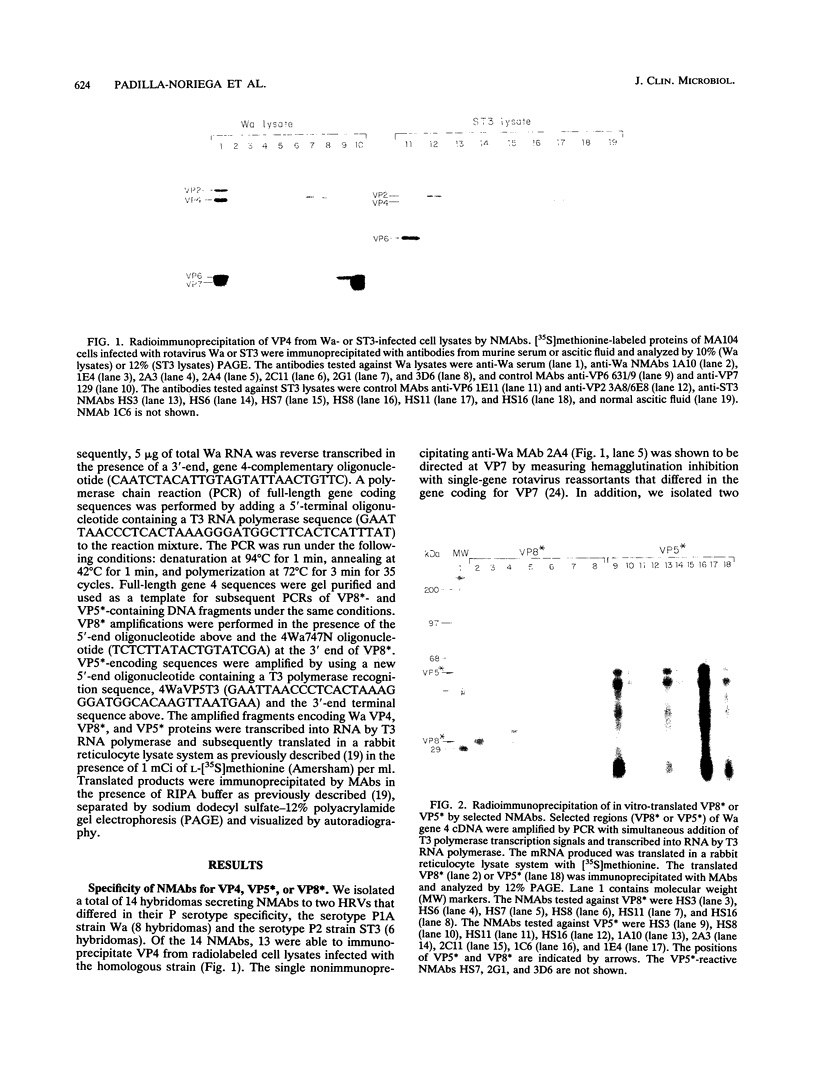
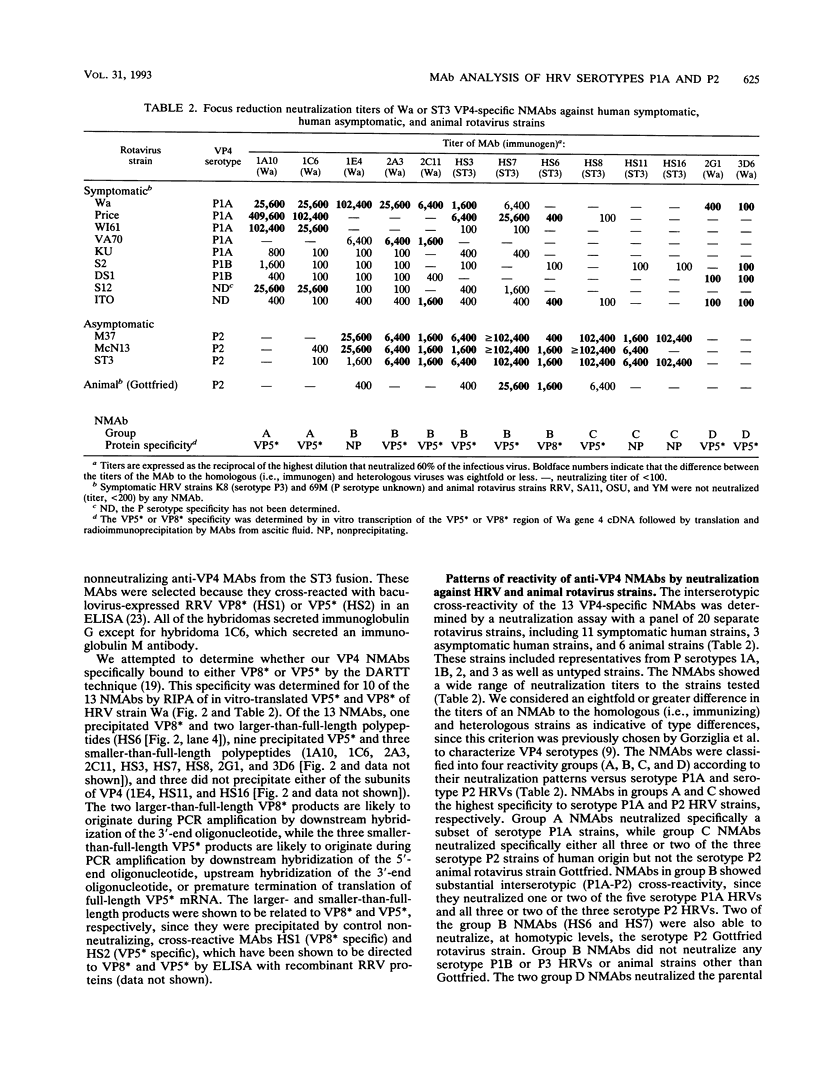
Abstract
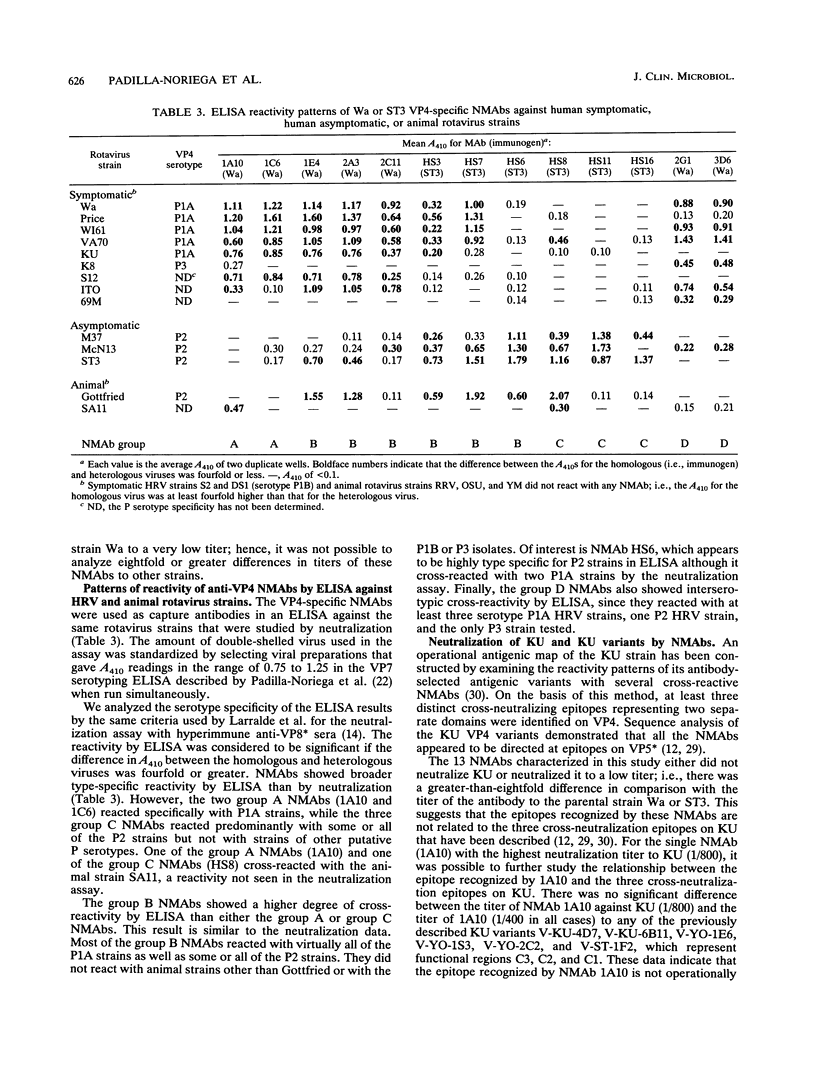
Three human rotavirus (HRV) VP4 serotypes and one subtype have been described on the basis of a fourfold or an eightfold-or-greater difference in neutralization titer when tested with hyperimmune antisera to recombinant VP4 or VP8* (serotypes P1A, P1B, P2, and P3). To start to analyze the antigenic basis underlying serotype specificity, we produced a library of 13 VP4-specific neutralizing monoclonal antibodies (NMAbs) to two HRVs, the serotype P1A strain Wa and the serotype P2 strain ST3, and characterized the reactivity of these NMAbs with a panel of serotypically diverse HRV strains by neutralization assay and enzyme-linked immunosorbent assay (ELISA). We characterized the serotypic specificity of the NMAbs by using a fourfold or an eightfold-or-greater difference in titer against the homologous (i.e., immunogen) and heterologous strains as a criterion for serotype. Some ST3-derived NMAbs reacted specifically with serotype P2 HRVs by ELISA and/or neutralization assay, while some Wa-derived NMAbs reacted specifically by ELISA and/or neutralization assay with some or all serotype P1A HRVs. Other Wa- and ST3-derived NMAbs reacted with some or all serotype P1A and P2 HRV strains by neutralization assay and ELISA. Most NMAbs did not react with serotype P1B or P3 strains. In previous studies, three distinct operationally defined epitopes have been identified on VP4 by examining the reactivity patterns of selected antigenic variants of HRV strain KU. At least one of the NMAbs described here recognizes an epitope unrelated to these previously identified epitopes, since it neutralized both KU and its variants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beards G., Xu L., Ballard A., Desselberger U., McCrae M. A. A serotype 10 human rotavirus. J Clin Microbiol. 1992 Jun;30(6):1432–1435. doi: 10.1128/jcm.30.6.1432-1435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G. F., Chalmers R. M., Fitzgerald T. A., Snodgrass D. R. Serological and genomic characterization of L338, a novel equine group A rotavirus G serotype. J Gen Virol. 1991 May;72(Pt 5):1059–1064. doi: 10.1099/0022-1317-72-5-1059. [DOI] [PubMed] [Google Scholar]
