Abstract
Efficient and enantiocontrolled synthesis of γ-hydroxy-α,β-unsaturated sulfones and esters are reported through the reaction of enantioenriched α-selenyl aldehydes with EWG-stabilized carbanions and then a one pot selenide oxidation, in situ epoxide formation, and final in situ epoxide opening.
While studying the metabolites of certain vitamin D3 analogs, we became interested in the synthesis of γ-hydroxy-α,β-unsaturated sulfones and esters. γ-Hydroxy-α,β-ethylenic sulfones have previously been used as substrates in stereocontrolled processes such as conjugate additions and cycloaddition reactions,1 as substrates for preparation of enatiomerically pure polypropionate chains or amino alcohol units,2 and also as intermediates in alkaloid syntheses.3 γ-Hydroxy-α,β-enoates have been used as sources of α,β-epoxyesters, which are highly versatile functionalities and can be converted into a number of compounds by opening the oxirane4 and as intermediates in natural product syntheses.5 Use of both of these γ-hydroxy-α,β-unsaturated systems as substrates for stereoselective radical reactions has been explored.6 General methodologies for the asymmetric synthesis of these systems, however, are limited in scope.7 Based on the utility of such systems in highly stereocontrolled processes1 and as intermediates in natural product syntheses,3,5 we set out to design a simple, asymmetric general strategy for their synthesis.
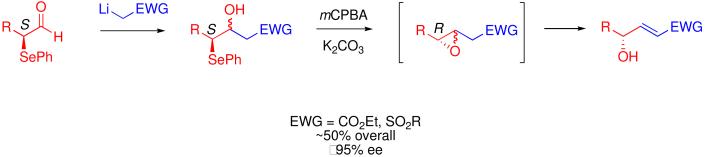
Recent reports on the organocatalytic, asymmetric α-selenenylation of aldehydes in high yields (>85%) and high enantiomeric excess (>95%)8 gave us an entry point to control absolute stereochemistry in our γ-hydroxy-α,β unsaturated systems. Such α-selenylated aldehydes could easily undergo an aldol type reaction with an electron withdrawing group (EWG)-stabilized carbanion to give a diastereomeric pair of γ-selenyl-β-hydroxy sulfones or esters. Oxidation of the selenide and treatment with mild base could give a β,γ-epoxide which, upon further treatment with base could rearrange into the desired enantiomerically enriched γ-hydroxy-α,β unsaturated ester or sulfone with the overall inversion of stereochemistry (Scheme 1).
Scheme 1.
Synthesis of γ-hydroxy-α,β-unsaturated sulfones and esters from α-selenyl aldehydes11
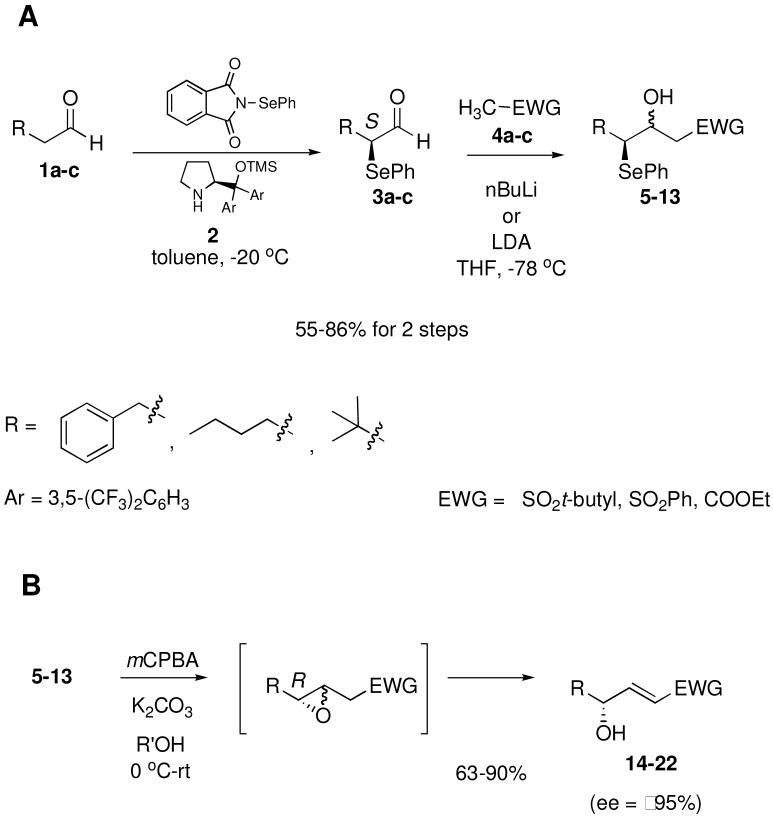
