Abstract
In previous works, our research group has successfully proved the use of subcellular vaccines based on poly(ε-caprolactone) (PEC) microparticles containing an antigenic extract of Brucella ovis (HS) against experimental brucellosis in both mice and rams. However, the successful exploitation of pharmaceutical products, and therefore of this product as veterinary vaccine, requires preservation of both biological activity and native structure in all steps of development from purification to storage. In this context, we have carried out an accelerated stability study to evaluate the relative stability of HS when loading in PEC microparticles. For this purpose, freeze-dried microparticles were stored at 40 ± 1°C and 75% RH as a preliminary analysis of a stability testing. The results showed that both physico-chemical (size, morphology, antigen content, release profile) and biological (integrity and antigenicity of the HS) properties were preserved after 6 months of storage. On the contrary, after 1 year of storage, the HS release profile was dramatically affected probably due to a progressive loss of the polymer microstructure. In addition, the degradation and loss of the antigenicity of the HS components was also evident by SDS-PAGE and immunoblotting analysis. In fact, after 12 months of storage, only the integrity and antigenicity of two of the major protective proteins of the HS antigenic complex were preserved.
Key words: adjuvant, brucellosis, microparticles, stability, vaccine
INTRODUCTION
Currently, the most universal system of prophylaxis used against brucellosis, and the only practical measure in countries with high incidence of this disease, consists on vaccination of animals (1,2). Controlled experiments and accumulated knowledge have demonstrated that the Brucella abortus strain 19 (S19) in cattle and Brucella melitensis strain Rev1 in sheep and goats are useful vaccines (3,4). However, all of these vaccines display some undesirable traits, mainly related to their incomplete avirulence in animals and humans (5,6), their abortifacient effect when used in pregnant animals (4,5). In addition, S19 and Rev1 vaccines interfere in the common serodiagnostic tests used to identify infected animals (2).
Another possibility to design and prepare new, sure, and effective vaccines may be the use of subcellular compounds (associated with a good adjuvant), rather than the whole live microorganism. This approach would allow overcoming the main drawbacks related with the use of live vaccines and to potentate the immune response to subcellular antigens, avoiding booster doses. For vaccination against brucellosis, the hot saline extract from the outer membrane of Brucella ovis (HS) was proposed. This antigenic extract has been shown to protect against B. ovis challenge in rams (7) and mice (1) either, by active immunization or by passive transfer of immune serum. Moreover, the existence of an important antigenic cross-reactivity between HS and components of the B. melitensis membrane (8,9) has also been demonstrated.
HS is a complex mixture of outer membrane proteins (Omps) and rough lipopolysaccharide (R-LPS) (10–12). This extract is especially rich in Omp25 (25 to 27 kDa), Omp31 (31 to 34 kDa) (13), and Omp22 (Omp3b; 22 kDa) (14). Other proteins such as group 2 (Omp2a and Omp2b [36 to 38 kDa]) and three lipoproteins (L-Omp10, L-Omp16, and L-Omp19) (13,15), which are expressed in all Brucella spp. (15), have also been identified. Among all the components, Omp31 seems to be of a particular interest in B. ovis infection, in which it may be the immunodominant antigen in the antibody responses of naturally and experimentally infected rams (16). Similarly, the outer membrane lipoproteins 16 and 19 have also demonstrated to provide great protection against B. ovis infection in mice (1).
In this context, our research group proposed the use of poly(ε-caprolactone) (PEC) microparticles as adjuvant for the controlled release of HS (17,18). These microparticles were prepared by the solvent extraction/evaporation method previously described (18), using TROMS (Total Recirculation One-Machine System) in order to avoid the use of aggressive homogenization techniques (i.e., ultrasounds and/or Ultraturrax) and, thus, minimize the degradation of the antigenic complex (19). This formulation was found to induce adequate immune response and protection against experimental brucellosis in both mice and rams which was similar to that observed for the reference vaccine Rev1 (18,20). In addition, this formulation was also able to induce protection against a challenge with B. melitensis in mice (21).
The successful exploitation of pharmaceutical products, and therefore of this product as veterinary vaccine, requires preservation of both biological activity and native structure in all steps of development from purification to storage. Thus, the aim of this study was to evaluate the relative stability of HS when loading in PEC microparticles as well as the polymer integrity. For this purpose, freeze-dried microparticles where stored at 40 ± 1°C and 75% RH as a preliminary analysis of a stability testing.