- Browning G. F., Fitzgerald T. A., Chalmers R. M., Snodgrass D. R. A novel group A rotavirus G serotype: serological and genomic characterization of equine isolate FI23. J Clin Microbiol. 1991 Sep;29(9):2043–2046. doi: 10.1128/jcm.29.9.2043-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Midthun K., Hoshino Y., Green K., Gorziglia M., Kapikian A. Z., Chanock R. M. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J Virol. 1986 Dec;60(3):972–979. doi: 10.1128/jvi.60.3.972-979.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Parea M., Arista S., Miranda P., Brüssow H., Hoshino Y., Flores J. Isolation and characterization of two distinct human rotavirus strains with G6 specificity. J Clin Microbiol. 1992 Jan;30(1):9–16. doi: 10.1128/jcm.30.1.9-16.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Green K., Nishikawa K., Taniguchi K., Jones R., Kapikian A. Z., Chanock R. M. Sequence of the fourth gene of human rotaviruses recovered from asymptomatic or symptomatic infections. J Virol. 1988 Aug;62(8):2978–2984. doi: 10.1128/jvi.62.8.2978-2984.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Hoshino Y., Buckler-White A., Blumentals I., Glass R., Flores J., Kapikian A. Z., Chanock R. M. Conservation of amino acid sequence of VP8 and cleavage region of 84-kDa outer capsid protein among rotaviruses recovered from asymptomatic neonatal infection. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7039–7043. doi: 10.1073/pnas.83.18.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Larralde G., Kapikian A. Z., Chanock R. M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Taniguchi K., Urasawa S. Identification of operationally overlapping and independent cross-reactive neutralization regions on human rotavirus VP4. J Gen Virol. 1990 Nov;71(Pt 11):2615–2623. doi: 10.1099/0022-1317-71-11-2615. