated, the decay pattern of PLGA 3A Mw maintained a sigma-shaped pattern (Fig. 5b) and the PI decreased (day 21, PI = 1.77 ± 0.05; day 56, PI = 1.24 ± 0.04) as already evidenced for the not-irradiated microspheres. The only variation in the drug release pattern, which was not significant (p = 0.11), was registered in the first week where the MP release increased from 16.9% to 20.1% (Fig. 4b).
CONCLUSION
MP was successfully encapsulated in biodegradable PLGA microspheres suitable for intra-articular administration in terms of particle size and kinetic of drug release. From the obtained experimental results, β-irradiation can be proposed as a method for terminal sterilization of such delivery systems. Indeed, the in vitro characterization of microspheres did not reveal a marked effect of β-irradiation at the dose of 25 KGy on either the microspheres made of uncapped and capped-end PLGA or the microencapsulated MP.
The results suggest that in our experimental condition, HPMC could protect PLGA from the chain scission induced by irradiation. If the radioprotecting effect of HPMC would be confirmed, the use of this excipient in the development of PLGA microspheres should be further investigated because it could overcome the current issues of aseptic preparation.
Acknowledgments
This research project was supported by the grant “Ricerca 2006 sui farmaci, sulle sostanze e sulle pratiche mediche utilizzabili a fini doping nelle attività sportive” from the Ministero della Salute (Rome, Italy). The authors would like to acknowledge Dr. Silvio Faragò of Centro Sperimentale della Seta (Milan, Italy) for the SEM investigations.
References
- 1.Lavelle W., Lavelle E. D., Lavelle L. Intra-articular injections. Med. Clin. N. Am. 2007;91:241–250. doi: 10.1016/j.mcna.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Gerwin N., Hops C., Lucke A. Intra-articular drug delivery in osteoarthritis. Adv. Drug Del. Rev. 2006;58:226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Derendorf H., Mollmann H., Gruner A., Haack D., Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin. Pharmacol. Ther. 1986;39:313–317. doi: 10.1038/clpt.1986.45. [DOI] [PubMed] [Google Scholar]
- 4.Ratcliffe J. H., Hunneyball I. M., Smith A., Wilson C. G., Davis S. S. Preparation and evaluation of biodegradable polymeric systems for the intra-articular delivery of drugs. J. Pharm. Pharmacol. 1984;36(7):431–434. doi: 10.1111/j.2042-7158.1984.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 5.World Anti-Doping Agency Prohibited List, version January 2007. www.wada-ama.org.
- 6.Horisawa E., Kubota K., Tuboi I., Sato K., Yamamoto H., Takeuchi H., Kawashima Y. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm. Res. 2002;19:132–139. doi: 10.1023/A:1014260513728. [DOI] [PubMed] [Google Scholar]
- 7.Hausberg A. G., Kenley R. A., DeLuca P. P. Gamma irradiation effects on molecular weight degradation of poly(DL-lactide-co-glycolide) microparticles. Pharm. Res. 1995;12:851–856. doi: 10.1023/A:1016256903322. [DOI] [PubMed] [Google Scholar]
- 8.Montanari L., Costantini M., Signorotti E. C., Valvo L., Cantucci M., Bartolomei M., Fattibene P., Onori S., Faucitano A., Conte B., Genta I. Gamma irradiation effects on poly (DL-lactide-co-glycolide) microspheres. J. Controll. Rel. 1998;56:219–229. doi: 10.1016/S0168-3659(98)00082-0. [DOI] [PubMed] [Google Scholar]
- 9.Y. W. Jo, B. H. Woo, A. M. Hazrati, and P. P. DeLuca. Use of Pharmasep unit for processing microspheres. AAPS PharmSciTech.2(1): article. http://www.aapspharmscitech.org (2001). [DOI] [PMC free article] [PubMed]