MATERIALS AND METHODS
Materials
Poly(ε-caprolactone) (PEC; Mw 42,500) and β-cyclodextrin hydrate were purchased from Aldrich-Chemical Company (USA). Polyvinylalcohol (PVA; Mw 115,000) and methylene chloride HPLC grade (DCM) were obtained from BDH-Supplies (England). Pluronic® F68, bicinchoninic acid solution (BCA) and copper (II) sulfate were achieved from Sigma (St. Louis, USA). MicroBCA protein assay kit was purchased from Pierce Chemical (Rockford, IL). Sodium hydroxide (NaOH) was obtained from Panreac Quimica (Spain). Acrylamide was obtained from Bio-Rad laboratories (CA, USA). Peroxidase-conjugate rabbit anti-sheep immunoglobulin G (heavy and light chain specific; RaSh H + L) was acquired from Nordic immunological Laboratories (Tilburg, The Netherlands). PVDF (polyvinylidene fluoride papers, pore size of 0.45 µm) sheets were from Schleicher & Schuell (Germany) and 4-chloro 1-naphtol was from Merck (Germany).
Extraction and Characterization of the Antigenic Extract
Hot saline extract was obtained from whole B. ovis Reo 198 as described previously (8) by suspending live cells in physiological saline (10 g of packed cells per 100 mL) and heating in flowing steam for 15 min. After centrifugation (12,000×g, 15 min) the supernatant was dialyzed for 5 days at 4°C against several changes of deionized water. The dialyzed material was centrifuged (60,000×g, 4 h) and the pellet (HS) dispersed in deionized water, before congelation and lyophilization. This antigenic extract (HS) was stored at room temperature. The batch of antigen used to prepare the vaccine formulation contained 48.7 ± 4.97% protein and 41.7 ± 4.74% rough lipopolysaccharide (R-LPS).
Preparation of HS-loaded Microparticles
HS-loaded microparticles (HS-MP) were prepared using TROMS (Total Recirculation One-Machine System) as described previously (22). Briefly, for the preparation of the microparticles, the antigenic extract was firstly mixed in a mortar for 30 min with β-cyclodextrin (HS/cyclodextrin ratio of 1 by weight) and the mixture was then dispersed in the aqueous phase containing a Pluronic® 6% w/v (inner aqueous phase, W1). Then, the organic phase (200 mg PEC in 5 mL methylene chloride) was injected through a needle with an inner diameter of 0.12 mm into a first vessel containing the inner aqueous phase by activation of the pumping system (pumping flow of 50 mL/min). This inner emulsion (W1/O) previously formed was forced to circulate through the system for 2 min under a turbulent regime (flow of 50 mL/min). After this step, this emulsion was injected via a needle (inner diameter of 0.12 mm) onto the second vessel containing 30 mL of an aqueous phase 0.5% PVA (W2 phase). The turbulent injection through a second needle (inner diameter of 0.17 mm) resulted in the formation of a multiple emulsion (W1/O/W2) which was forced to circulate in the system for 4 min in order to be homogenized. The resulting W1/O/W2 emulsion was stirred for at least 2 h at room temperature conditions to allow the evaporation of the organic solvent. Microparticles were washed three times with distilled water by centrifugation at 4°C (25,000×g, 15 min). Finally, microparticles were dispersed in water, frozen at −80°C and lyophilized (Genesis 12EL, Virtis).
Storage Conditions
A 12-month accelerated stability test was carried out after preparation of microparticles. For this purpose, microparticles containing HS (HS-MP) were packaged in sealed vials and stored in a climatic chamber (VC0033, Heraeus) maintained at 40°C ± 1 and 75% RH. The samples were withdrawn periodically and evaluated for morphology, antigen content, and release profile, integrity and antigenicity of the released HS.
Characterization of Microparticles
Particle Size Measurements
Microparticles were sized by laser diffractometry using a Mastersizer-S® laser sizer (Malvern Instruments, Malvern, UK). The average particle size was expressed as the volume mean diameter (Vmd) in micrometers (µm). For each time point, six samples were analyzed in triplicate.
Scanning Electron Microscopy
The morphology of microparticles was examined by scanning electron microscopy (SEM). Microparticles were mounted on double-faced adhesive tape on metal stubs, coated with gold to a thickness of 16 nm (Emitech K550 equipment). Observations were performed in a Zeiss DSM 940 A with a digital imaging capture system (DISS de Point Electronic GmBh).
HS Content
The quantification of the HS content was carried out after the alkaline hydrolysis of the polymer using 0.1 N NaOH. For this purpose, 10 mg of the freeze-dried microparticles, accurately weighted, were digested overnight in 1 mL of 0.1 N NaOH under magnetic stirrer. The samples were centrifuged (25,000×g, 15 min) and the antigen concentration in the supernatants was determined by the BCA protein assay.