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Taniguchi K., Urasawa T., Urasawa S. Preparation and characterization of a neutralizing monoclonal antibody directed to VP4 of rotavirus strain K8 which has unique VP4 neutralization epitopes. Arch Virol. 1991;121(1-4):153–162. doi: 10.1007/BF01316751. [DOI] [PubMed] [Google Scholar]
- Larralde G., Li B. G., Kapikian A. Z., Gorziglia M. Serotype-specific epitope(s) present on the VP8 subunit of rotavirus VP4 protein. J Virol. 1991 Jun;65(6):3213–3218. doi: 10.1128/jvi.65.6.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdins I., Sonza S., Dyall-Smith M. L., Coulson B. S., Holmes I. H. Demonstration of an immunodominant neutralization site by analysis of antigenic variants of SA11 rotavirus. J Virol. 1985 Oct;56(1):317–319. doi: 10.1128/jvi.56.1.317-319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liprandi F., Rodriguez I., Piña C., Larralde G., Gorziglia M. VP4 monotype specificities among porcine rotavirus strains of the same VP4 serotype. J Virol. 1991 Mar;65(3):1658–1661. doi: 10.1128/jvi.65.3.1658-1661.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G. A., Rennels M. B., Lim Y., Krall G., Kapikian A. Z., Levine M. M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988 Jun;7(6):388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Yamanaka M. Y., Dang M. N., Greenberg H. B. DNA amplification-restricted transcription-translation: rapid analysis of rhesus rotavirus neutralization sites. Proc Natl Acad Sci U S A. 1990 Jan;87(2):518–522. doi: 10.1073/pnas.87.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Clark H. F., Blavat G., Greenberg H. B. Reassortant rotaviruses containing structural proteins vp3 and vp7 from different parents induce antibodies protective against each parental serotype. J Virol. 1986 Nov;60(2):491–496. doi: 10.1128/jvi.60.2.491-496.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Noriega L., Arias C. F., López S., Puerto F., Snodgrass D. R., Taniguchi K., Greenberg H. B. Diversity of rotavirus serotypes in Mexican infants with gastroenteritis. J Clin Microbiol. 