Abstract
Biliary tract cancers are relatively common malignant gastrointestinal tumors in the elderly. Claudins are integral components of tight junctions that play important roles in maintaining epithelial cell polarity, controlling paracellular diffusion, and regulating cell growth and differentiation. The expression profile of claudins has been extensively characterized, but few reports exist on their expression in the normal and neoplastic biliary tract. Our aim was therefore to study claudins by IHC reactions in normal and neoplastic biliary tract samples. We detected that claudin expressions differ in the normal sample groups: the normal gallbladder strongly expressed claudin-2, -3, -4, and -10, but only weak reactions were seen in normal intrahepatic bile ducts. Although each cancer type expressed several claudins with various intensities, only claudin-4 presented especially strong immunoreactions in extrahepatic bile duct cancers and gallbladder carcinomas, whereas claudin-1 and -10 presented in intrahepatic bile duct cancers. Comparing the normal and carcinoma groups, the most significant decrease was detected in the expression of claudin-10. In conclusion, the expression pattern of claudins is different in the various parts of the normal and neoplastic biliary tract; moreover, an unequivocal decrease was detected in the carcinomas compared with their corresponding normal samples. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 57:113–121, 2009)
Keywords: cholangiocarcinoma, gallbladder cancer, claudins, tight junction
Biliary tract cancers, becoming relatively common malignant gastrointestinal tumors in the elderly, comprise carcinomas of the intrahepatic and extrahepatic bile ducts and the gallbladder (Chuang et al. 2004; Kakar and Ferrell 2007; Blechacz and Gores 2008). Bile duct carcinomas or cholangiocarcinomas equally affect both sexes and are frequently associated with primary sclerosing cholangitis; other risk factors include alcohol abuse, smoking, viral infection, and rarely, ulcerative colitis (Bajor et al. 2001; Hong et al. 2005; Kakar and Ferrell 2007). Twenty percent to 30% of bile duct cancers originate from the intrahepatic bile ducts—this type is on the increase in Western countries and in Japan (Obama et al. 2005)—and the rest have extrahepatic origin [intrahepatic bile duct cancer (IBDC) and extrahepatic bile duct cancer (EBDC), respectively] (Kakar and Ferrell 2007). Gallbladder carcinoma (GBC) is the fifth most common malignancy of the gastrointestinal tract, primarily affecting women. The etiological role of gallstones in gallbladder cancer is equivocal (Kakar and Ferrell 2007; Lewis et al. 2007). Pancreatobiliary maljunction (PBM), on the other hand, is a high-risk factor for the development of all biliary tract cancers (Kobayashi et al. 2006).
Claudins, a family of transmembrane proteins, were identified as indispensable components of tight junctions (TJs) (Furuse et al. 1998; Morita et al. 1999). Along with adherens junctions and desmosomes, TJs play an important role in the maintenance of epithelial cell polarity (Rodriguez-Boulan and Nelson 1989; Miyoshi and Takai 2005). The TJ membrane protein complex constitutes a barrier against paracellular diffusion of solutes and restricts lateral diffusion of the lipid and protein components of the cell membrane (Gumbiner 1993; Anderson and Van Itallie 1995; Ivanov et al. 2005). Moreover, TJs were observed to regulate the growth and differentiation of cultured cells (Balda and Matter 1998; Tsukita et al. 1999), and they play a role in epithelial–mesenchymal transition (EMT) (Gonzalez-Mariscal et al. 2007). The claudin family has 24 members to date, which are shown to be differentially expressed in various types of tissues. The claudin expression profile of several organs (e.g., colon, pancreas, breast, esophagus, and prostate) has been extensively characterized (Swisshelm et al. 2005; Hewitt et al. 2006; Borka et al. 2007; Chiba et al. 2008). Also, a specific claudin expression pattern has been attributed to various tumor types (e.g., claudin-1, -4, and -7 show increased IHC reaction in pancreatic adenocarcinomas, whereas claudin-3 remains negative) (Soini 2005; Swisshelm et al. 2005; Borka et al. 2007). Importantly, selective expression of claudins by different tumors designates them as promising markers for differential diagnosis (Morin 2005; Halasz et al. 2006; Kominsky 2006; Soini and Talvensaari-Mattila 2006; Soini et al. 2006; Oliveira and Morgado-Diaz 2007); for example, claudin-1 differentiates between seropapillary and endometrioid adenocarcinoma of the uterus (Sobel et al. 2006), and claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas (Lódi et al. 2006; Nishino et al. 2008).