The HS loading was calculated as the amount of antigen entrapped per milligram microparticles. Each sample was assayed in triplicate.
In vitro Release Studies
Microparticles (30 mg) were added to 1 mL PBS (10 mM, pH 7.4) and dispersed using a vortex. Release study was conducted at 37 ± 1°C under horizontal agitation during 28 days. At definite time intervals, sample tubes were centrifuged (25,000 × g, for 15 min) and the protein content was determined by micro-BCA assay and performed in a 96-well multiscanner autoreader (Labsystems iEMS Reader MF). Dissolution medium was replaced after each determination. Unloaded microspheres were used as control and subjected to the same procedure. Release profiles were calculated in terms of cumulative release, and plotted versus time.
Study of the Structural Integrity and Antigenicity of the Entrapped HS
The antigen structural integrity and antigenicity of the HS-loaded microparticles were assessed by SDS-PAGE and immunoblotting, respectively.
HS-loaded microparticles were dissolved in DCM and the organic solvent evaporated under nitrogen gas. Batches with mannitol were previously washed with distilled water as described above. The HS released was recovered and suspended in the electrophoretic sample buffer (Tris–HCl 62.5 mM (pH 6.8), 10% glycerol, 2% SDS, 5% β-mercaptoethanol, and 0.05% bromophenol blue). For SDS-PAGE, samples were analyzed by using a 15% acrylamide slabs with the discontinuous buffer system of Laemmli and gels stained with alkaline-silver for proteins. Immunoblotting was carried out as described previously (8) with a pool of sera from rams naturally infected with B. ovis and with peroxidase-conjugated goat anti-rabbit IgG (Nordic) and 4-chloro,1-naphtol as chromogen.
The apparent molecular masses of the proteins were determined by comparing their electrophoretic mobility with that of the molecular mass marker (rainbow-colored protein molecular weight marker, Amersham Pharmacia Biotech, Freiburg, Germany).
Statistical Analysis
To assess statistical significance an ANOVA test (Tukey’s DHS test) was made and P < 0.05 was considered as a statistically significant difference. Data are expressed as the mean ± S.D. of at least three experiments.
RESULTS
Physico-chemical Characteristics of Microparticles
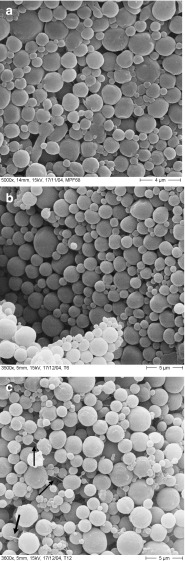
HS-containing microparticles (HS-MP), prepared by TROMS, displayed an initial mean size of 2.00 ± 0.10 µm. By SEM, these microparticles displayed a spherical shape and smooth surface (Fig. 1a).
Fig. 1.
Surface scanning electron micrographs of HS-loaded poly(ɛ-caprolactone) microparticles prepared by TROMS. a Fresh microparticles; b microparticles stored at 40°C/75% RH for 6 months; c microparticles stored at 40°C/75% RH for 12 months
In order to explore the stability of this microparticle vaccine, different batches of HS-MP were stored in a climatic chamber at 40°C/75% RH. As a first parameter of quality, the mean size of stored microparticles was determined at different times (Table I). As expected, no significant variations of the mean size of microparticles were observed. This observation was confirmed by SEM (Fig. 1). Thus, after 6 months of storage, HS-MP appeared with a perfectly defined structure, with no evidence of apparent changes in their surface (Fig. 1b). After 12 months of storage at 40°C/75% RH, microparticles continued to be spherical and displayed a smooth surface without any sign of fusion between particles. However, the presence of structure-like fibers connecting microparticles was observed (Fig. 1c, black arrows).
Table I.
