Abstract
Neurons within the spinal cord can support several forms of plasticity, including response–outcome (instrumental) learning. After a complete spinal transection, experimental subjects are capable of learning to hold the hindlimb in a flexed position (response) if shock (outcome) is delivered to the tibialis anterior muscle when the limb is extended. This response-contingent shock produces a robust learning that is mediated by ionotropic glutamate receptors (iGluRs). Exposure to nociceptive stimuli that are independent of limb position (e.g., uncontrollable shock; peripheral inflammation) produces a long-term (>24 h) inhibition of spinal learning. This inhibition of plasticity in spinal learning is itself a form of plasticity that requires iGluR activation and protein synthesis. Plasticity of plasticity (metaplasticity) in the CNS has been linked to group I metabotropic glutamate receptors (subtypes mGluR1 and mGluR5) and activation of protein kinase C (PKC). The present study explores the role of mGluRs and PKC in the metaplastic inhibition of spinal cord learning using a combination of behavioral, pharmacological, and biochemical techniques. Activation of group I mGluRs was found to be both necessary and sufficient for metaplastic inhibition of spinal learning. PKC was activated by stimuli that inhibit spinal learning, and inhibiting PKC activity restored the capacity for spinal learning. Finally, a PKC inhibitor blocked the metaplastic inhibition of spinal learning produced by a group I mGluR agonist. The data strongly suggest that group I mGluRs control metaplasticity of spinal learning through a PKC-dependent mechanism, providing a potential therapeutic target for promoting use-dependent plasticity after spinal cord injury.
Keywords: spinal cord injury, spinal learning, use-dependent plasticity, nociception, NMDA, AMPA
Introduction
Long-term plasticity in the CNS manifests at multiple levels, from molecular changes at individual synapses to alterations in learning and behavior. The ionotropic glutamate receptors (iGluRs) AMPA receptor (AMPAR) and NMDA receptor (NMDAR) play an essential role in plasticity. Signaling through these receptors can produce lasting changes in synaptic efficacy resulting in long-term potentiation (LTP) or long-term depression (LTD) of postsynaptic potentials (Malinow and Malenka, 2002). The threshold for inducing synaptic plasticity is, itself, subject to modulation. Certain forms of stimulation can shift the threshold for plasticity so that stimuli that would normally induce LTP come to induce LTD, or have no effect. This plasticity of plasticity or “metaplasticity” (Abraham and Bear, 1996; Abraham and Tate, 1997) is associated with trafficking of ionotropic glutamate receptors (Hellier et al., 2007). iGluR function can be affected by group I metabotropic glutamate receptors (mGluR1 and mGluR5) and activation of downstream protein kinase C (PKC). Direct pharmacological manipulation of these signaling systems affects metaplasticity within the CNS.
Most studies of CNS plasticity and metaplasticity have focused on the hippocampus. However, these phenomena are observed throughout the CNS, including the spinal cord (Parker and Grillner, 1999; Bevan and Parker, 2004; Grau et al., 2006). Comparatively little is known about the mechanisms of spinal metaplasticity, despite evidence that recovery of function after spinal cord injury requires concurrent augmentation of adaptive plasticity (e.g., reacquisition of stepping) and diminution of maladaptive plasticity (e.g., plasticity within pain pathways). Here, we explore the pharmacological and biochemical mechanisms of spinal metaplasticity using a simple spinal learning paradigm as the primary behavioral measure (Grau et al., 1998). The task requires that spinally transected rats learn to maintain the hindlimb in a flexed position to terminate shock delivered to the same leg [response-contingent shock (Buerger and Fennessy, 1970)]. With this simple preparation, it can be shown that stimulation alters the capacity for spinal learning in a bidirectional manner that is reminiscent of hippocampal metaplasticity (Grau et al., 2006). Training with response-contingent shock on one limb facilitates learning on the contralateral limb (Crown and Grau, 2001). Conversely, if nociceptive stimulation is delivered in a manner that is independent of leg position (e.g., uncontrollable shock or peripheral inflammation), the spinal cord demonstrates a lasting inhibition of learning (Grau et al., 1998; Ferguson et al., 2006; Hook and Grau, 2007; Hook et al., 2008). Elsewhere, we found that this bidirectional spinal plasticity correlates with spinal levels of brain-derived neurotrophic factor (BDNF) (Gómez-Pinilla et al., 2007), interacts with locomotor plasticity (Bigbee et al., 2007), and affects recovery of function after spinal cord injury (Grau et al., 2004; Hook et al., 2007).
The present study tests whether group I mGluRs and downstream activation of PKC regulate the capacity for spinal learning. Using a combination of behavioral, pharmacological, and biochemical methods, we found that mGluR1, mGluR5, and PKC are necessary for metaplastic inhibition of spinal learning. Group I mGluR activation was sufficient to generate a long-term inhibition of spinal learning and did so through a PKC-dependent mechanism.
Materials and Methods
Subjects.
Male Sprague Dawley rats obtained from Harlan served as subjects. The rats were 100–120 d of age and weighed between 400 and 460 g. Subjects were individually housed, maintained on a 12 h light/dark cycle, and given ad libitum access to food and water. All experiments were performed in accordance with the National Institutes of Health (NIH) standards for the care and use of laboratory animals (NIH publication no. 80-23) and were approved by the University Laboratory Animal Care Committee at Texas A&M University.
Surgery and intrathecal cannulization.
Surgery and intrathecal cannulization were performed using procedures described in detail previously (Grau et al., 1998; Ferguson et al., 2003, 2006). Briefly, subjects were pretreated with an intraperitoneal injection of warm 0.9% saline (2.5 ml) and atropine (40 mg/kg) followed by anesthesia with pentobarbital (50 mg/kg, i.p.). Subjects' backs were shaved, cleaned with iodine, and a longitudinal incision was made extending from about cervical vertebra 6 to thoracic vertebra 4 (T4). The tissue in front of T2 was cleared away, and the spinal cord was transected using a cautery. The void produced by the transection was filled with Oxycel (Parke-Davis) and a segment of polyethylene tubing (25 cm; PE-10) fitted with 0.23 mm (diameter) stainless-steel wire (SWGX-090; Small Parts) was inserted 9 cm caudally into the subarachnoid space between the dura and the spinal cord. The exposed end of tubing was secured to the adjacent tissue using cyanoacrylate. The guide wire was then gently pulled from the tubing and the wound was closed using Michel clips (Fine Science Tools).
After spinalization, subjects were hydrated with an intraperitoneal injection of 2.5 ml of warm 0.9% saline and placed in temperature-controlled environment (∼25.5°C). To prevent injury to the hindlimbs during recovery, the rear legs of spinalized animals were maintained in a normal flexed position by two pieces of porous tape (Orthaletic; width, 1.3 cm) gently wrapped once around the rat's body. Experiments were begun 24 h after transection. Previous work has indicated that comparable spinal learning is observed in acute and chronic spinal preparations, suggesting that spinal shock only has a minimal effect on this form of spinal learning (Grau et al., 1998; Bigbee et al., 2007).
Spinal transections were confirmed by (1) inspecting the cord during the operation to ensure that no spared fibers bridged the transection site and that the rostral and caudal stumps of the cord completely retracted, (2) observing the behavior of the subjects after recovery to ensure that they exhibited paralysis below the level of the forepaws and did not vocalize to leg shock, and (3) examining the spinal cord postmortem in a randomly selected subset of the subjects.
Shock-induced inhibition of spinal learning.
The procedures used to generate a deficit in instrumental learning/adaptive plasticity in the spinal cord have been described in detail previously (Grau et al., 1998; Crown and Grau, 2001; Crown et al., 2002a; Ferguson et al., 2003, 2006; Joynes et al., 2003; Joynes and Grau, 2004; Patton et al., 2004). Briefly, a shock electrode constructed from a modified fuse clip was coated with electrode paste and attached to the tail with a length of Orthaletic tape. Leads from the fuse clip were attached to a BRS/LVE shock generator (model SG-903) and 6 min of intermittent uncontrollable shock (80 ms duration; 1.5 mA AC; mean interstimulus interval of 2 s) was delivered. This procedure has been found to generate reliable metaplastic inhibition of spinal learning that lasts at least 48 h (Crown et al., 2002b). This metaplastic inhibition occurs on both legs and is observed if uncontrollable shock is applied to the hindlimb or the tail.
Spinal cord learning procedures.