- 10.Bittner B., Mäder K., Kroll C., Borchert H. H., Kissel T. Tetracycline HCl-loaded poly(DL-lactide-co-glycolide) microspheres prepared by a spray-drying technique: influence of γ-irradiation on radical formation and polymer degradation. J. Controll. Rel. 1999;59:23–32. doi: 10.1016/S0168-3659(98)00170-9. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Sancho C., Herrero-Vanrell R., Negro S. Study of gamma-irradiation effects on aciclovir. poly(DL-lactic-co-glycolic) acid microspheres for intravitreal administration. J. Controll. Rel. 2004;99(21):41–52. doi: 10.1016/j.jconrel.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Loo J. S. C., Ooi C. P., Boey F. Y. C. Degradation of poly(lactide-co-glycolide) (PLGA) and poly(L-lactide) (PLLA) by electron beam radiation. Biomater. 2005;26:1359–1376. doi: 10.1016/j.biomaterials.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Montanari L., Cilurzo F., Selmin F., Conti B., Genta I., Poletti G., Orsini F., Valvo L. Poly(lactide-co-glycolide) microspheres containing bupivacaine: comparison between gamma and beta irradiation effects. J. Controll. Rel. 2003;90:281–190. doi: 10.1016/S0168-3659(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 14.Bushell J. A., Claybourn M., Williams H. E., Murphy D. M. EPR and ENDOR study of γ- and β-radiation sterilization in poly(lactide-co-glycolide) polymers and microspheres. J. Controll. Rel. 2005;110:49–57. doi: 10.1016/j.jconrel.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Friess W., Schlapp M. Sterilization of gentamicin containing collagen/PLGA microparticle composites. Eur. J. Pharm. Biopharm. 2006;63:176–187. doi: 10.1016/j.ejpb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Mauduit J., Bukh N., Vert M. Gentamycin/poly(lactic acid) blends aimed at sustained release local antibiotic therapy administered per-operatively. I. The case of gentamycin base and gentamycin sulfate in poly(D,L-lactic acid) oligomers. J. Controll. Rel. 1993;23:209–220. doi: 10.1016/0168-3659(93)90002-M. [DOI] [Google Scholar]
- 17.Sansdrap P., Moës A. J. Influence of manufacturing parameters on the size characteristics and the release profiles of nifedipine from poly(DL-lactide-co-glycolide) microspheres. Int. J. Pharm. 1993;98(1–3):157–174. doi: 10.1016/0378-5173(93)90052-H. [DOI] [Google Scholar]
- 18.Giovagnoli S., Blasi P., Schoubben A., Rossi C., Ricci M. Preparation of large porous biodegradable microspheres by using a simple double-emulsion method for capreomycin sulfate pulmonary delivery. Int. J. Pharm. 2007;333:103–111. doi: 10.1016/j.ijpharm.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi W. I., HamLin W. E., Metha S. C. Infrared attenuated total reflectance (ATR) method for observing the water-mediated surface phase reversion of methylprednisolone II to I during dissolution. J. Pharm. Sci. 1969;58(9):1145–1146. doi: 10.1002/jps.2600580926. [DOI] [PubMed] [Google Scholar]
- 20.Kane M. P., Tsuji K. Radiolytic degradation scheme for cobalt-60-irradiated corticosteroids. J. Pharm. Sci. 1983;72:30–35. doi: 10.1002/jps.2600720108. [DOI] [PubMed] [Google Scholar]
- 21.F. Cilurzo, F. Selmin, P. Minghetti, L. Montanari, C. Lenardi, F. Orsini, and G. Poletti, Comparison between gamma and beta irradiation effects on hydroxypropylmethylcellulose and gelatin hard capsules. AAPS PharmSciTech 6(4): article 73. http://www.aapspharmscitech.org (2005). [DOI] [PMC free article] [PubMed]
- 22.Maggi L., Segale L., Ochoa Maciste E., Buttafava A., Faucitano A., Conte U. Chemical and physical stability of hydroxypropylmethylcellulose matrices containing diltiazem hydrochloride after gamma irradiation. J. Pharm. Sci. 2003;92:131–140. doi: 10.1002/jps.10271. [DOI] [PubMed] [Google Scholar]
- 23.Montanari L., Cilurzo F., Conti B., Genta I., Groppo A., Valvo L., Faucitano A., Buttafava A. Gamma irradiation effects and EPR investigation on poly(lactide-co-glycolide) microspheres containing bupivacaine. Il Farmaco. 2002;57:427–433. doi: 10.1016/S0014-827X(02)01220-X. [DOI] [PubMed] [Google Scholar]