Influence of the Time of Storage at 40°C/75% RH on the Mean Size of Microparticles and Antigen Content of Microparticles
| Time of storage in months (40°C/75% RH) | Mean size (µm) | HS loading (µg/mg microparticles) |
|---|---|---|
| 0 | 2.00 ± 0.10 | 13.90 ± 1.04 |
| 1 | 2.04 ± 0.05 | 13.34 ± 0.13 |
| 3 | 2.10 ± 0.08 | 12.57 ± 0.50 |
| 6 | 2.15 ± 0.32 | 13.21 ± 0.66 |
| 9 | 2.11 ± 0.10 | 13.76 ± 0.41 |
| 12 | 2.15 ± 0.21 | 14.04 ± 0.28 |
Data express the mean ± SD (n = 6)
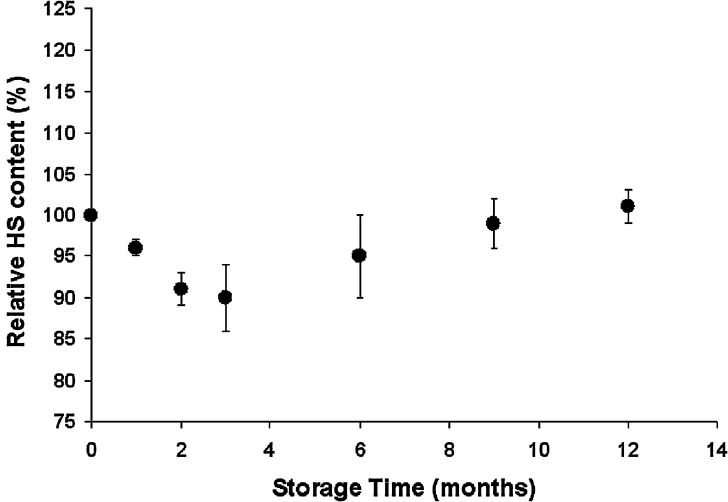
The initial amount of HS loaded in microparticles was calculated to be 13.9 ± 1.04 µg/mg, which represented an encapsulation efficiency of about 70%. Figure 2 shows the evolution of the HS relative content in microparticles as a function of time of storage at 40°C/75% RH. As expected, HS-MP did not show a significant diminution on the HS loading during storage. In fact, the HS content was maintained within the range between 101% and 90% of the initial antigen content, which can be considered within the variation due to the precision of the analytical technique (±14.7% (23)).
Fig. 2.
Evolution of the HS relative content in microparticles as a funtion of time of storage at 40°C/75% RH. Data expressed as the mean ± SD (n = 6) of the percentage of the initial HS content in microparticles (13.9 µg/mg, which was considered 100%)
In vitro Release of HS from Microparticles
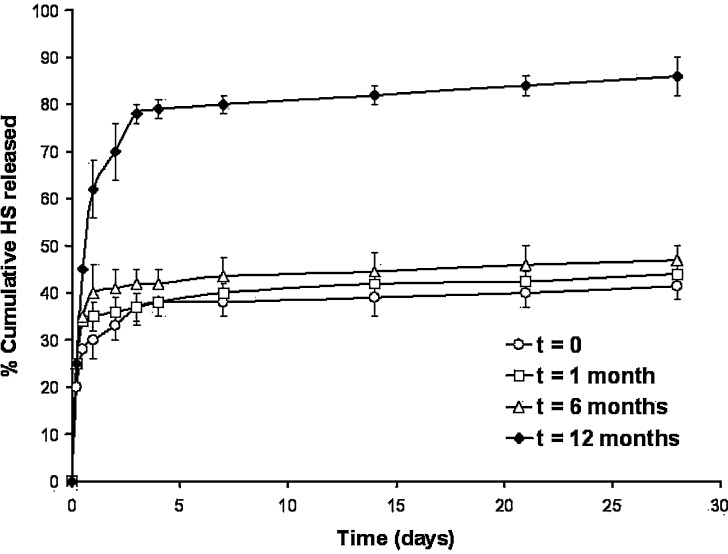
Figure 3 shows the release profiles of HS from microparticles (expressed as a cumulative release vs. time). HS-MP released the antigen in a biphasic way, characterized by an initial short release within the first 12 h (burst effect), followed by a more continuous and sustained release of the loaded antigen. Concerning freshly prepared microparticles, they displayed an initial burst effect of about 25% whereas, at the end of the experiment (4 weeks) they had released close to 40% of the total encapsulated HS. During the first 6 months of storage at 40°C/75% HR, no significant differences on the profiles of antigen release were observed; although the burst effect slightly increased (around 35% of the loaded HS for microparticles stored 6 months). On the other hand, the second part of the release profile was quite similar to that observed for freshly microparticles. Thus, after the 28-day release experiment, the amount of HS released was found to be between 45%, for microparticles stored during 1 month, to 47%, for microparticles stored 6 months.
Fig. 3.
Influence of the time of storage on the release profiles of HS from microparticles. (empty circle) HS released from fresh microparticles, (empty square) HS released from microparticles after 1 month of storage at 40°C/75% RH, (empty upright triangle) HS released from microparticles after 6 months of storage at 40°C/75% RH, (empty diamond) HS released from microparticles after 12 months at 40°C/75% RH. Empty microparticles were used as blank. Data express the mean of the percentage of HS released ±SD, n = 3
On the contrary, 1 year after storage, the release profile of HS from microparticles dramatically changed. In this case, microparticles displayed a fast release rate with an initial burst effect of about 50% of the loaded HS, followed by a more rapid release period in which 85% of the loaded HS was released after 28 days.
