Abstract
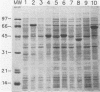
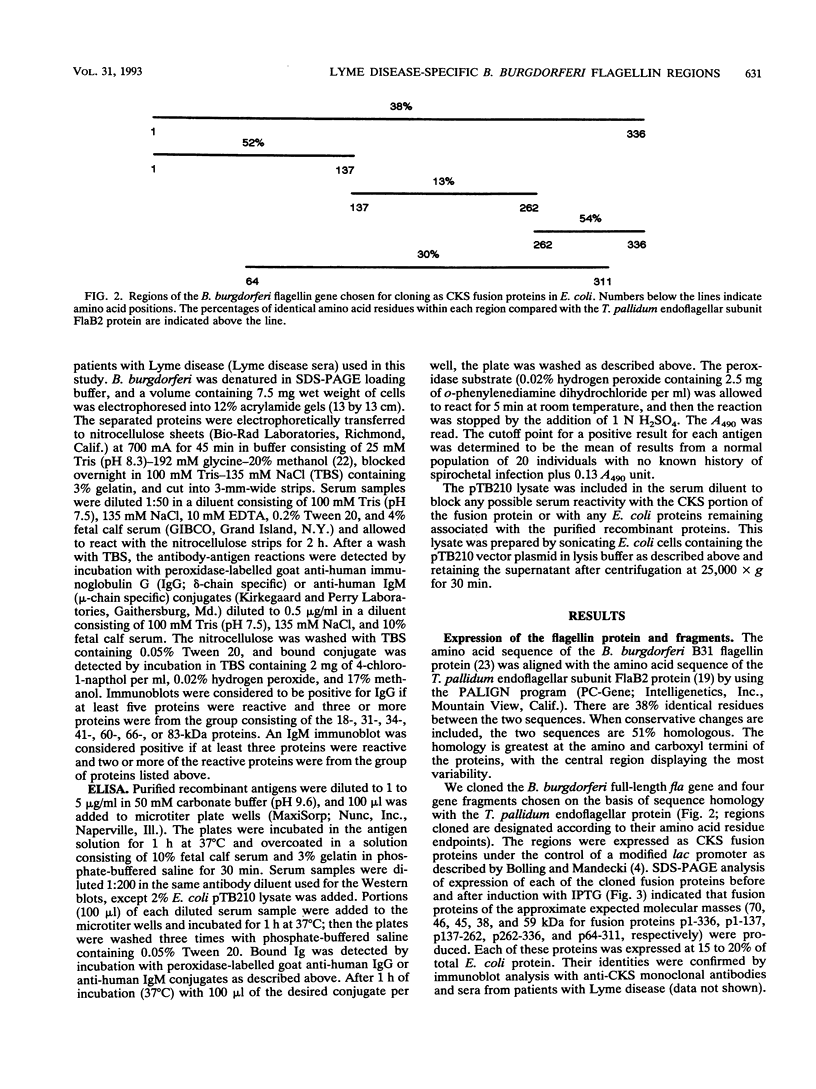
Selected regions of the Borrelia burgdorferi flagellin gene (fla) that exhibit high or low homology with related genes from other bacterial species were amplified by the polymerase chain reaction and expressed as fusion proteins in Escherichia coli. Purified fusion proteins were assayed for antibody reactivity in a microtiter plate enzyme-linked immunosorbent assay with sera from Lyme disease patients as well as syphilitic and normal sera. Immunoglobulin G antibody from Lyme disease patient sera reacted predominantly with the central portion of the protein. The region of the flagellin protein encompassing amino acids 64 to 311 detected nearly all of the immunoglobulin G-positive Lyme sera and only reacted with 1 of 26 syphilis patient serum samples. In contrast, 12 of 26 syphilis patient serum samples and 2 of 47 normal serum samples reacted with the amino terminus of the flagellin protein, whereas 4 of 26 syphilis patient serum samples and 7 of 47 normal serum samples reacted with the carboxyl terminus. The central region containing amino acids 64 to 311 may be employed diagnostically to differentiate antibodies to B. burgdorferi from antibodies to Treponema pallidum. In addition, this region also was recognized by immunoglobulin M in the Lyme patient sera, indicating its potential usefulness as a marker for early Lyme disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G. Laboratory aspects of Lyme borreliosis. Clin Microbiol Rev. 1988 Oct;1(4):399–414. doi: 10.1128/cmr.1.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berland R., Fikrig E., Rahn D., Hardin J., Flavell R. A. Molecular characterization of the humoral response to the 41-kilodalton flagellar antigen of Borrelia burgdorferi, the Lyme disease agent. Infect Immun. 1991 Oct;59(10):3531–3535. doi: 10.1128/iai.59.10.3531-3535.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling T. J., Mandecki W. An Escherichia coli expression vector for high-level production of heterologous proteins in fusion with CMP-KDO synthetase. Biotechniques. 1990 May;8(5):488–492. [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Collins C., Peltz G. Immunoreactive epitopes on an expressed recombinant flagellar protein of Borrelia burgdorferi. Infect Immun. 1991 Feb;59(2):514–520. doi: 10.1128/iai.59.2.514-520.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann G. S., Jacobs E., Deutzmann R., Göbel U. B. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J Bacteriol. 1991 Feb;173(4):1452–1459. doi: 10.1128/jb.173.4.1452-1459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicki R. L., Steere A. C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988 Apr;157(4):790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson M., Stiernstedt G., Granström M., Asbrink E., Wretlind B. Comparison of flagellum and sonicate antigens for serological diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis. 1990 Mar;9(3):169–177. doi: 10.1007/BF01963833. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Barbour A. G. Enzyme-linked immunosorbent assays for Lyme disease: reactivity of subunits of Borrelia burgdorferi. J Infect Dis. 1989 Jan;159(1):43–49. doi: 10.1093/infdis/159.1.43. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Miller J. N., Anderson J. F., Riviere G. R. Cross-reactivity of nonspecific treponemal antibody in serologic tests for Lyme disease. J Clin Microbiol. 1990 Jun;28(6):1276–1279. doi: 10.1128/jcm.28.6.1276-1279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K., Yamashita I., Vonderviszt F. Structure of the core and central channel of bacterial flagella. Nature. 1989 Dec 7;342(6250):648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- Pallesen L., Hindersson P. Cloning and sequencing of a Treponema pallidum gene encoding a 31.3-kilodalton endoflagellar subunit (FlaB2). Infect Immun. 1989 Jul;57(7):2166–2172. doi: 10.1128/iai.57.7.2166-2172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Moter S. E., Simon M. M., Ebnet K., Heiberger A., Kramer M. D. The Borrelia burgdorferi flagellum-associated 41-kilodalton antigen (flagellin): molecular cloning, expression, and amplification of the gene. Infect Immun. 1990 Jun;58(6):1711–1719. doi: 10.1128/iai.58.6.1711-1719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Schierz G., Preac-Mursic V., Weber K., Pfister H. W., Einhäupl K. Serological diagnosis of erythema migrans disease and related disorders. Infection. 1984 Sep-Oct;12(5):331–337. doi: 10.1007/BF01651147. [DOI] [PubMed] [Google Scholar]
- Zöller L., Burkard S., Schäfer H. Validity of western immunoblot band patterns in the serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1991 Jan;29(1):174–182. doi: 10.1128/jcm.29.1.174-182.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]