In order to examine the scope of this reaction, we chose three aldehydes (3-phenylpropanal, hexanal, and 3,3-dimethylbutanal) and three EWG-stabilized methyl groups (t-butyl methyl sulfone, phenyl methyl sulfone, and ethyl acetate). Using the methodology of Tiecco and Marini8b to make α-selenyl aldehydes 3a-c, we were able to react the crude α-selenyl aldehyde9 with lithiated EWG-stabilized methyl groups 4a-c to give diastereomeric compounds 5-13 (Scheme 1, A) in 55-86% yields. γ-Selenyl-β-hydroxy sulfones and esters 5-13 underwent oxidation and spontaneous cyclization with mCPBA and K2CO3 to give the transient β,γ-epoxide which immediately rearranged in situ to yield exclusively the γ-hydroxy-(E)-α,β-unsaturated sulfone or ester 14-22 (Scheme 1, B) in 63-90% yields and excellent ee's (≥95%). The (E)-geometry of the new carbon-carbon double bond in products 14-22 was confirmed by 1H NMR spectoscopy (Jα,β = 14-16 Hz). α-Selenyl aldehyde 3c derived from 3,3-dimethylbutanal underwent reaction sequence A (Scheme 1) in high yield with all three EWG-stabilized carbanions (4a-c), but γ-selenyl-β-hydroxy ester 13 derived from reaction with ethyl aceate (4c) decomposed under the reaction conditions used in reaction sequence B (Scheme 1). Also, substituted EWG-stabilized methyl groups such as propionates were explored, but no substantial selectivity in E/Z double bond formation was seen.
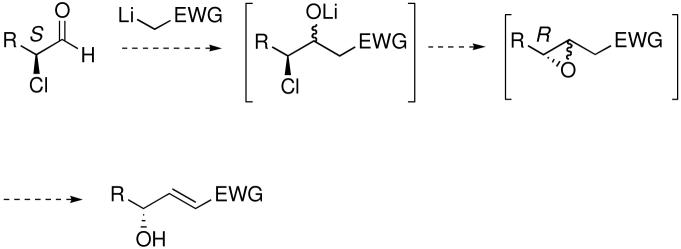
Our success using α-selenenylated aldehydes led us to investigate other leaving groups alpha to the aldehyde and whether a one-pot 3-step procedure might be possible (Scheme 2). Given the precedent for the enantioselective organocatalytic α-chlorination of aldehydes10 we decided to explore the feasibility of such a system in the reaction sequence. Individually, each step in the reaction sequence with an α-chloro aldehyde appeared to work, but a one-pot procedure could not be achieved successfully. Ultimately this strategy was not pursued due to the instability and volatility of the α-chlorinated aldehydes and the superior results with the α-selenyl systems.
Scheme 2.
Plan for one pot procedure
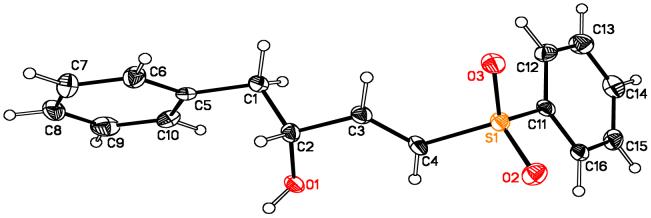
Confirmation of absolute stereochemistry was first achieved by reducing α-selenyl aldehydes 3a-c with NaBH4 and comparing optical rotations with published values.8b An x-ray crystal structure was also obtained for γ-hydroxy-α,β-unsaturated sulfone 15, which confirms the R configuration of the γ-hydroxy carbon (Figure 1).