The expression pattern of claudins in the biliary tract has been studied before (Lódi et al. 2006; Laurila et al. 2007); however, it has not yet been fully characterized, and the utility of claudins in differentiating IBDC, EBDC, and GBC from each other and from pancreatic adenocarcinoma has not been explored. Therefore, in this study, we evaluated the expression of claudins-1, -2, -3, -4, -7, -8, and -10 in IBDC, EBDC, and GBC by IHC.
Materials and Methods
Surgically removed, formalin-fixed, paraffin-embedded bile duct carcinomas from 62 patients (11 IBDCs, 17 EBDCs, and 34 GBCs) were analyzed for claudin expression. Twelve normal intrahepatic bile duct samples from portal tracts, 12 normal extrahepatic bile duct samples, and 33 normal gallbladder samples were selected as healthy control samples. None of the patients received chemotherapy or radiotherapy before surgery. The study was approved by the Regional Ethical Committee (172/2003).
The median age of patients was 65 years, and the male-female ratio was 24/38. The bile duct carcinoma and GBC samples were classified as well differentiated (G1), moderately differentiated (G2), and poorly differentiated (G3) tumors according to the Cancer Grading Manual (Guzman and Chejfec 2007) criteria (Table 1). The specimens presented glandular structures of varying sizes; poorly differentiated (G3) samples mostly showed solid growth pattern with small- to medium-sized cells. No acute inflammation was noted in the tissue specimens.
Table 1.
Classification of the biliary tract samples according to the Cancer Grading Manual (see Guzman and Chejfec 2007)
| Groups | G1 | G2 | G3 |
|---|---|---|---|
| IBDC | 1/11 | 6/11 | 5/11 |
| EBDC | 0/17 | 12/17 | 5/17 |
| GBC | 1/34 | 19/34 | 14/34 |
Numbers of the samples in each group according to grade. IBDC, intrahepatic bile duct cancer; EBDC, extrahepatic bile duct cancer; GBC, gallbladder cancer.
Tissue Microarray
In all cases, hematoxylin–eosin–stained slides of the formalin-fixed, paraffin-embedded materials were used to select representative tumor regions. The region of interest in the donor paraffin blocks was cored twice with a 2.0-mm-diameter needle and transferred into a recipient paraffin block with a total capacity of 24 cores. The Tissue Micro-Array Builder instrument was used for the procedure (Histopathology; Pécs, Hungary). The multiblocks were incubated twice for 5 min at 56C to improve adhesion between cores and the paraffin of the recipient block. Cores from 62 tumor and 57 normal sample donor blocks were placed in 15 tissue microarray (TMA) recipient blocks [3 NIBD (normal intrahepatic bile duct) + NEBD (normal extrahepatic bile duct) + NGB (normal gallbladder), 3 NGB, 2 IBDC, 1 EBDC, 3 GBC, and 3 mixed blocks from cancer groups]. Each TMA block contained duplicates or triplicates of the selected samples and two to three controls [corresponding tumor and normal samples for normal and tumor blocks, respectively, and hepatocellular carcinoma (HCC) for all]. The morphology of the selected tissues was controlled on the 3- to 4-μm-thick whole TMA sections after hematoxylin–eosin staining.