Instrumental testing with controllable leg shock in spinalized animals was conducted using an apparatus similar to that used in previous studies (Grau et al., 1998). Briefly, rats were loosely restrained in Plexiglas tubes [20.0 cm (length) × 7.0 cm (internal diameter)]. Two slots [6.0 cm (length) × 1.7 cm (width)] were cut in the sides and base of the tube, allowing both hindlegs to hang freely. Shock was delivered using a BRS/LVE shock generator (model SG-903). Leg shock was applied by attaching one lead from the shock generator to a wire inserted through the skin over the tibia 1.5 cm above the ankle. The other lead was attached to a 2.5 cm stainless-steel pin that was inserted 0.4 cm into the tibialis anterior muscles 1.7 cm above the other electrode. Leg position was monitored using a contact electrode constructed from a 7.0 cm, 0.018“ stainless-steel rod that was taped to the plantar surface of the subject's foot using cloth tape (Orthaletic; 1.3 cm; Johnson & Johnson). A heat shrink covering (2.5 cm) covered the end of the rod and electrically insulated this circuit from the rat. A fine wire [0.01 mm2 (36 AWG)] was attached to the end of the rod, extending from the rear of the foot to a digital input monitored by a Macintosh computer. A plastic rectangular dish [11.5 (w) × 19 (l) × 5 (d)] containing a NaCl solution was placed ∼7.5 cm below the restraining tube, submerging the tip of the contact electrode. A drop of soap was added to the solution to reduce surface tension. A ground wire was connected to a 1 mm stainless-steel rod that was placed in the solution. When the contact electrode attached to the rat's paw contacted the solution, it completed a circuit monitored by the computer. The state of this circuit was sampled at 30 Hz.
Flexion force was measured before instrumental testing using a strain gauge connected to the foot by a monofilament plastic line (“4 lb test” Stren; DuPont). The strain gauge had previously been calibrated by determining the relationship between voltage and force in newtons. The data revealed a linear relationship that allowed us to convert voltage to force. Shock intensity was adjusted to produce a flexion force of a fixed value.
Behavioral measures.
The spinal learning apparatus provides three outputs: time in solution, flexion number, and flexion duration. The computer recorded the amount of time that the contact electrode was in contact with the solution (time in solution). Whenever the electrode left the solution, the number of flexion responses was increased by 1 (flexion number). To observe learning across trials, the training session was divided into 30, 1 min training bins. From time in solution and flexion number, we derived flexion duration using the following equation: flexion durationi = (60 s − time in solutioni)/(flexion numberi + 1), where i was the current training bin. Previous work has demonstrated that flexion duration is uniquely sensitive to response–outcome (instrumental) learning (Grau et al., 1998), whereas flexion number and time in solution can change in the absence of instrumental learning (Church et al., 1976). For this reason, flexion duration was used as the primary assay of spinal plasticity. Flexion number was evaluated as a measure of motor performance and is displayed in the supplemental material (available at www.jneurosci.org).
Drug experiments.
Intrathecal drugs were delivered using a 10 μl Hamilton syringe that was attached to the exposed end of the intrathecal cannulas. Controlled delivery of drug over several minutes was achieved using a syringe pump (model 11; Harvard Apparatus). For the group I mGluR experiments, drugs were purchased from Tocris, and subjects were given one of four doses of drug dissolved in 3 μl of vehicle. The vehicle was 0.9% saline for the mGluR5 antagonist 2-methyl-6-(phenylethylnyl)pyridine (MPEP) and the general group I mGluR agonist 3,5-dihydroxyphenylglycine (DHPG). The mGluR1 antagonist 7-(hydroxy-imino)cycloproa[b]chromen-1a-carboxylate ethyl ester (CPCCOEt) was dissolved in 100% dimethylsulfoxide (DMSO). The PKC inhibitor bisindolylmaleimide (BIM) (Sigma-Aldrich) was delivered in 10 μl of 100% DMSO and the PKC inhibitor chelerythrine (Calbiochem) was delivered in 10% DMSO in 0.9% saline. Because DMSO has been shown to have electrophysiological effects and effects on NMDA-dependent activation of the spinal locomotor networks (Tsvyetlynska et al., 2005), we were concerned that the DMSO vehicle per se could impact the test results. To examine whether vehicle treatment impacted the results, we merged the vehicle data from all experiments. This resulted in a large dataset containing 114 subjects. Even with this relatively high sample size, ANOVA failed to find any significant effect of DMSO versus 0.9% saline (p > 0.05). Analysis of effect size and power calculations revealed that DMSO does not have an effect that can be resolved using reasonable sample sizes for animal research (η2 = 0.007; 1 − β = 0.77). Together, these results suggest that, for all intents and purposes, DMSO does not affect spinal learning in this preparation.
Protein kinase C activity assay.
PKC activity was measured in tissue homogenate containing spinal segments L4–S2 from shocked and unshocked rats (N = 24). Previous work has isolated spinal learning to these segments (Liu et al., 2005). At 10 min, 1 h, and 24 h after the shock stimulus, tissue was flash frozen in liquid nitrogen and homogenized in ice-cold lysis buffer consisting of 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, supplemented with a protease inhibitor mixture (Sigma-Aldrich; P8340; 1:100) and sodium orthovanadate (Na3VO), a phosphatase inhibitor. Lysates were incubated on ice for 30 min and cleared by centrifugation at 30,000 × g, 4°C for 30 min. The protein concentration in cord lysates was determined using the micro-BCA protein assay (Pierce). Kinase activity was measured using the StressXpress Nonradioactive PKC Kinase Assay kit (no. EKS-420; Assay Designs). Given the sensitivity of the assay and the small amount of total tissue collected, our initial analyses examined activity in the total protein fraction. Briefly, 2 μg of cord lysate was mixed with dilution buffer and incubated on a microtiter plate for 1 h at room temperature in the presence of ATP. A proprietary PKC substrate (Assay Designs) was precoated on the wells of the microtiter plate. PKC standards were diluted and incubated in the same manner to generate a standard curve for quantification of activity. Next, the microtiter plate was incubated with a phosphospecific IgG antibody to label all the phosphorylated sites resulting from active PKC in the samples. Plates were incubated with anti-rabbit IgG: HRP-conjugated antibody, and colorimetric reactions were initiated by adding ABTS substrate solution. Colorimetric reactions were terminated after 6 min, and absorbance was read on an ELISA microplate reader at 415 nm (BioTek ELX808). Fresh lysis buffer (without cord lysate) was included in the assay as a blank, and cord lysate without ATP served as the negative control. All samples were assayed in triplicate and the ELISA was performed two times. Analyses of the standard concentrations confirmed that optical density was linearly related to concentration (r = 0.96; p < 0.001) within the range of interest. All analyses were conducted on the mean concentration obtained for each subject averaged across the two assays (n = 4 subjects/group).
Statistics.
The experimental designs were conceived a priori and analyzed as balanced, factorial mixed designs using SPSS 16 GLM repeated measures (for additional details, see supplemental Table 1, available at www.jneurosci.org as supplemental material). ANOVA and trend analysis (by polynomial contrast) were used to determine whether the impact of experimental treatments was better represented by linear relationships or higher polynomials when there was a continuous independent variable (e.g., dose or time). ANCOVA was used as a control in rare cases in which ANOVA detected differences on baseline response performance. Group differences were evaluated using one-way ANOVA and Duncan's post hoc tests when appropriate. The reported sample sizes (n) reflect the number of subjects per group for every factorial combination of the between-subjects factors. Sample sizes for each experiment were determined by power analysis on pilot data. In all analyses, significance was evaluated at p < 0.05 and power at 1 − β ≥ 0.8. In all graphical representation, the group means were derived by averaging over time and then averaging across groups. Error bars reflect the between-groups SEM.
Results
All results are reported using flexion duration as the outcome. Previous work has demonstrated that this measure is highly sensitive to spinal cord learning and metaplastic inhibition (Grau et al., 1998, 2006). Here, as in previous studies, a failure to learn was accompanied by an increase in response number (Grau et al., 2006). This is important because it suggests that the failure to learn does not reflect a performance deficit. Because some readers may wish to examine the relationship between flexion duration and performance, the response number data have been made available in the supplemental material (available at www.jneurosci.org).
Group I mGluRs are necessary for metaplastic inhibition of spinal learning
The two group I mGluRs subtypes, mGluR1 and mGluR5, have similar roles in hippocampal plasticity (Gubellini et al., 2003); however, there are reports of divergent effects on some forms of spinal plasticity (El Manira et al., 2002; Mills et al., 2002; Kettunen et al., 2003). Given these observations, it is difficult to predict whether mGluR1 and mGluR5 would have the same or different roles in metaplasticity of spinal learning. We addressed this issue by independently testing the impact of both a mGluR1 and mGluR5 antagonist. For both experiments, drug was delivered intrathecally before metaplastic inhibition of spinal learning with uncontrollable shock. All subjects were tested 24 h later, a time point that is associated with a robust spinal learning deficit under these stimulus conditions (Crown et al., 2002b).