Figure 1.
Crystal structure of 15 (confirms R configuration at the γ-hydroxy carbon atom).
Further proving the value of this synthetic method, scale up of the procedure to one gram was readily accomplished with no erosion of ee (97%) and in 59% overall yield for γ-hydroxy-α,β-unsaturated sulfone 14.11
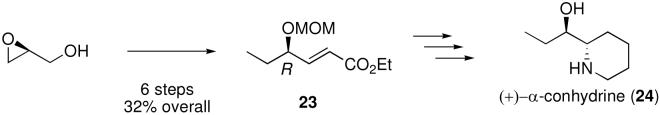
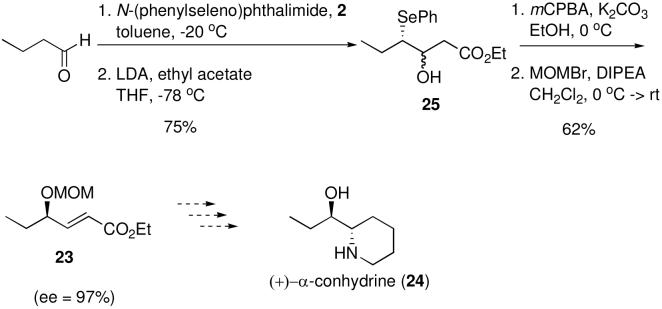
γ-Hydroxy ether-α,β-unsaturated ester 23 (Scheme 3) is a key intermediate in a recent synthesis of the biologically active alkaloid (+)-α-conhydrine (24).5a Intermediate 23 was prepared in 6 steps and 32% overall yield from (S)-glycidol.5a Using the methodology described here we have prepared intermediate ester 23 in only 4 steps and in 47% overall yield and 97% ee (Scheme 4).
Scheme 3.
Synthesis of (+)-α-conhydrine (24) using key intermediate γ-hydroxy ether-α,β-unsaturated ester 235a
Scheme 4.
Formal synthesis of (+)-α-conhydrine (24)
In summary, we report an efficient and generalized procedure for the synthesis of γ-hydroxy-α,β-unsaturated sulfones and esters of high enantiomeric purity that is easily scaled to amounts >1 gram. This 3-step process works on a variety of substrates with high overall yields (~50%) and excellent control of absolute stereochemistry (ee values ≥95%). We have shown the application of this methodology to a formal synthesis of the natural product (+)-α-conhydrine. Undoubtedly this methodology could be expanded to the preparation of other γ-hydroxy-α,β-unsaturated compounds with other EWGs, such as nitro or cyano.
Supplementary Material
Table 1.
Reaction results from Scheme 1
| compound (A, B) | R | EWG | A (yield%) | B (yield%) | ee |
|---|---|---|---|---|---|
| 5, 14 | Bn (1a) | SO2t-butyl (4a) | 84 | 70 | 99* |
| 6, 15 | Bn (1a) | SO2Ph (4b) | 79 | 90 | 95** |
| 7, 16 | Bn (1a) | COOEt (4c) | 77 | 73 | 96* |
| 8, 17 | n-butyl (1b) | SO2t-butyl (4a) | 86 | 91 | 95** |
| 9, 18 | n-butyl (1b) | SO2Ph (4b) | 85 | 86 | 97* |
| 10, 19 | n-butyl (1b) | COOEt (4c) | 50 | 67 | 95* |
| 11, 20 | t-butyl (1c) | SO2t-butyl (4a) | 82 | 63 | 96** |
| 12, 21 | t-butyl (1c) | SO2Ph (4b) | 75 | 69 | 99* |
| 13. 22 | t-butyl (1c) | COOEt (4c) | 85 | N/A | N/A |
ee values determined using chiral HPLC.
ee values determined through 1H NMR analysis of crude reaction mixture of γ-hydroxy alcohols with (S)-Mosher acid chloride.
Acknowledgment
We thank the NIH (CA 93547) for financial support and the ACS (predoctoral fellowship to K.S.P.).