From a statistical point of view, no significant differences were found between fresh microparticles and 1 month or 6 months post-storage ones (P > 0.05). In contrast, the release profile after 12 months was significantly different from fresh microparticles (P < 0.05).
Structural Integrity and Antigenicity of the Entrapped HS
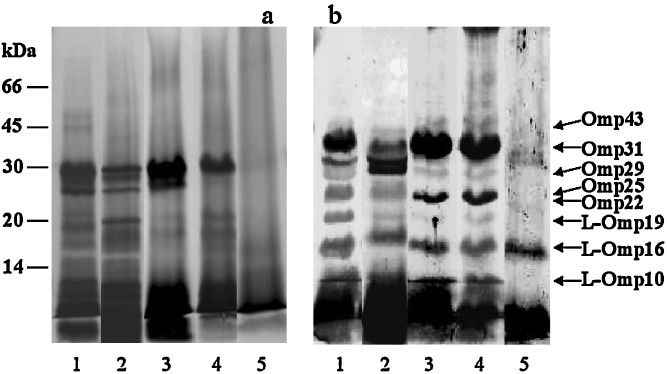
The integrity or protein profile and antigenicity of both the intact HS and the total HS extracted from microparticles after storage is presented in Fig. 4. The results from SDS-PAGE analysis of the extracted HS from microparticles (Fig. 4, Panel a) revealed that the integrity of proteins was preserved. This observation was confirmed by immunoblotting, where the HS extracted from microparticles demonstrated reactivity against a pool of sera from naturally infected rams with B. ovis. Thus, the antigenicity of the HS is well preserved after 6 months of storage, with the presence of the more immunogenic proteins: Omp31, Omp25, Omp22, L-Omp10, L-Omp16, and L-Omp19 among others. All of these results suggested that, the major antigenic proteins were not altered after 6 months of storage (Fig. 4, panel b, lane 4). On the contrary, after 1 year of storage, the degradation of the HS components was evident. In fact, both SDS-PAGE and immunoblotting revealed less intense bands for Omp25, Omp29, and Omp31, whereas no presence of L-Omp10 and L-Omp19 was found.
Fig. 4.
Influence of storage conditions (40°C/75% RH) on the integrity and antigenicity of the HS loaded in microparticles. For SDS-PAGE a silver stain for proteins was employed. Western-blot b was revealed with a pool of sera from B. ovis infected rams. The gels were loaded with the equivalent to 12 µg HS/well. Loading order: lane 1, Free HS; lane 2, HS extracted from microparticles prior to storage; lanes 3–5, HS extracted from microparticles after storage at 1, 6 and 12 months, respectively
DISCUSSION
Recently, a new subcellular vaccine containing an antigenic extract from B. ovis (hot saline; HS) loaded in poly(ε-caprolactone) microparticles as adjuvant (17,18), was developed. In this work, we wanted to gain insight about the stability of this vaccine. For subcellular vaccines, structural integrity and antigenicity of antigens have been considered key factors determining their immunogenicity or efficacy (24,25).
PEC is a semicrystalline biodegradable polyester polymer that degrades slowly and does not generate an acid environment unlike the PLA/PLG polymers (26). Although the permeability of macromolecules in PEC is low, such low permeability may be sufficient enough for protein delivery (27). Other advantages of PEC include its hydrophobicity, its in vitro stability, and its low cost.
Concerning the new vaccine formulation (HS-MP), our results showed that the physico-chemical characteristics of these microparticles were similar during all the period of the study. Thus, no clear changes on the size and HS loading of microparticles, stored at 40 ± 1°C and a relative humidity of 75%, were detected for at least 12 months. These findings are in accordance with Yang et al. (28) who described that the content of nitrendipine from the microspheres stored at 40°C/75% RH was unchanged for at least 3 months, due to the protective effect of the dispersion state of the drug for the polymer used as matrix of microparticles.
Concerning the morphology of microparticles, after 6 months of storage, no evident differences in the shape or appearance of these formulations were visualized (Fig. 1) when compared to fresh microparticles. However, after 1 year of storage, the presence of polymer fibers was an evidence of the degradation of these microparticles.