Impact of a mGluR1 antagonist
To test whether mGluR1 is necessary for inhibiting spinal learning, we used the noncompetitive antagonist CPCCOEt at doses that are known to affect spinal nociceptive plasticity (Neugebauer et al., 1999). Immediately after drug administration, subjects were given 6 min of uncontrollable shock (0 or 1.5 mA) to the tail in a balanced, factorial design (n = 6), and spinal learning was evaluated 24 h later.
To determine whether drug treatment affected the baseline capacity to make a response, we evaluated the shock intensity needed to generate a comparable (0.4 N) flexion force across groups and the duration of the first flexion response at the start of testing (n = 6 subjects/group). The mean shock intensity ranged from 0.66 (±0.07) to 0.76 (±0.06) mA and the mean duration of the initial response (+SE) ranged from 0.14 (±0.01) to 0.16 (±0.01) s. Independent ANOVAs failed to reveal significant main effects or interactions on either measure, indicating that the capacity to make a response was not significantly different across groups, all F values <2.84, p > 0.05
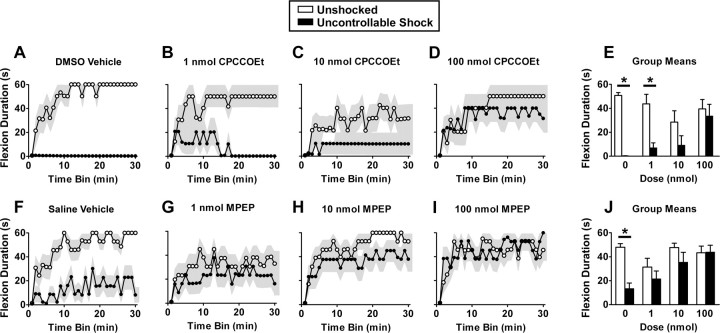
The impact of the mGluR1 antagonist CPCCOEt on metaplasticity of spinal learning is depicted in Figure 1A–E. Vehicle-treated subjects learned to maintain the leg in a flexed position, increasing flexion duration over time, whereas uncontrollable shock produced a metaplastic inhibition of spinal learning (Fig. 1A). The mGluR1 antagonist blocked induction of this learning deficit in a dose-dependent manner (Fig. 1A–E). Confirming these observations, an ANOVA on flexion duration revealed a significant main effect of shock (F(1,40) = 26.67; p < 0.0001). The interactions for shock by dose (F(3,40) = 3.21; p < 0.05) and dose by time (F(87,1160) = 1.31; p < 0.05) also reached significance. No other terms reached significance (all F values <1.30; p > 0.05).
Figure 1.
Group I mGluRs are necessary for metaplastic inhibition of spinal learning. Unshocked subjects demonstrated typical spinal learning, increasing flexion duration over the 30 min testing interval (A, F). Brief exposure to uncontrollable shock produced a deficit in spinal learning that was apparent at test at 24 h (A, F). Antagonists to the group I mGluRs, mGluR1 (CPCCOEt in 100% DMSO) (A–E) or GluR5 (MPEP in 0.9% saline) (F–J) restored spinal learning in dose-dependent manner. A–D and F–I depict performance over time; E and J depict group means. The shaded region represents SEM over time; error bars represent SEM for group means (n = 6 subjects/group for CPCCOEt; n = 8 subjects/group for MPEP). *p < 0.05 from unshocked.
Impact of a mGluR5 antagonist
To test the role of mGluR5, we delivered the specific antagonist MPEP (Tocris) to spinalized subjects (n = 8) before uncontrollable shock. Immediately after drug administration, subjects were given 6 min of uncontrollable shock (0 or 1.5 mA) to the tail in a balanced, factorial design. Twenty-four hours later, subjects were prepared as described in Materials and Methods, and spinal learning was evaluated.
To evaluate whether drug treatment affected the capacity to perform the target response, we analyzed the duration of the initial response and the shock intensity necessary to induce a 0.4 N change in flexion force. The duration of the initial response (+SE) ranged from 0.14 (±0.01) to 0.18 (±0.02) s and the shock intensity necessary to elicit a 0.4 N flexion force (+SE) ranged from 0.57 (±0.06) to 0.67 (±0.04) mA. Independent ANOVAs failed to reveal any significant main effects or interactions (all F values <4.0; p > 0.05).
The impact of MPEP on flexion duration is depicted in Figure 1F–J. Saline-treated, unshocked animals demonstrated normal spinal learning (Fig. 1F). Animals that were given saline and uncontrollable shock had impaired learning, demonstrating shock-induced metaplastic inhibition of spinal learning (Fig. 1F). MPEP blocked the shock-induced learning deficit in a dose-dependent manner, restoring the capacity for spinal learning to the level of the unshocked group (Fig. 1F–J). Confirming these observations, an ANOVA on flexion duration revealed a significant main effect of shock (F(1,56) = 4.57; p < 0.05). Both the main effect of dose and the shock by dose interaction reached significance (both F(3,56) > 2.76; p < 0.05). In addition, the main effect of time (F(29,1624) = 13.30; p < 0.001) reached significance. No other main effects or interactions were significant (all F values <1.32; p > 0.05).
Antagonism of mGluR1 but not mGluR5 facilitates acquisition of spinal learning
The observation that mGluR1 and mGluR5 antagonists restore spinal learning suggests that exposure to uncontrollable stimulation engages a group I mGluR-mediated process that inhibits spinal learning. To further test this hypothesis, we asked whether an antagonist would foster acquisition of the learned response. Subjects were given either CPCCOEt or vehicle immediately followed by spinal training. Trend analysis was used to statistically evaluate the slope of acquisition over time. As shown in Figure 2A, CPCCOEt fostered acquisition of the learned response. As in previous studies (Gómez-Pinilla et al., 2007), facilitation was most evident in the first 5 min, before asymptotic performance. An ANOVA revealed a significant effect of time (F(29,406) = 15.31; p < 0.0001). Although neither the main effect of drug treatment, nor the time by drug interaction were significant (both F values <1.28; p > 0.05), polynomial contrast revealed a significant linear component for the time by drug interaction over the full training interval (F(1,406) = 10.34). Neither the quadratic nor cubic contrasts were significant (both F values <1.53). The significant linear component indicates that the acquisition curve for the CPCCOEt group had a steeper overall slope than the vehicle-treated control. To test whether this steeper slope was driven by step function-like changes in learning in individual subjects, we performed a t test on the time until the first 60 s flexion duration (Gallistel et al., 2004). This analysis failed to yield a significant effect, suggesting that the significant increase in the slope of the learning curves in CPCCOEt subjects was attributable to increases in slope of individual learning curves. Graphical analysis confirmed this (data not shown). MPEP, however, did not have a significant effect on spinal learning (Fig. 2B) (all p > 0.05). Together, the mGluR antagonist findings indicate that mGluR1 plays a direct role in acquisition of spinal cord learning, whereas both mGluR1 and mGluR5 play a role in metaplastic inhibition of spinal cord learning. This suggests that acquisition of spinal learning involves pathways that are differentially engaged by mGluR1 and mGluR5, whereas metaplastic inhibition involves common pathways. PKC activation is one well established common pathway activated by both mGluR1 and mGluR5 (for review, see Neugebauer, 2002) providing a potential target for restoring spinal cord plasticity.
Figure 2.
mGluR1 but not mGluR5 antagonism acutely facilitates acquisition of spinal learning. The mGluR1 antagonist CPCCOEt (in 100% DMSO) increased the rate of spinal learning when subjects were tested immediately after drug exposure (A). This effect was most pronounced in the first 5 min of testing, a time point that is associated with molecular plasticity in spinal learning (Gómez-Pinilla et al., 2007). The mGluR5 antagonist MPEP (in 0.9% saline) did not improve spinal learning (B). The shaded region represents SEM over time (n = 8 subjects/group).
Activation of group I mGluRs generates lasting inhibition of spinal learning
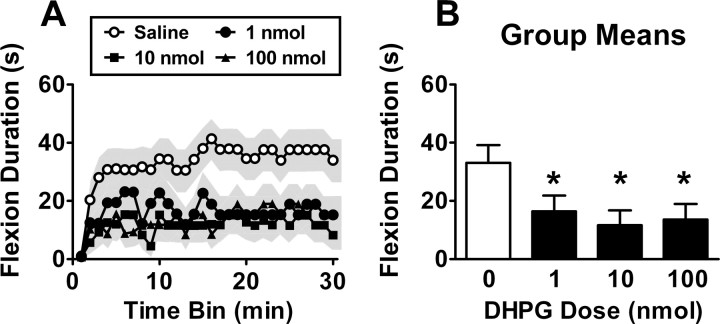
The antagonist experiments indicate that group I mGluRs are necessary for metaplastic inhibition of spinal cord learning. To test whether engaging these receptors is sufficient to inhibit spinal learning, we used the agonist DHPG. This compound has been shown in hippocampal culture to induce internalization of iGluRs and LTD, suggesting that it can have lasting effects on cellular plasticity. If mGluRs underlie metaplastic inhibition of spinal learning, DHPG should induce a lasting effect that mirrors the established time course of shock-induced inhibition (Crown et al., 2002b). We therefore delivered DHPG at a range of doses (n = 16) and tested spinal learning 24 h after.