1990 Jun;28(6):1114–1119. doi: 10.1128/jcm.28.6.1114-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Noriega L., Fiore L., Rennels M. B., Losonsky G. A., Mackow E. R., Greenberg H. B. Humoral immune responses to VP4 and its cleavage products VP5* and VP8* in infants vaccinated with rhesus rotavirus. J Clin Microbiol. 1992 Jun;30(6):1392–1397. doi: 10.1128/jcm.30.6.1392-1397.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Green K. Y. Human rotavirus strain 69M has a unique VP4 as determined by amino acid sequence analysis. Virology. 1991 May;182(1):407–412. doi: 10.1016/0042-6822(91)90691-4. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Stoner-Ma D. L., Estes M. K., Greenberg H. B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985 Aug;22(2):286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Svensson L., Padilla-Noriega L., Taniguchi K., Greenberg H. B. Lack of cosegregation of the subgroup II antigens on genes 2 and 6 in porcine rotaviruses. J Virol. 1990 Jan;64(1):411–413. doi: 10.1128/jvi.64.1.411-413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Maloy W. L., Nishikawa K., Green K. Y., Hoshino Y., Urasawa S., Kapikian A. Z., Chanock R. M., Gorziglia M. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J Virol. 1988 Jul;62(7):2421–2426. doi: 10.1128/jvi.62.7.2421-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Morita Y., Urasawa T., Urasawa S. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J Virol. 1987 May;61(5):1726–1730. doi: 10.1128/jvi.61.5.1726-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa S., Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985 May;66(Pt 5):1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Kobayashi N., Gorziglia M., Urasawa S. Nucleotide sequence of VP4 and VP7 genes of human rotaviruses with subgroup I specificity and long RNA pattern: implication for new G serotype specificity. J Virol. 1990 Nov;64(11):5640–5644. doi: 10.1128/jvi.64.11.5640-5644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]