Footnotes
Supporting Information Available: Experimental procedures and spectra of γ-hydroxy-α,β-unsaturated sulfones and esters. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Barton DL, Conrad PC, Fuchs PL. Tetrahedron Lett. 1980;21:1811–1814..Trost BM, Seoane P, Mignani S, Acemoglu M. J. Am. Chem. Soc. 1989;111:7487–7500.
- (2).Carretero JC, Dominguez E. J. Org. Chem. 1993;58:1596–1600.. de Blas J, Carretero JC, Dominguez E. Tetrahedron Lett. 1994;35:4603–4606.. Dominguez E, Carretero JC. Tetrahedron. 1994;50:7557–7566..
- (3).Rojo J, Garcia M, Carretero JC. Tetrahedron. 1993;49:9787–9800.. Carretero JC, Arrayas RG. J. Org. Chem. 1998;63:2993–3005.. de Vincent J, Arrayas RG, Canada J, Carretero JC. Synlett. 2000:53–56.. Iradier F, Arrayas RG, Carretero JC. Org. Lett. 2001;3:2957–2960. doi: 10.1021/ol0162163.. Krafft ME, Kyne GM, Hirosawa C, Schmidt P, Abboud KA, L'Helias M. Tetrahedron. 2006;62:11782–11792..
- (4).Rodriguez S, Vidal A, Monroig JJ, Gonzalez FV. Tetrahedron Lett. 2004;45:5359–5361.. Rodriguez S, Kneeteman M, Izquierodo J, Lopez I, Gonzalez FV, Peris G. Tetrahedron. 2006;62:11112–11123.. Lopez I, Rodriguez S, Izquierodo J, Gonzalez FV. J. Org. Chem. 2007;72:6614–6617. doi: 10.1021/jo0709955..
- (5).Jamieson AG, Sutherland A. Org. Lett. 2007;9:1609–1611. doi: 10.1021/ol070424z.. Kotkar SP, Suryavanshi GS, Sudalai A. Tet. Asym. 2007;18:1795–1798.. Varseev GN, M. ME. Org. Lett. 2007;9:1461–1464. doi: 10.1021/ol070049a.. Taber DF, Reddy PG, Arneson KO. J. Org. Chem. 2008;73:3467–3474. doi: 10.1021/jo702600v..
- (6).Morikawa T, Washio Y, Harada S, Hanai R, Kayashita T, Nemoto H, Shiro M, Taguchi T. J. Chem. Soc. Perkin Trans. I. 1995:271–281.. Charlton MA, Green JR. Can. J. Chem. 1997;75:965–974.. Ogura K, Kayano A, Akazome M. Bull. Chem. Soc. Jpn. 1997;70:3091–3101..
- (7).Ogura K, Shibuya N, Iida H. Tetrahedron Lett. 1981;22:1519–1522.. Pyne SG, Spellmeyer DC, Chen S, Fuchs PL. J. Am. Chem. Soc. 1982;104:5728–5740.. Fuchs PL, Hutchinson DK. J. Am. Chem. Soc. 1985;107:6137–6138.. Ryu I, Kusumoto N, Ogawa A, Kambe N, Sonoda N. Organometallics. 1989;8:2279–2281.. Dominguez E, Carretero JC. Tetrahedron. 1990;46:7197–7206.. Burgess K, Cassidy J, Henderson I. J. Org. Chem. 1991;56:2050–2058.. Trost BM, Grese TA. J. Org. Chem. 1991;56:3189–3192.. Wnuk SF, Dalley NK, Robins M. Can. J. Chem. 1991;69:2104–2111.. Barton DL, Gero SD, Quiclet-Sire B, Samadi M. Tetrahedron Asymmetry. 1994;5:2123–2126.. Zoller T, Uguen D. Eur. J. Org. Chem. 1999:1545–1550.. de la Rosa VG, Ordonez M, Llera JM. Tetrahedron Asymmetry. 2000;2000:2991–3001.. Marcus J, Van Meurs PJ, Van den Nieuwendijk MCH, Porchet M, Brussee J, Van der Gen A. Tetrahedron. 2000;56:2491–2495.. Pedersen TM, Hansen EL, Kane J, Rein T, Helquist P, Nourby PO, Tanner D. J. Am. Chem. Soc. 2001;123:9738–9742. doi: 10.1021/ja005809q.. Rodriguez S, Vidal A, Monroig JJ, Gonzalez FV. Tetrahedron Lett. 2004;45:5359–5361..