To determine whether DHPG affected response vigor, we analyzed the shock intensity necessary to elicit a 0.4 N change in flexion force and the duration of the initial response. The mean shock intensity ranged from 0.53 (±0.04) to 0.73 (±0.08) mA and the mean duration of the initial response (+SE) ranged from 0.14 (±0.01) to 0.17 (+0.01) s. Independent ANOVAs failed to reveal any significant main effects or interactions on either outcome (all F values <2.76; p > 0.05).
The impact of drug treatment on spinal learning is illustrated in Figure 3. Saline-treated subjects learned to maintain the leg in a flexed position over time (Fig. 3A). DHPG delivered 24 h before testing impaired spinal instrumental learning (Fig. 3A,B). Confirming these effects, an ANOVA revealed a significant main effect of drug (F(3,56) = 3.02; p < 0.05). A Duncan's post hoc analysis on the main effect of drug revealed that the vehicle-treated group exhibited superior performance (longer flexion durations) relative to the DHPG-treated groups (p < 0.05). No other main effects or interactions reached significance (all p > 0.05).
Figure 3.
A group I mGluR agonist induces lasting metaplastic inhibition of spinal learning. Subjects that were given DHPG (in 0.9% saline) had impaired spinal learning at test at 24 h, as evident by impaired performance over time (A) and group means (B). The shaded region represents SEM over time; error bars represent SEM for group means (n = 16 subjects/dose). *p < 0.05 from vehicle.
PKC activation by stimulus conditions that inhibit spinal learning
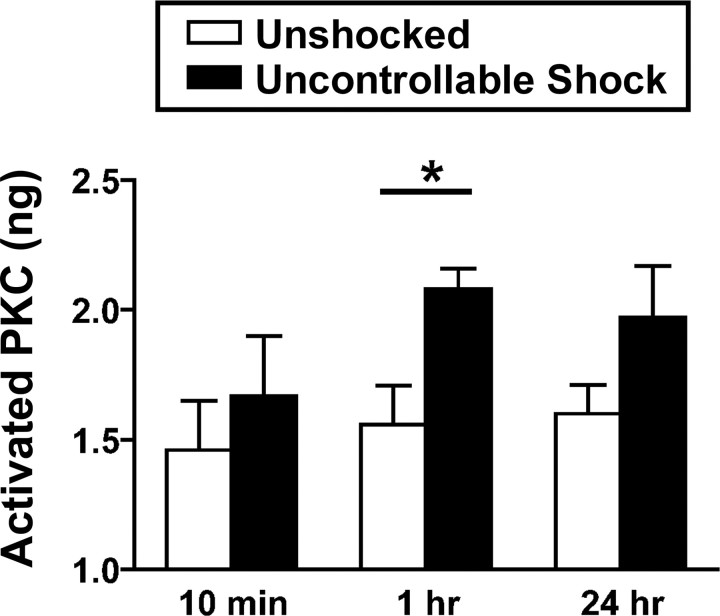
The pharmacological data provide links between group I mGluRs and metaplastic inhibition of spinal learning. The most striking finding was that a single bolus of DHPG produced a lasting deficit in spinal learning that was apparent 24 h later. To our knowledge, this is the first drug manipulation identified that directly inhibits spinal learning for a lasting interval. This suggests that group I mGluRs engage cellular changes that produce lasting alterations of spinal plasticity. Group I mGluRs are known to activate a G-protein-linked cascade involving PKC (Hermans and Challiss, 2001). To test whether PKC activation is associated with inhibition of spinal learning, we examined whether uncontrollable shock increases PKC activation using an ELISA. Subjects (N = 24) received uncontrollable shock or nothing and the L4–S2 tissue was collected 10 min, 1 h, or 24 h later. Previous work has shown that instrumental learning occurs within the L4–S2 segments (Crown et al., 2002b; Liu et al., 2005).
Shock treatment increased PKC activity in the lumbar-sacral cord (Fig. 4). The main effect of shock was significant (F(1,18) = 6.84; p < 0.05). Neither the main effect of time nor its interaction with shock treatment reached statistical significance (both F values <1.27; p > 0.05). Orthogonal planned comparisons of the shocked and unshocked groups at each time point revealed a robust effect at 1 h (F(1,6) = 8.77; p < 0.05) but not at 10 min or 24 h (both F values <2.54; p > 0.05).
Figure 4.
Activation of PKC in the spinal cord by uncontrollable shock. ELISA revealed that uncontrollable shock produced a significant increase in PKC activity that reached statistical significance by 1 h after shock exposure. Error bars represent SEM for group means (n = 4 subjects/group). *p < 0.05.
PKC activity is necessary for metaplastic inhibition of spinal learning
The finding of PKC activation after uncontrollable shock suggests that PKC may play a role in metaplastic inhibition of spinal learning. To directly test this hypothesis, we delivered two structurally distinct PKC inhibitors and tested for restoration of spinal cord learning. We first used the staurosporine-derivative BIM, GF 109203X, at a range of doses that have been shown to inhibit PKC (Herbert et al., 1990; Toullec et al., 1991) and affect pain behavior when administered intrathecally (Yashpal et al., 1995; Hua et al., 1999; Heinke and Sandkühler, 2005). To confirm PKC specificity, we performed an independent experiment with the structurally distinct PKC inhibitor chelerythrine at a similarly effective dose (Hua et al., 1999). The capacity for spinal learning was tested 24 h later.
To determine whether drug treatment affected baseline behavioral reactivity, we examined both the shock intensity needed to elicit a 0.4 N flexion force and the duration of the first flexion response. Neither BIM nor chelerythrine affected performance on either measure (all F values <3.0; p > 0.05).
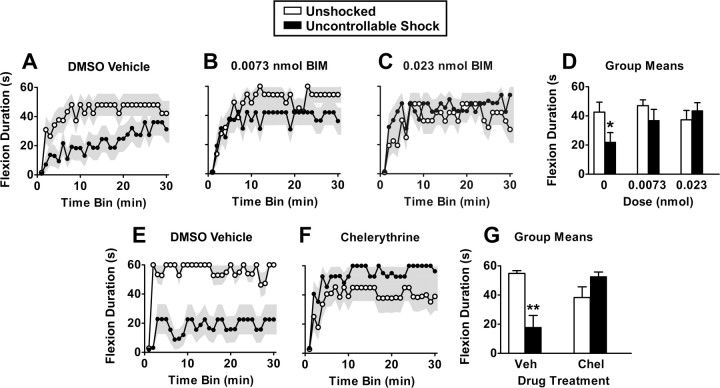
Unshocked controls exhibited a progressive increase in flexion duration indicative of instrumental learning. Rats that received the vehicle before shock treatment exhibited poor learning (Fig. 5A,E). Treatment with either BIM (Fig. 5A–D) or chelerythrine (Fig. 5E–G) attenuated this shock-induced learning deficit. ANOVA on the BIM data confirmed that the linear component of the drug by shock interaction was significant (F(1,48) = 5.28; p < 0.05). There was also a significant effect of time (F(29,1392) = 19.32; p < 0.0001). No other variable approached statistical significance (all F values <1.69; p > 0.05). Post hoc comparisons of the group means using Duncan's multiple range test confirmed that vehicle-treated shocked subjects differed from the unshocked controls (both vehicle and BIM, 0.0073 nmol) and the shocked group that received 0.023 nmol of BIM. No other differences were significant (all p > 0.05).
Figure 5.
PKC activation is necessary for metaplastic inhibition of spinal learning. Uncontrollable shock produced a metaplastic inhibition of spinal learning at testing at 24 h (A, E). Spinal learning was restored by two mechanistically distinct PKC inhibitors BIM (A–D) and chelerythrine (E–G) delivered in 100 and 10% DMSO, respectively. Group means are depicted in D and G. The shaded region represents SEM over time; error bars represent SEM for group means (n = 8 subjects/group). *p < 0.05 from all groups except for shocked subjects in middle dose; **p < 0.05 from all other groups.
ANOVA on the chelerythrine data revealed a significant linear effect of time (F(1,28) = 19.97; p < 0.001). There was also a significant drug by shock interaction (F(1,28) = 17.20; p < 0.001). Post hoc comparisons with Duncan's multiple range test revealed that the vehicle-treated, shocked subjects had significantly lower performance than all other groups.