In fact, the release profile of HS from PEC microparticles exhibited a burst phase of antigen release in the early stages of testing amounting to approximately 25% of the protein loading (for fresh microparticles). This first release step was followed by a low and constant rate of HS release occurring in our in vitro conditions for more than 1 month. This burst release of protein was normally considered to be due to the surface-located protein (29). On the other hand, this release profile has been considered as especially well-adapted for vaccine release requiring a high initial dose for the priming of immune responses and a slow continuous release to induce the booster doses after a delay-time (30). More particularly, in our case, this vaccine has been proved effective against experimental infections with B. ovis, B. abortus, and B. melitensis in mice (18,21) and B. ovis in rams (20).
When microparticles were stored at 40°C/75 RH for 6 months, no significant modifications in the HS release profiles were observed (see Fig. 3). However, after 1 year of storage, the HS release profile dramatically changed. In this case, the burst effect was about 50% and at the end of the experiment more than 80% of the loaded antigen has been released. This fact can be directly related to the degradation of the polymer and the presence of fibers as visualized by SEM (Fig. 1c). Thus, the rapid release observed for HS when microparticles were stored at 40°C/75% HR for 1 year can be related to a modification of the polymer microstructure, which could be understood as follows. When the temperature rises, the mobility of the chain segments in the non-crystalline regions would increase, contributing to a high porosity of the microparticles. Since the diffusant could penetrate more easily in the amorphous domain of the polymer, the release rate of HS from microparticles would increase. Another simultaneous phenomenon can be the decrease of the crystallinity of the polymer due to a progressive transformation of crystalline domains in amorphous regions. This fact has been reported by Mallapragada et al. (31) who described that the release of metronidazole from semicrystalline poly(vinyl alcohol) devices was depended on the crystallization conditions of the polymer. In any case, several authors reported the effect of crystalline microstructure on drug-release behavior from biodegradable polymers, concluding that the higher the crystallinity, the slower the release rate (32–34).
Concerning the HS integrity during the 12-month storage at 40°C/75% RH, SDS-PAGE analysis demonstrated the absence of additional bands in gels obtained with the loaded HS, in comparison to the native HS. However, after 1 year of storage, bands corresponding to Omp31, Omp29, Omp25, L-Omp19, and L-Omp10 were found to be less strong or were absents. In addition, the biological activity or antigenicity of the HS was assessed through immunoblotting, showing that the encapsulated HS was recognized by a pool of sera from naturally infected rams. Likewise, after 6 months of storage, SDS-PAGE and immunoblotting showed that both the integrity and the antigenicity of the HS were preserved. Unfortunately, the loss of antigenic properties of HS was clearly evident after 1 year of storage. In fact, only the integrity and antigenicity of two of the protective proteins, L-Omp16 and Omp29, still remained.
CONCLUSION
In summary, the results confirmed the preservation of the major antigenic proteins of HS and their antigenicity properties following encapsulation in poly(ɛ-caprolactone) microparticles by TROMS. On the other hand, the stability study confirmed that HS-MP is stable for at least 6 months when stored at 40°C/75% RT. During this time, no evidence of significant alterations on the physico-chemical and biological properties of HS were found. Thus, we can suppose that under the standard storage conditions (25°C/60% HR), this formulation would be stable for at least a similar period of time. Obviously, this is a preliminary study and additional work has to be conducted to establish the expiration date of this product after manufacture.
Acknowledgments
This work was supported by grants from the “Ministerio de Educación y Cultura de España” (Grants AGL2000-0299-C03 and AGL2004-07088-C03), Instituto de Salud Carlos III (Red Temática de Investigación en Brucelosis, Ref. No. G03/201), Fundación Ma Francisca Roviralta and Fundación Universitaria de Navarra.