IHC
Claudin-1, -2, -3, -4, -7, -8, and -10 and cytokeratin-7 were analyzed by IHC. IHC analysis of the different tumor groups was performed using the antibodies and conditions as shown in Table 2. The IHC reactions were performed on 3- to 4-μm-thick formalin-fixed paraffin-embedded sections obtained from the TMA blocks. After the deparaffination steps, slides were washed in PBS (pH 7.4) and treated for 30 min in a retrieval solution (Target Retrieval Solution cat S1699; DAKO, Glostrup, Denmark) in a microwave oven. Reactions were carried out in a Ventana ES automated immunostainer (Ventana Medical Systems; Tucson, AZ). The reagents and secondary antibody from the Ventana detection kit (iView DAB Detection Kit, cat 760-091; Ventana) were used as provided by the manufacturer. Negative and positive controls were included for all antibodies. The positive controls are shown in Table 2.
Table 2.
Primary antibodies
| Dilution | Positive control | Host and clonality | Company | Catalog no. | |
|---|---|---|---|---|---|
| Claudin-1 | 1:100 | Normal cutis | Rabbit polyclonal | Zymed | 18-7362 |
| Claudin-2 | 1:80 | Normal colon | Mouse monoclonal | Zymed | 18-7363 |
| Claudin-3 | 1:80 | Normal colon | Rabbit polyclonal | Zymed | 34-1700 |
| Claudin-4 | 1:100 | Normal colon | Mouse monoclonal | Zymed | 18-7341 |
| Claudin-7 | 1:80 | Normal mammary gland | Rabbit polyclonal | Zymed | 34-9100 |
| Claudin-8 | 1:80 | Normal renal tubules | Rabbit monoclonal | Zymed | 40-2600 |
| Claudin-10 | 1:60 | Normal renal tubules | Rabbit polyclonal | Zymed | 38-8400 |
| CK 7 | 1:300 | Normal bile ducts | Mouse monoclonal | DAKO | M7018 |
Zymed, San Francisco, CA.
Semiquantitative IHC Analysis
After performing the IHC reactions, digitized slides of the tissue microarrays were produced using a Mirax Midi Scanner (3DHISTECH; Budapest, Hungary). Scanned slides were manually evaluated by the pathologist with respect to (a) the intensity of the immunoreaction and (b) the percentage of immunopositive cells. The intensity of the immunoreactions was scored as none, weak, moderate, or strong. The percentage of immunopositive cells was determined in the whole area of interest. In the case of cores containing a mixture of tumor and normal tissue, tumor and normal cells were counted separately.
Statistical Analysis
Statistical analysis was performed using SPSS 15.0 software (SPSS, Chicago, IL). The Mann-Whitney test was performed to compare the differences in claudin expression in the selected pairs of the sample groups (NIBD-IBDC, NEBD-EBDC, NGB-GBC, IBDC-EBDC, IBDC-GBC, and EBDC-GBC). The Bonferroni-Holmes test was used as an additional test for corrections. p<0.05 was considered statistically significant. The correlation between intensity and immunopositivity of an area was calculated by Spearman's rank correlation; p<0.01 was defined as a significant difference between the studied variables. SPSS 15.0 software (SPSS) was used to show the distinction of groups by discriminant analysis.
Results
General Characterization of Claudin Immunoreactions
In the normal biliary epithelia, in accordance with the corresponding positive control tissues (Table 2), immunostaining of claudin-1, -3, -4, -8, and -10 appeared only on plasma membranes. In addition to the membranous staining, cytoplasmic immunoreactions of claudin-2 were evident in both the normal biliary epithelia and in the normal colon control. Furthermore, cytoplasmic claudin-7 immunostaining also appeared in the gallbladder epithelial cells but not in the control mammary gland epithelium. The cell surface reactions of claudin-3, -8, and -10 were often restricted to the apical membrane domain of biliary epithelial cells; of these, only claudin-8 exhibited the same apical restriction in the positive control tissue (normal renal tubules). The other claudins were distributed more or less evenly across the apical, lateral, and basal membrane domains. Similar observations were made on biliary tract cancers, with the following remarks: (a) the immunoreactions extended to the entire cell surface in the case of claudin-8 and -10 and (b) additional cytoplasmic staining of claudin-10 appeared in the tumor cells.