To test for significant differences between the two inhibitors, we merged the datasets from the BIM and chelerythrine experiments and then used ANOVA to test for a differential effect of the PKC inhibitors. There were no significant differences between the two drugs (all p > 0.05), suggesting that the different PKC inhibitors were similarly effective.
The findings provide strong support for a role of PKC in metaplastic inhibition of spinal learning. We found similar effects using both BIM and chelerythrine, two mechanistically distinct, selective PKC inhibitors (Herbert et al., 1990; Toullec et al., 1991) that have well documented behavioral and biochemical effects when administered in the spinal cord (Yashpal et al., 1995; Hua et al., 1999; Granados-Soto et al., 2000; Guo et al., 2004). However, this does not exclude the possibility that other kinases may also contribute to these effects, because off-target drug effects or reduced PKC activity could affect signaling across several interlinked pathways.
PKC activation is not necessary for spinal learning
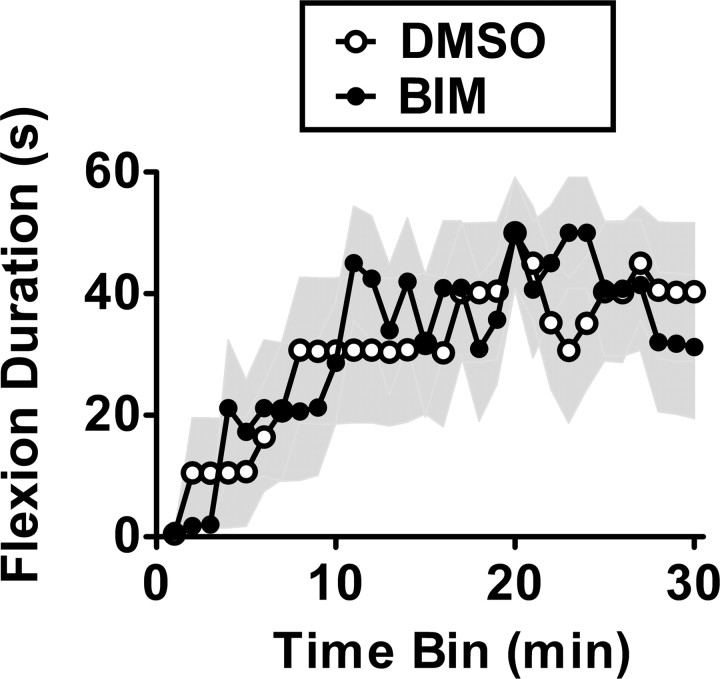
Group I mGluR antagonism selectively blocked the long-term consequences of uncontrollable stimulation, but did not impair learning. Having demonstrated that PKC inhibition also attenuates the consequences of uncontrollable stimulation, we tested its effect on spinal learning. We delivered BIM (0.023 nmol) and assessed spinal learning 15 min later. Vehicle-treated rats exhibited a progressive increase in flexion duration over the 30 min of training (Fig. 6). BIM did not significantly alter the learning over time or the group means (31.8 ± 9.6 for BIM vs 30.7 ± 10.6 for vehicle) (all p > 0.9). This suggests that PKC is involved in metaplastic inhibition of spinal learning but has little effect on the rate of spinal learning itself.
Figure 6.
PKC activation does not affect acquisition of spinal learning. The rate of acquisition of spinal learning was not significantly affected by the PKC inhibitor BIM (in 100% DMSO). The shaded region represents SEM over time (n = 8 subjects/group).
Group I mGluRs inhibit spinal learning through PKC activation
Given that group I mGluRs and PKC were both implicated in metaplastic inhibition of spinal learning, we tested whether mGluR-induced inhibition of spinal learning depends on PKC. Previous studies suggest that DHPG activates PKC in a BIM-reversible manner (Camodeca et al., 1999; Fundytus et al., 2001; Giles et al., 2007). To test whether metaplastic inhibition of spinal learning by DHPG (Fig. 3) depends on PKC, we delivered BIM 15 min before DHPG and assayed spinal learning 24 later.
Spinally transected rats received BIM (0.023 nmol) or its vehicle (100% DMSO), followed by DHPG (100 nmol) or its vehicle (0.9% saline). Baseline flexion vigor, and instrumental learning, was then assessed as described above. Subjects that received two vehicle injections or BIM plus saline were able to acquire the instrumental response (Fig. 7A). Treatment with DMSO plus DHPG produced a marked learning deficit (Fig. 7B). This finding provided an independent replication of the earlier data in which a deficit was observed after administration of DHPG. Pretreatment with the PKC inhibitor BIM blocked the detrimental effects of DHPG (Fig. 7B,C). An ANOVA on the flexion duration data yielded a significant trials by BIM by DHPG three-way interaction (p < 0.05). Post hoc comparisons (Duncan's) confirmed that the DHPG-treated group had significantly lower performance than the other three (p < 0.05). No other differences were significant.
Figure 7.
Group I mGluRs inhibit spinal learning via a PKC-dependent mechanism. Replicating previous findings (Fig. 3), the group I mGluR agonist DHPG was found to induce metaplastic inhibition of spinal learning at test at 24 h (A, B). Delivery of the PKC inhibitor BIM (in 100% DMSO) before DHPG (in 0.9% saline) blocked this effect, restoring the capacity for spinal learning (B, C). To achieve a balanced design, all subjects received both vehicles. The shaded region represents SEM over time; error bars represent SEM for group means (n = 10 subjects/group).
Together with the previous experiments, the findings strongly suggest that metaplastic inhibition of spinal learning involves group I mGluR activation and engagement of intracellular pathways involving PKC.
Discussion
The present findings demonstrate that group I mGluR and PKC activity alter the requirements for future synaptic modification in the spinal cord. When exposed to uncontrollable shock, the spinal cord develops a lasting impairment in behavioral plasticity (Grau et al., 1998; Crown and Grau, 2001; Crown et al., 2002b; Ferguson et al., 2003, 2006; Joynes and Grau, 2004; Patton et al., 2004; Washburn et al., 2007). Pharmacological activation of mGluRs mimicked the effects of uncontrollable shock, whereas mGluR antagonism at the time of training facilitated learning. On stimulation, mGluRs act through intracellular signaling pathways to elevate PKC activity (Pisani et al., 1997; Ugolini et al., 1997; Skeberdis et al., 2001). Uncontrollable shock increased PKC activity in the spinal cord, and the long-term effects of uncontrollable shock were blocked by group I mGluR antagonists and a PKC inhibitor. In parallel, the mGluR–PKC pathway was implicated in pharmacologically induced metaplasticity because spinal cord neurons exposed to an mGluR agonist and a PKC inhibitor together maintained intact learning ability.
DHPG is the first drug demonstrated to produce a lasting impairment of spinal learning. Manipulation of other spinal receptors, including iGluRs, serotonergic, noradrenergic, GABAergic, and opioid systems, has only transient effects (for review, see Grau et al., 2006). Long-lasting synaptic modifications in the CNS commonly involve potentiation or depression of iGluR responses through posttranslational receptor modification and trafficking. Group I mGluR agonists produce long-lasting PKC-dependent potentiation of NMDAR and AMPAR responses in spinal cord motoneurons (Ugolini et al., 1997). In the dorsal horn of transverse spinal slices, group I mGluRs signal through PKC to increase tyrosine phosphorylation of NMDAR subunit NR2B, which is associated with increased channel conductance and calcium influx (Ali and Salter, 2001; Guo et al., 2004). NMDAR potentiation through mGluR–PKC signaling is observed in hippocampal preparations and Xenopus oocyte systems as well (Aniksztejn et al., 1992; Skeberdis et al., 2001). The correspondence between the present findings and these electrophysiological and biochemical data provides avenues for additional investigation; however, predictions are not straightforward, because the specific neuronal circuits underlying flexion response learning are not known. mGluR activation can lead to simultaneous potentiation and depression in distinct parts of the spinal circuit as well as changes in cellular excitability and network properties (Jones and Headley, 1995; Zhong et al., 2000; Marchetti et al., 2003; Clem et al., 2008), although not all of these effects involve PKC (Heinke and Sandkühler, 2005).