- (8).Sunden H, Rios R, Cordova A. Tetrahedron Lett. 2007;48:7865–7869.. Tiecco M, Carlone A, Sternativo S, Marini F, Bartoli G, Melchiorre P. Angew. Chem. Int. Ed. 2007;46:6882–6885. doi: 10.1002/anie.200702318..
- (9).Column chromatography of the α-selenyl aldehydes, even using florisil led to erosion of enantiomeric purity.
- (10).Brochu MP, Brown SP, MacMillan DWC. J. Am. Chem. Soc. 2004;126 doi: 10.1021/ja049562z.. Halland N, Braunton A, Bachmann S, Marigo M, Jorgensen KA. J. Am. Chem. Soc. 2004;126:4790–4791. doi: 10.1021/ja049231m..
- (11).Procedure for the synthesis of 14. A solution of 3-phenylpropanal, 1a, (90%, 1.2 mL, 9.4 mmol) and catalyst 2 (0.7 g, 1.2 mmol) in toluene (20 mL) was stirred at rt under Ar for 30 min. The reaction mixture was cooled to -20 °C and N-(phenylseleno)phthalimide (3.4 g, 11.2 mmol) was added. After stirring 2 h the contents of the flask were filtered and rinsed with hexanes, the solvent evaporated (avoid heat!) and the crude mixture (3a) dried under vacuum for 1 h to get rid of all toluene. To an ice cooled solution of methyl t-butyl sulfone, 4a, (4.0 g, 29.4 mmol) in anhydrous THF (30 mL) under Ar was added n-BuLi (1.5 M in hexanes, 18.8 mL, 28.1 mmol) dropwise and the mixture stirred for 30 min. The reaction mixture was cooled to -78 °C and a solution of crude 3a (9.4 mmol) in 10 mL THF was added via cannula. After stirring at -78 °C for 1 h the mixture was quenched by addition to water (50 mL) and extracted with Et2O (3 × 30 mL). The organics were combined, rinsed with brine (30 mL), dried over MgSO4, filtered, concentrated and chromatographed (25% EtOAc in hexanes) to give 5 (3.4 g, 84% yield), which was used directly in the next step. To a solution of 5 (2.5 g, 5.9 mmol) in MeOH (20 mL) was added K2CO3 (3.2 g, 23.5 mmol) and the reaction mixture was stirred for 20 min. The mixture was cooled to 0 °C and mCPBA (77%, 2.6 g, 11.8 mmol) was added. The solution was allowed to warm slowly to rt and stirred a total of 1.5 h. The reaction mixture was then added to a sat. solution of NaHCO3 (25 mL) and extracted with CH2Cl2 (3 × 30 mL). The organics were combined, dried over MgSO4, filtered, concentrated, and chromatographed (30% EtOAc in hexanes) to give 14 (1.1 g, 70% yield) as a white solid. [α]D24 -1.9 (c 1.35, CHCl3); IR (neat, cm-1) 3483 (brs), 3061 (w), 2980 (w), 2929 (w), 1630 (w), 1453 (w), 1297 (m), 1272 (m), 1200 (w), 1108 (m), 835 (w), 702 (w); 1H NMR (CDCl3, 400 MHz) δ 7.27 (m, 5H), 6.95 (dd, 1H, J = 14.8, 3.6 Hz), 6.53 (dd, 1H, J = 14.8, 2.0 Hz), 4.65 (m, 1H), 2.99 (dd, 1H, J = 14.0, 5.2 Hz), 2.84 (dd, 1H, J = 13.6, 8.0 Hz), 2.09 (brs, 1H), 1.91 (s, 9H); 13C NMR (CDCl3, 100 MHz) δ 151.05, 136. 23, 129.42, 128.82, 127.14, 123.47, 71.26, 58.42, 42.85, 23.22; HRMS: calcd for C14H20O3S [MH+]: 269.1211, found 269.1211; MP = 81-83 °C
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.