It should be noted that claudin-8 immunoreactions on paraffin sections were generally very weak; however, despite the faint immunostaining, some significant differences between the sample groups were found, and the presence of the protein was further confirmed by immunofluorescence on frozen sections (data not shown).
Evaluation of Claudin Immunoreactions
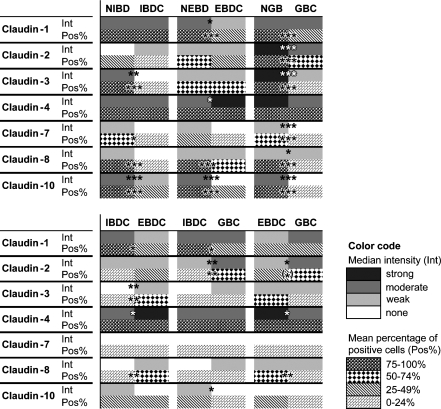
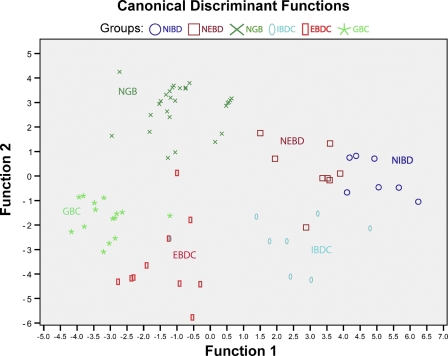
Immunoreactions were evaluated with regard to two parameters: immunoreaction intensity and the percentage of immunopositive cells. The results of both analyses are summarized in Figure 1 and shown in more detail in Supplementary Figure 1. Discriminant analysis based on all intensity and percent positivity data of the performed immunoreactions resulted in a neat separation of the six sample groups (normal intrahepatic and extrahepatic bile ducts, normal gallbladder, and carcinomas of the corresponding locations; Figure 2).
Figure 1.
An overview of claudin immunoreaction results. Median intensity (Int) and mean percent positivity (Pos%) scores of the normal and tumor sample groups are represented with colors and patterns, respectively (for color code, see legend in the figure). Cancers are compared with their corresponding normal epithelia in top panel, whereas the cancer groups are compared with one another in bottom panel. Significant differences are marked by asterisks. NIBD, normal intrahepatic bile duct; NEBD, normal extrahepatic bile duct; NGB, normal gallbladder; IBDC, intrahepatic bile duct cancer; EBDC, extrahepatic bile duct cancer; GBC, gallbladder cancer. (*), nearly significant (p=0.056); *, significant at 0.05>p>0.01; **, significant at 0.01>p>0.001; ***, significant at p<0.001.
Figure 2.
Discriminant analysis. The six studied groups (NIBD, NEBD, NGB, IBDC, EBDC, GBC) are neatly separated by discriminant analysis based on claudin-1, -2, -3, -4, -7, -8, and -10 immunoreaction intensity and percent immunopositivity data.
Pairwise statistical comparisons of the three normal and the three cancer groups, with regard to both evaluation parameters, were made from two viewpoints: (a) cancers were compared with their normal sites of origin and (b) cancer types were compared with one another (Figures 1A and 1B, respectively).
Significant differences in the cancer vs normal comparisons exceeded in number those found in the cancer vs cancer comparisons: immunoreaction intensity, the percent of immunopositive cells, or both was significantly different in 14/21 cancer vs normal comparisons (of which 11 were strongly significant in at least one aspect), whereas this proportion was 10/21 in the case of cancer vs cancer comparisons (of which none were strongly significant). Eleven of 24 significant differences were manifested in both aspects of the immunoreactions, indicating a positive correlation between the two parameters, which was statistically confirmed in the case of claudin-1, -2, -3, -4, -7, and -10 (Spearman's rank correlation, p≤0.001 for all).