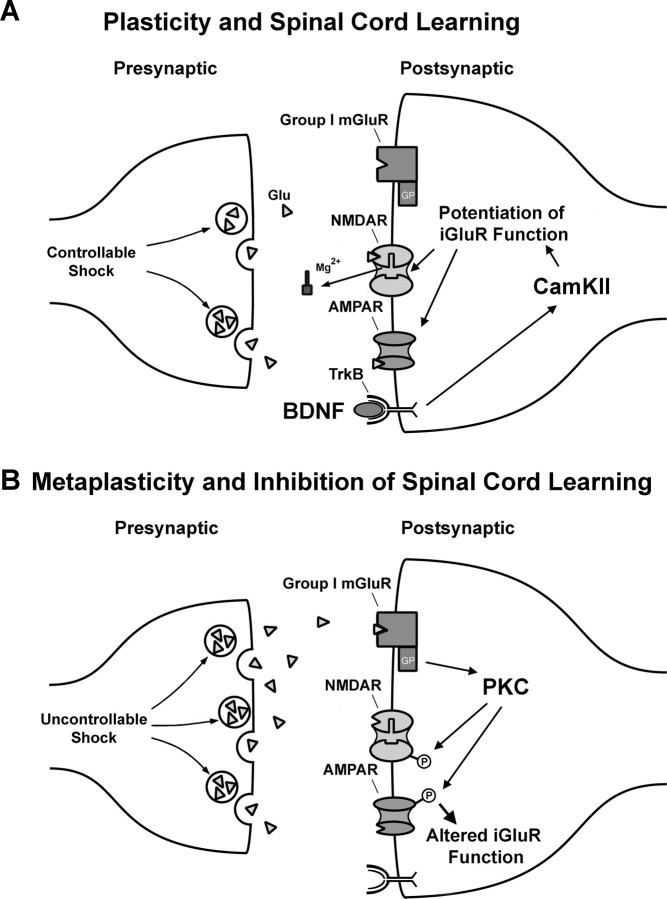
A hypothetical postsynaptic mechanism for metaplasticity of spinal learning
Based on the hypothesis that mGluRs control metaplasticity of spinal learning by altering NMDAR function, we can propose a cellular/molecular model that provides numerous targets for restoring adaptive spinal plasticity (Fig. 8). According to the model, response-contingent shock yields a patterned, highly regulated release of glutamate that activates NMDARs (Joynes et al., 2004) and downstream signaling pathways (Fig. 8A). Spinal learning performance correlates with the mRNA levels of calcium/calmodulin-dependent kinase II (CaMKII) and cAMP response element binding protein (CREB), and BDNF facilitates spinal learning (Baumbauer et al., 2007b; Gómez-Pinilla et al., 2007). These molecules are linked to NMDAR-mediated long-term potentiation (Yin and Tully, 1996; Poncer et al., 2002; Bramham and Messaoudi, 2005), suggesting a mechanism for spinal learning with response-contingent shock.
Figure 8.
A proposed cellular/molecular model of the plasticity (A) and inhibitory metaplasticity (B) of spinal cord learning. Previous work has revealed that iGluR activation is necessary for spinal learning and that molecules that facilitate iGluR-mediated plasticity, such as BDNF and CaMKII, also facilitate spinal learning (for details, see text). Together, these data suggesting that the pattern of glutamate release during response-contingent shock induces iGluR-mediated plasticity, which supports learning (A). However, iGluRs are also implicated in inhibition of spinal learning by uncontrollable nociceptive stimulation. Based on the present results, we propose that high levels of glutamate produced by uncontrollable shock activate mGluRs, resulting in PKC activation and long-term alteration of iGluR function resulting in a spinal learning deficit (B).
NMDARs detect coincident presynaptic and postsynaptic activity and allow calcium influx eventually leading to altered synaptic strength. Stimulation above or below a certain threshold produces potentiation or depression, respectively, by generating different patterns of postsynaptic calcium influx (Abraham and Bear, 1996). If, as suggested above, NMDAR conductances are altered by increased PKC phosphorylation after DHPG or uncontrollable shock, the threshold for synaptic modification may shift downward (Fig. 8B). Spinal cord synapses would lose the ability to encode more subtle stimulation patterns and adapt to the imposed response contingency. Blockade of these changes with antagonists of either mGluRs (Fig. 1) or NMDAR (Ferguson et al., 2006) protects learning capacity by preventing this inhibitory metaplasticity.
Links to spinal nociception
Spinal mGluRs are involved in nociceptive processing (Coderre and Melzack, 1992; Fisher and Coderre, 1996a,b; Stanfa and Dickenson, 1998; Neugebauer et al., 1999; Bordi and Ugolini, 2000; Karim et al., 2001; Fisher et al., 2002; Fundytus et al., 2002; Mills et al., 2002; Zhang et al., 2002). Activation of group I mGluRs induces spontaneous nociceptive behaviors in rats (Fisher and Coderre, 1996a). Moreover, group I mGluR antagonists reduce nociceptive responses in a number of paradigms including sciatic nerve ligation (Fisher et al., 2002), inflammation by carrageenan/kaolin, formalin (Stanfa and Dickenson, 1998; Karim et al., 2001; Zhang et al., 2002), and intradermal capsaicin (Neugebauer et al., 1999).
The present findings suggest that spinal mGluRs not only affect nociceptive processing but also modify the capacity for spinal learning. This supports other work linking metaplastic inhibition of spinal learning with nociceptive plasticity. Inhibition of spinal learning can be induced by incision injury or intradermal carrageenan and uncontrollable shock produces a tactile hyperreactivity (Ferguson et al., 2006; Young et al., 2007). In addition, inhibition of spinal learning involves receptor systems known to modulate pain, including neurokinin (Baumbauer et al., 2007a), κ-opioid (Joynes and Grau, 2004; Washburn et al., 2008), GABAA (Ferguson et al., 2003), and 5-HT receptors (Crown and Grau, 2005). Removing descending inhibition with a dorsolateral funiculus transection increases nociceptive reactivity (Eide and Hole, 1993; Liu et al., 1997) and renders spinal learning vulnerable to metaplastic inhibition (Crown and Grau, 2005). This suggests that descending inhibitory tracts passing through the dorsolateral funiculus help to maintain the balance of spinal plasticity, preventing both plasticity within pain pathways and metaplastic inhibition of spinal learning.
Plasticity in NMDAR-mediated synaptic transmission provides a framework for understanding the relationship between nociception and metaplastic inhibition. Both mGluR and PKC have been linked to the enhancement of nociceptive reactivity observed after peripheral inflammation and the sensitization of spinal nociceptive pathways (Woolf, 1983; Coderre and Melzack, 1985, 1992; Fisher and Coderre, 1996b; Fundytus et al., 2002). Enhanced nociception in models of neuropathic pain is attributed to an increased contribution of the NMDAR to sensory processing in the spinal cord (Willis, 2001). Phosphorylation of the NR2B subunit increases channel open probability and raises the overall excitability of the system. Because uncontrollable stimulation also enhances nociceptive reactivity, it is possible that these treatments induce overlapping mechanisms. Thus, modified NMDAR currents could both enhance immediate responses to stimulation and alter the stimulus threshold for spinal plasticity, negatively impacting the ability to produce new adaptive responses.
Implications for management of metaplasticity after spinal cord injury
Damage to descending inhibitory tracts after spinal cord injury yields dysregulation of spinal plasticity, rendering spinal learning vulnerable to metaplastic inhibition (Crown and Grau, 2005). Alterations in spinal metaplasticity could have profound effects on recovery of function by altering the ability of the spinal cord to regain proper function (Edgerton et al., 2001; Hook and Grau, 2007; Courtine et al., 2008). Recent findings indicate that uncontrollable shock undermines recovery of function after spinal contusion injury (Grau et al., 2004; Hook et al., 2007). Attempts to control nociceptive transmission with an opioid yielded little benefit and may have enhanced histopathological damage (Hook et al., 2007).
There is variability in the rate and level to which patients recover after spinal cord injury. The factors dictating recovery have yet to be fully elucidated. One critical variable may be metaplastic modulation of glutamatergic plasticity. Glutamate is widely thought to exacerbate cell death and associated functional losses after spinal cord injury (for review, see Beattie, 2004; Park et al., 2004). Recent data indicate that glutamate-mediated cell death may involve plasticity and trafficking of iGluRs (Hermann et al., 2001; Beattie et al., 2002; Ogoshi et al., 2005; Stellwagen et al., 2005; Ferguson et al., 2008). Moreover, the group I mGluRs have been implicated in both neuropathic pain and functional losses after spinal cord injury (Mukhin et al., 1996; Agrawal et al., 1998; Mills and Hulsebosch, 2002; Mills et al., 2002). The precise role of spinal metaplasticity in these effects remains a topic for additional study.
Conclusion and future directions
One advantage of a simple spinal learning task is that it provides a high-throughput model for screening experimental therapeutics that alter spinal metaplasticity. To the extent that metaplasticity plays a role in recovery of function after spinal cord injury, this could prove to be a powerful tool for the field of spinal cord injury research (Grau et al., 2006). Using a simple spinal learning task, the present findings suggest that group I mGluRs generate metaplastic inhibition of spinal learning through a PKC-dependent mechanism. Based on these observations, we hypothesize that controlling mGluR induction of PKC should facilitate adaptive plasticity in the spinal cord across a broad range of injury conditions. This could yield functional benefits after spinal cord injury by promoting use-dependent plasticity.