References
- 1.Bowden R. A., Cloeckaert A., Zygmunt M. S., Dubray G. Outer-membrane protein- and rough lipopolysaccharide-specific monoclonal antibodies protect mice against Brucella ovis. J. Med. Microbiol. 1995;43:344–347. doi: 10.1099/00222615-43-5-344. [DOI] [PubMed] [Google Scholar]
- 2.MacMillan A. P. Conventional serological tests. In: Nielsen K. H., Duncan J. R., editors. Animal Brucellosis. Boca Raton, Florida: CRC; 1990. pp. 153–197. [Google Scholar]
- 3.Alton G. G., Elberg S. S. Rev1 Brucella melitensis vaccine: a review of ten years of study. Vet. Bull. 1967;371:793–800. [Google Scholar]
- 4.De Rosa G., Larobina D., La Rotonda M. I., Musto P., Quaglia F., Ungaro F. How cyclodextrin incorporation affects the properties of protein-loaded PLGA-based microspheres: the case of insulin/hydroxypropyl-β-cyclodextrin system. J. Contr. Rel. 2005;102:71–83. doi: 10.1016/j.jconrel.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Blasco J. M., Díaz R. Brucella melitensis Rev1 vaccine as a cause of human brucellosis. Lancet. 1993;342:805. doi: 10.1016/0140-6736(93)91571-3. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti P. L. Relationship between animal and human disease. In: Young E. J., Corbel M. J., editors. Brucellosis: Clinical and Laboratory Aspects. Boca Raton, Florida: CRC; 1989. pp. 41–51. [Google Scholar]
- 7.Blasco J. M., Gamazo C., Winter A. J., Jiménez de Bagüés M. P., Marín C., Barberán M., Moriyón I., Alonso-Urmeneta B., Díaz R. Evaluation of whole cell and subcellular vaccines against Brucella ovis in rams. Vet. Immunol. Immunopathol. 1993;37:257–270. doi: 10.1016/0165-2427(93)90198-D. [DOI] [PubMed] [Google Scholar]
- 8.Gamazo C., Winter A. J., Moriyón I., Riezu-Boj J. L., Blasco J. M., Díaz R. Comparative analysis of proteins extracted by hot saline or release spontaneously into outer membrane blebs from field strains of Brucella ovis and Brucella melitensis. Infect. Immun. 1989;57:1419–1426. doi: 10.1128/iai.57.5.1419-1426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riezu-Boj J. I., Moriyón I., Blasco J. M., Gamazo C., Díaz R. Antibody response to Brucella ovis outer membrane proteins in ovine brucellosis. Infect. Immun. 1990;58:489–494. doi: 10.1128/iai.58.2.489-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowden R. A., Estein S. M., Zygmunt M. S., Dubray G., Cloeckaert A. Identification of protective outer membrane antigens of Brucella ovis by passive immunization of mice with monoclonal antibodies. Microbes. Infect. 2000;2:481–488. doi: 10.1016/S1286-4579(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez de Bagüés M. P., Elzer P. H., Barberán M., Blasco J. M., Marín C. M., Gamazo C., Winter A. J. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect. Immun. 1994;62:632–638. doi: 10.1128/iai.62.2.632-638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cloeckaert A., de Wergifosse P., Dubray G., Limet J. N. Identification of seven surface-exposed Brucella outer membrane proteins by use of monoclonal antibodies: immunogold labelling for electron microscopy and enzyme-linked immunosorbent assay. Infect. Immun. 1990;58:3980–3987. doi: 10.1128/iai.58.12.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cloeckaert A., Vizcaíno N., Paquet J. -Y., Bowden R. A., Elzer P. H. Major outer membrane proteins of Brucella spp.: past, present and future. Vet. Microbiol. 2002;90:229–247. doi: 10.1016/S0378-1135(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 14.Salhi I., Boigegrain R. A., Machold J., Weise C., Cloeckaert A., Rouot B. Characterization of new members of the Group 3 outer membrane protein family of Brucella spp. Infect. Immun. 2003;71:4326–4332. doi: 10.1128/IAI.71.8.4326-4332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tibor A., Decelle B., Letesson J. J. Outer membrane proteins Omp10, Omp16, and Omp19 of Brucella spp. are lipoproteins. Infect. Immun. 1999;67:4960–4962. doi: 10.1128/iai.67.9.4960-4962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kittelberger R., Hilbink F., Hansen M. F., Ross G. P., de Lisle G. W., Cloeckaert A., de Bruyn J. Identification and characterization of immunodominant antigens during the course of infection with Brucella ovis. J. Vet. Diagn. Invest. 1995;7:210–218. doi: 10.1177/104063879500700208. [DOI] [PubMed] [Google Scholar]
- 17.Murillo M., Gamazo C., Irache J. M., Goñi M. M. Polyester microparticles as a vaccine delivery system for brucellosis: influence of the polymer on release, phagocytosis and toxicity. J. Drug Target. 2002;10:211–219. doi: 10.1080/10611860290022642. [DOI] [PubMed] [Google Scholar]
- 18.Murillo M., Grilló M. J., Reñé J., Marín C. M., Barberán M., Goñi M. M., Blasco J. M., Irache J. M., Gamazo C. A Brucella ovis antigenic complex bearing poly-epsilon-caprolactone microparticles confer protection against experimental brucellosis in mice. Vaccine. 2001;19:4099–4106. doi: 10.1016/S0264-410X(01)00177-3. [DOI] [PubMed] [Google Scholar]
- 19.García del Barrio G., Novo F. J., Irache J. M. Loading of plasmid DNA into PLGA microparticles using TROMS (Total Recirculation One-Machine System): evaluation of its integrity and controlled release properties. J. Control. Rel. 2003;86:123–130. doi: 10.1016/S0168-3659(02)00371-1. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz M. P., Estevan M., Marín C. M., De Miguel M. J., Grilló M. J., Barberán M., Irache J. M., Blasco J. M., Gamazo C. Brucella outer membrane complex-loaded microparticles as a vaccine against Brucella ovis in rams. Vaccine. 2006;24:1897–1905. doi: 10.1016/j.vaccine.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Estevan M., Gamazo C., Grilló M. J., García del Barrio G., Blasco J. M., Irache J. M. Experiments on a sub-unit vaccine encapsulated in microparticles and its efficacy against Brucella melitensis in mice. Vaccine. 2006;24:4179–4187. doi: 10.1016/j.vaccine.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Estevan M., Gamazo C., González-Gaitano G., Irache J. M. Optimization of the entrapment of bacterial cell envelope extracts into microparticles for vaccine delivery. J. Microencapsul. 2006;23:169–181. doi: 10.1080/02652040500435253. [DOI] [PubMed] [Google Scholar]
- 23.Instructions BCA Protein Assay Reagent Kit. Pierce Biotechnology, Rockford, USA, 2002.