Comparison of Cancers With the Corresponding Normal Tissues
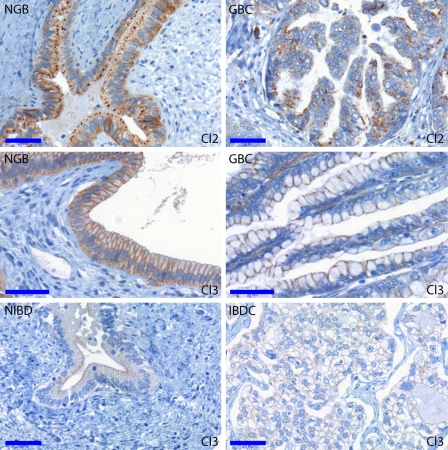
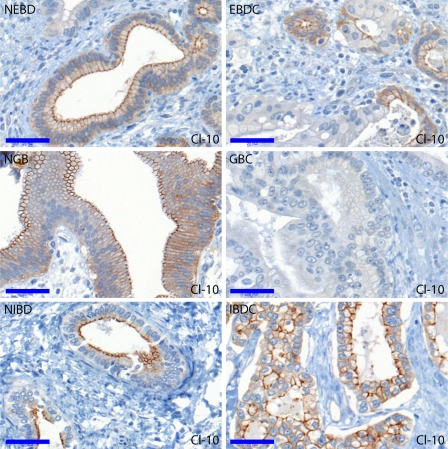
Representative photomicrographs comparing cancers with the corresponding normal tissues are shown in Figures 3 (claudin-2 and -3) and 4 (claudin-10). In most cancer vs normal comparisons, the investigated claudins were found to be expressed more intensively and/or by a higher percentage of cells in the normal tissues (see Figure 1A). Cytokeratin-7, used as a biliary epithelial marker, also showed decreased immunostaining in cancers, especially in poorly differentiated areas (data not shown). Significantly lower expression, regarding at least one evaluation parameter, of claudins in the various cancers were observed as follows: claudin-3, -7, -8, and -10 in IBDC; claudin-1, -8, and -10 in EBDC; and claudin-1, -2, -3, -7, -8, and -10 in GBC. In other words, claudin-2 was found to be downregulated in GBC only; claudin-1, -3, and -7 were downregulated in two cancer types; and claudin-8 and -10 were downregulated in all the three cancer types. The most generalized decrease of claudin expression was noted in the case of gallbladder carcinoma, with all investigated claudins but claudin-4 showing diminished immunostaining relative to the normal gallbladder epithelium. This is partly explained by the fact that the highest claudin expressions among the normal samples were observed in the NGB group. Claudin-4, as an exception, was not downregulated in any of the cancer types analyzed; rather, its immunostaining was even found more intense in EBDC compared with NEBD.
Figure 3.
Claudin-2 and -3 immunoreactions in the NGB, GBC, NIBD, and IBDC groups. Claudin-2 was most intensely expressed in the normal gallbladder and significantly diminished in GBC compared with NGB. Claudin-3 was strongly expressed on the normal gallbladder epithelium, but only weak expression was seen in the normal intrahepatic bile ducts. A significant drop was found in claudin-3 positivity in the case of gallbladder and intrahepatic bile duct carcinomas compared with their corresponding normal regions. Bar = 0.05 mm.
Figure 4.
Claudin-10 immunoreactions in the NEBD, NGB, NIBD, EBDC, GBC, and IBDC groups. Claudin-10 decreased significantly in all corresponding pairs of normal vs carcinoma comparisons, although it was relatively strongly expressed in IBDC compared with both EBDC and GBC. Bar = 0.05 mm.