Footnotes
This work was supported by National Institute of Health Grants NS041548 [National Institute of Neurological Disorders and Stroke (NINDS)] and HD058412 (National Institute of Child Health and Human Development) to J.W.G. A.R.F. is supported by NINDS Grant F32NS053059. We thank Todd C. Sacktor for advice about PKC inhibitors.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Agrawal SK, Theriault E, Fehlings MG. Role of group I metabotropic glutamate receptors in traumatic spinal cord white matter injury. J Neurotrauma. 1998;15:929–941. doi: 10.1089/neu.1998.15.929. [DOI] [PubMed] [Google Scholar]
- Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L, Otani S, Ben Ari Y. Quisqualate metabotropic receptors modulate NMDA currents and facilitate induction of long-term potentiation through protein kinase C. Eur J Neurosci. 1992;4:500–505. doi: 10.1111/j.1460-9568.1992.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Jr, Joynes RL. Intrathecal administration of neurokinin 1 and neurokinin 2 receptor antagonists undermines the savings effect in spinal rats seen in an instrumental learning paradigm. Behav Neurosci. 2007a;121:186–199. doi: 10.1037/0735-7044.121.1.186. [DOI] [PubMed] [Google Scholar]
- Baumbauer KM, Young EE, Hoy KC, Jr, Abood A, Joynes RL. Administration of a Ca-super2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor prevents the learning deficit observed in spinal rats after noncontingent shock administration. Behav Neurosci. 2007b;121:570–578. doi: 10.1037/0735-7044.121.3.570. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Beattie MS. Inflammation and apoptosis: linked therapeutic targets in spinal cord injury. Trends Mol Med. 2004;10:580–583. doi: 10.1016/j.molmed.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Bevan S, Parker D. Metaplastic facilitation and ultrastructural changes in synaptic properties are associated with long-term modulation of the lamprey locomotor network. J Neurosci. 2004;24:9458–9468. doi: 10.1523/JNEUROSCI.3391-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigbee AJ, Crown ED, Ferguson AR, Roy RR, Tillakaratne NJ, Grau JW, Edgerton VR. Two chronic motor training paradigms differentially influence acute instrumental learning in spinally transected rats. Behav Brain Res. 2007;180:95–101. doi: 10.1016/j.bbr.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi F, Ugolini A. Involvement of mGluR(5) on acute nociceptive transmission. Brain Res. 2000;871:223–233. doi: 10.1016/s0006-8993(00)02467-7. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Buerger AA, Fennessy A. Learning of leg position in chronic spinal rats. Nature. 1970;225:751–752. doi: 10.1038/225751a0. [DOI] [PubMed] [Google Scholar]
- Camodeca N, Breakwell NA, Rowan MJ, Anwyl R. Induction of LTD by activation of group I mGluR in the dentate gyrus in vitro. Neuropharmacology. 1999;38:1597–1606. doi: 10.1016/s0028-3908(99)00093-3. [DOI] [PubMed] [Google Scholar]
- Church RM, Getty DJ, Lerner ND. Duration discrimination by rats. J Exp Psychol Anim Behav Process. 1976;2:303–312. doi: 10.1037//0097-7403.2.4.303. [DOI] [PubMed] [Google Scholar]
- Clem RL, Celikel T, Barth AL. Ongoing in vivo experience triggers synaptic metaplasticity in the neocortex. Science. 2008;319:101–104. doi: 10.1126/science.1143808. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. Increased pain sensitivity following heat injury involves a central mechanism. Behav Brain Res. 1985;15:259–262. doi: 10.1016/0166-4328(85)90181-0. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Preserving and restoring behavioral potential within the spinal cord using an instrumental training paradigm. J Neurophysiol. 2001;86:845–855. doi: 10.1152/jn.2001.86.2.845. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Evidence that descending serotonergic systems protect spinal cord plasticity against the disruptive effect of uncontrollable stimulation. Exp Neurol. 2005;196:164–176. doi: 10.1016/j.expneurol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord. II. Evidence for central mediation. Physiol Behav. 2002a;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord: IV. Induction and retention of the behavioral deficit observed after noncontingent shock. Behav Neurosci. 2002b;116:1032–1051. doi: 10.1037//0735-7044.116.6.1032. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Leon RD, Harkema SJ, Hodgson JA, London N, Reinkensmeyer DJ, Roy RR, Talmadge RJ, Tillakaratne NJ, Timoszyk W, Tobin A. Retraining the injured spinal cord. J Physiol. 2001;533:15–22. doi: 10.1111/j.1469-7793.2001.0015b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide PK, Hole K. The role of 5-hydroxytryptamine (5-HT) receptor subtypes and plasticity in the 5-HT systems in the regulation of nociceptive sensitivity. Cephalalgia. 1993;13:75–85. doi: 10.1046/j.1468-2982.1993.1302075.x. [DOI] [PubMed] [Google Scholar]
- El Manira A, Kettunen P, Hess D, Krieger P. Metabotropic glutamate receptors provide intrinsic modulation of the lamprey locomotor network. Brain Res Brain Res Rev. 2002;40:9–18. doi: 10.1016/s0165-0173(02)00184-4. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Washburn SN, Crown ED, Grau JW. GABA(A) receptor activation is involved in noncontingent shock inhibition of instrumental conditioning in spinal rats. Behav Neurosci. 2003;117:799–812. doi: 10.1037/0735-7044.117.4.799. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Cell death after spinal cord injury is exacerbated by rapid TNSα-inducing trafficking of GluR2-lacking AMPARs to the plasma membrane. J Neurosci. 2008;28:11391–11400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ. Comparison of nociceptive effects produced by intrathecal administration of mGluR agonists. Neuroreport. 1996a;7:2743–2747. doi: 10.1097/00001756-199611040-00067. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ. The contribution of metabotropic glutamate receptors (mGluRs) to formalin-induced nociception. Pain. 1996b;68:255–263. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- Fisher K, Lefebvre C, Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 2002;73:411–418. doi: 10.1016/s0091-3057(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Yashpal K, Chabot JG, Osborne MG, Lefebvre CD, Dray A, Henry JL, Coderre TJ. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR(1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 2001;132:354–367. doi: 10.1038/sj.bjp.0703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Osborne MG, Henry JL, Coderre TJ, Dray A. Antisense oligonucleotide knockdown of mGluR1 alleviates hyperalgesia and allodynia associated with chronic inflammation. Pharmacol Biochem Behav. 2002;73:401–410. doi: 10.1016/s0091-3057(02)00831-6. [DOI] [PubMed] [Google Scholar]
- Gallistel CR, Fairhurst S, Balsam P. The learning curve: implications of a quantitative analysis. Proc Natl Acad Sci U S A. 2004;101:13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles PA, Trezise DJ, King AE. Differential activation of protein kinases in the dorsal horn in vitro of normal and inflamed rats by group I metabotropic glutamate receptor subtypes. Neuropharmacology. 2007;53:58–70. doi: 10.1016/j.neuropharm.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gómez-Pinilla F, Huie JR, Ying Z, Ferguson AR, Crown ED, Baumbauer KM, Edgerton VR, Grau JW. BDNF and learning: evidence that instrumental training promotes learning within the spinal cord by up-regulating BDNF expression. Neuroscience. 2007;148:893–906. doi: 10.1016/j.neuroscience.2007.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Soto V, Kalcheva I, Hua X, Newton A, Yaksh TL. Spinal PKC activity and expression: role in tolerance produced by continuous spinal morphine infusion. Pain. 2000;85:395–404. doi: 10.1016/S0304-3959(99)00281-X. [DOI] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behav Neurosci. 1998;112:1366–1386. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Washburn SN, Hook MA, Ferguson AR, Crown ED, Garcia G, Bolding KA, Miranda RC. Uncontrollable stimulation undermines recovery after spinal cord injury. J Neurotrauma. 2004;21:1795–1817. doi: 10.1089/neu.2004.21.1795. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behav Cogn Neurosci Rev. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Costa C, Tropepi D, Bernardi G, Conquet F, Calabresi P. Corticostriatal LTP requires combined mGluR1 and mGluR5 activation. Neuropharmacology. 2003;44:8–16. doi: 10.1016/s0028-3908(02)00214-9. [DOI] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinke B, Sandkühler J. Signal transduction pathways of group I metabotropic glutamate receptor-induced long-term depression at sensory spinal synapses. Pain. 2005;118:145–154. doi: 10.1016/j.pain.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Grosshans DR, Coultrap SJ, Jones JP, Dobelis P, Browning MD, Staley KJ. NMDA receptor trafficking at recurrent synapses stabilizes the state of the CA3 network. J Neurophysiol. 2007;98:2818–2826. doi: 10.1152/jn.00346.2007. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Grau JW. An animal model of functional electrical stimulation: evidence that the central nervous system modulates the consequences of training. Spinal Cord. 2007;45:702–712. doi: 10.1038/sj.sc.3102096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW. The impact of morphine after a spinal cord injury. Behav Brain Res. 2007;179:281–293. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behav Neurosci. 2008;122:233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Chen P, Yaksh TL. Inhibition of spinal protein kinase C reduces nerve injury-induced tactile allodynia in neuropathic rats. Neurosci Lett. 1999;276:99–102. doi: 10.1016/s0304-3940(99)00818-6. [DOI] [PubMed] [Google Scholar]