- 24.McGee J. P., Singh M., Li X. M., Qiu H., O’Hagan D. T. The encapsulation of a model protein in poly(d,l lactide-co-glycolide) microparticles of various sizes: an evaluation of process reproducibility. J. Microencapsul. 1997;14:197–210. doi: 10.3109/02652049709015333. [DOI] [PubMed] [Google Scholar]
- 25.O’Hagan D. T., McGee J. P., Boyle R., Gumaer D., Li X. M., Potts B., Wang C. Y., Koff W. C. The preparation, characterization and pre-clinical evaluation of an orally administered HIV-1 vaccine, consisting of a branched peptide immunogen entrapped in controlled release microparticles. J. Control. Rel. 1995;36:75–84. doi: 10.1016/0168-3659(95)00052-A. [DOI] [Google Scholar]
- 26.Abe H. Thermal degradation of environmentally degradable poly(hydroxyalkanoic acid)s. Macromol. Biosci. 2006;6:469–486. doi: 10.1002/mabi.200600070. [DOI] [PubMed] [Google Scholar]
- 27.Pitt C. G. Poly(ε-caprolactone) and its copolymers. In: Chasin M., Langer R., editors. Biodegradable Polymers as Drug Delivery Systems. New York: Marcel Dekker; 1990. pp. 71–120. [Google Scholar]
- 28.Yang M., Cui F., You B., Wang L., Zhang L., Kawashima Y. A novel pH-dependent gradient-release delivery system for nitrendipine I. Manufacturing, evaluation in vitro and bioavailability in healthy dogs. J. Control. Rel. 2004;98:219–229. doi: 10.1016/j.jconrel.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 29.Yan C., Resau J. H., Heweston J., West M., Rill W. L., Kende M. Characterisation and morphological analysis of protein loaded poly(lactide-co-glycolide) microparticles prepared by water-in-oil-in-water emulsion technique. J. Control. Rel. 1994;32:231–241. doi: 10.1016/0168-3659(94)90233-X. [DOI] [Google Scholar]
- 30.Benoit M. A., Baras B., Gillard J. Preparation and characterization of protein-loaded poly(ε-caprolactone) microparticles for oral vaccine delivery. Int. J. Pharm. 1999;184:73–84. doi: 10.1016/S0378-5173(99)00109-X. [DOI] [PubMed] [Google Scholar]
- 31.Mallapragada S. K., Peppas N. A., Colombo P. Crystal dissolution-controlled release systems. II. Metronidazole release from semicrystalline poly(vinyl alcohol) systems. J. Biomed. Mat. Res. 1997;36:125–130. doi: 10.1002/(SICI)1097-4636(199707)36:1<125::AID-JBM15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Jeong J. C., Lee J., Cho K. Effects of crystalline microstructure on drug release behaviour of poly(ε-caprolactone) microspheres. J. Control. Rel. 2003;92:249–258. doi: 10.1016/S0168-3659(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 33.Lin W. J., Yu C. C. The effect of solvent removal conditions on performance and release property of protein-loaded microparticles. J. Microencapsul. 2002;19:767–773. doi: 10.1080/02652040210162540. [DOI] [PubMed] [Google Scholar]
- 34.Sinha V. R., Bansal K., Kaushik R., Kumria R., Trehan A. Poly-ɛ-caprolactone microspheres and nanospheres: an overview. Int. J. Pharm. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]