- Jones MW, Headley PM. Interactions between metabotropic and ionotropic glutamate receptor agonists in the rat spinal cord in vivo. Neuropharmacology. 1995;34:1025–1031. doi: 10.1016/0028-3908(95)00055-b. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Grau JW. Instrumental learning within the spinal cord: III. Prior exposure to noncontingent shock induces a behavioral deficit that is blocked by an opioid antagonist. Neurobiol Learn Mem. 2004;82:35–51. doi: 10.1016/j.nlm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Ferguson AR, Crown ED, Patton BC, Grau JW. Instrumental learning within the spinal cord: V. Evidence the behavioral deficit observed after noncontingent nociceptive stimulation reflects an intraspinal modification. Behav Brain Res. 2003;141:159–170. doi: 10.1016/s0166-4328(02)00372-8. [DOI] [PubMed] [Google Scholar]
- Joynes RL, Janjua K, Grau JW. Instrumental learning within the spinal cord: VI. The NMDA receptor antagonist, AP5, disrupts the acquisition and maintenance of an acquired flexion response. Behav Brain Res. 2004;154:431–438. doi: 10.1016/j.bbr.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RW., IV Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettunen P, Hess D, El Manira A. mGluR1, but not mGluR5, mediates depolarization of spinal cord neurons by blocking a leak current. J Neurophysiol. 2003;90:2341–2348. doi: 10.1152/jn.01132.2002. [DOI] [PubMed] [Google Scholar]
- Liu GT, Ferguson AR, Crown ED, Bopp AC, Miranda RC, Grau JW. Instrumental learning within the rat spinal cord: localization of the essential neural circuit. Behav Neurosci. 2005;119:538–547. doi: 10.1037/0735-7044.119.2.538. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Wang R, Nie H, Zhang RX, Qiao JT, Dafny N. Effects of intrathecal monoamine antagonists on the nociceptive c-Fos expression in a lesioned rat spinal cord. Int J Neurosci. 1997;91:169–180. doi: 10.3109/00207459708986374. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Taccola G, Nistri A. Distinct subtypes of group I metabotropic glutamate receptors on rat spinal neurons mediate complex facilitatory and inhibitory effects. Eur J Neurosci. 2003;18:1873–1883. doi: 10.1046/j.1460-9568.2003.02924.x. [DOI] [PubMed] [Google Scholar]
- Mills CD, Hulsebosch CE. Increased expression of metabotropic glutamate receptor subtype 1 on spinothalamic tract neurons following spinal cord injury in the rat. Neurosci Lett. 2002;319:59–62. doi: 10.1016/s0304-3940(01)02551-4. [DOI] [PubMed] [Google Scholar]
- Mills CD, Johnson KM, Hulsebosch CE. Group I metabotropic glutamate receptors in spinal cord injury: roles in neuroprotection and the development of chronic central pain. J Neurotrauma. 2002;19:23–42. doi: 10.1089/089771502753460213. [DOI] [PubMed] [Google Scholar]
- Mukhin A, Fan L, Faden AI. Activation of metabotropic glutamate receptor subtype mGluR1 contributes to post-traumatic neuronal injury. J Neurosci. 1996;16:6012–6020. doi: 10.1523/JNEUROSCI.16-19-06012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V. Metabotropic glutamate receptors—important modulators of nociception and pain behavior. Pain. 2002;98:1–8. doi: 10.1016/s0304-3959(02)00140-9. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Chen PS, Willis WD. Role of metabotropic glutamate receptor subtype mGluR1 in brief nociception and central sensitization of primate STT cells. J Neurophysiol. 1999;82:272–282. doi: 10.1152/jn.1999.82.1.272. [DOI] [PubMed] [Google Scholar]
- Ogoshi F, Yin HZ, Kuppumbatti Y, Song B, Amindari S, Weiss JH. Tumor necrosis-factor-alpha (TNF-alpha) induces rapid insertion of Ca2+-permeable alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/kainate (Ca-A/K) channels in a subset of hippocampal pyramidal neurons. Exp Neurol. 2005;193:384–393. doi: 10.1016/j.expneurol.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- Parker D, Grillner S. Activity-dependent metaplasticity of inhibitory and excitatory synaptic transmission in the lamprey spinal cord locomotor network. J Neurosci. 1999;19:1647–1656. doi: 10.1523/JNEUROSCI.19-05-01647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BC, Hook MA, Ferguson AR, Crown ED, Grau JW. The behavioral deficit observed following noncontingent shock in spinalized rats is prevented by the protein synthesis inhibitor cycloheximide. Behav Neurosci. 2004;118:653–658. doi: 10.1037/0735-7044.118.3.653. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Enhancement of NMDA responses by group I metabotropic glutamate receptor activation in striatal neurones. Br J Pharmacol. 1997;120:1007–1014. doi: 10.1038/sj.bjp.0700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, Esteban JA, Malinow R. Multiple mechanisms for the potentiation of AMPA receptor-mediated transmission by α-Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2002;22:4406–4411. doi: 10.1523/JNEUROSCI.22-11-04406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeberdis VA, Lan J, Opitz T, Zheng X, Bennett MV, Zukin RS. mGluR1-mediated potentiation of NMDA receptors involves a rise in intracellular calcium and activation of protein kinase C. Neuropharmacology. 2001;40:856–865. doi: 10.1016/s0028-3908(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Dickenson AH. Inflammation alters the effects of mGlu receptor agonists on spinal nociceptive neurones. Eur J Pharmacol. 1998;347:165–172. doi: 10.1016/s0014-2999(98)00098-3. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Tsvyetlynska NA, Hill RH, Grillner S. Role of AMPA receptor desensitization and the side effects of a DMSO vehicle on reticulospinal EPSPs and locomotor activity. J Neurophysiol. 2005;94:3951–3960. doi: 10.1152/jn.00201.2005. [DOI] [PubMed] [Google Scholar]
- Ugolini A, Corsi M, Bordi F. Potentiation of NMDA and AMPA responses by group I mGluR in spinal cord motoneurons. Neuropharmacology. 1997;36:1047–1055. doi: 10.1016/s0028-3908(97)00103-2. [DOI] [PubMed] [Google Scholar]
- Washburn SN, Patton BC, Ferguson AR, Hudson KL, Grau JW. Exposure to intermittent nociceptive stimulation under pentobarbital anesthesia disrupts spinal cord function in rats. Psychopharmacology (Berl) 2007;192:243–252. doi: 10.1007/s00213-007-0707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn SN, Maultsby ML, Puga DA, Grau JW. Opioid regulation of spinal cord plasticity: evidence the kappa-2 opioid receptor agonist GR89696 inhibits learning within the rat spinal cord. Neurobiol Learn Mem. 2008;89:1–16. doi: 10.1016/j.nlm.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann N Y Acad Sci. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Pitcher GM, Parent A, Quirion R, Coderre TJ. Noxious thermal and chemical stimulation induce increases in 3H-phorbol 12,13-dibutyrate binding in spinal cord dorsal horn as well as persistent pain and hyperalgesia, which is reduced by inhibition of protein kinase C. J Neurosci. 1995;15:3263–3272. doi: 10.1523/JNEUROSCI.15-05-03263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Hillyer J, Joynes RL. Local anesthetic treatment significantly attenuates acute pain responding but does not prevent the neonatal injury-induced reduction in adult spinal behavioral plasticity. Behav Neurosci. 2007;121:1073–1081. doi: 10.1037/0735-7044.121.5.1073. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lu Y, Chen Y, Westlund KN. Group I metabotropic glutamate receptor antagonists block secondary thermal hyperalgesia in rats with knee joint inflammation. J Pharmacol Exp Ther. 2002;300:149–156. doi: 10.1124/jpet.300.1.149. [DOI] [PubMed] [Google Scholar]
- Zhong J, Gerber G, Kojić L, Randić M. Dual modulation of excitatory synaptic transmission by agonists at group I metabotropic glutamate receptors in the rat spinal dorsal horn. Brain Res. 2000;887:359–377. doi: 10.1016/s0006-8993(00)03066-3. [DOI] [PubMed] [Google Scholar]