Differences Between the Cancer Types and Their Potential Diagnostic Benefit
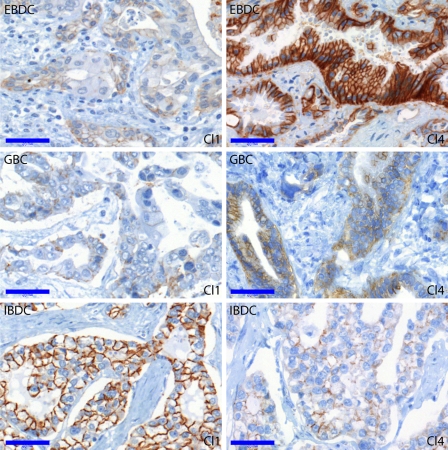
Significant differences between the three cancer types, regarding either evaluation parameter, were found in the case of claudin-1, -2, -3, -4, -8, and -10 but not in the case of claudin-7 (see Figure 1B). Photomicrographs showing the visually most obvious differences in claudin expression between the cancer groups (strong claudin-1 in IBDC vs EBDC and GBC; strong claudin-4 in EBDC vs IBDC and GBC) are presented in Figure 5. For the purpose of discrimination between the three cancer types based on claudin expression, a possible decision algorithm is proposed below.
Figure 5.
Claudin-1 and -4 immunoreactions in EBDC, GBC, and IBDC. Claudin-1 immunostaining was more intense, although non-significantly, in IBDC compared with both EBDC and GBC. Claudin-4 was most intense in the extrahepatic bile duct carcinomas and significantly fainter in IBDC and GBC. Bar = 0.05 mm.
First, GBCs may be differentiated from both cholangiocarcinomas on the basis of claudin-2 immunoreaction intensity and percent positivity. Intermediate or strong claudin-2 immunoreaction in >25% of cells vs weak or no claudin-2 immunoreaction in <25% of cells separated GBC from IBDC + EBDC, respectively, with a specificity of 85% and sensitivity of 72% for GBC (Fisher exact test, p=0.0003). Intrahepatic and extrahepatic cholangiocarcinomas may be distinguished by their claudin-4 immunoreaction intensity: classifying cholangiocarcinomas with strong claudin-4 as EBDC and those with less intense (none, weak, or intermediate) claudin-4 as IBDC yielded an 80% specificity and a 91% sensitivity for EBDC (Fisher exact test, p=0.02).
Applying an even more qualitative approach, the following generalizations can be made. IBDCs were characterized by the presence of claudin-1, -2, -4, and -10 besides the absence or scantness of claudin-3 and -8. EBDCs were typically hallmarked by a stronger expression of claudin-4 and -8 but a weaker expression of the other claudins, especially of claudin-2, relative to IBDC and GBC. Finally, GBCs usually exhibited a stronger claudin-2 expression compared with the other groups, besides a moderate presence of claudin-1 and -4, but the absence or scantness of claudin-10. However, numerous exceptions to these tendencies were noted; all differences found to be statistically significant despite individual deviations are indicated in Figure 1B. Actual distributions of immunoreaction intensities and percent positivities in the cancer groups are detailed in Supplementary Figure 1.
Finally, it is of note that, in the case of EBDC and GBC, the expression of claudin-1 seemed to inversely correlate with increasing tumor grade. Claudin-1 was almost not expressed in the poorly differentiated (Grade 3) subgroups of these tumors, whereas well- and moderately differentiated (Grades 1 and 2) EBDCs and GBCs showed weak but positive membrane reaction.
Discussion
The role of TJ proteins in cell polarity, growth, and differentiation has been described in several studies. TJs have also been implicated in cell proliferation and cancer (Cheung et al. 2005; Higashi et al. 2007). However, most studies have focused on the TJ plaque proteins, which play a direct role in cell motility, thus taking part in actin cytoskeleton rearrangement (Gonzalez-Mariscal et al. 2007). Fewer reports discuss the expression of different types of claudins, although these TJ components would merit similar interest because of their ability to modulate epithelial permeability barrier properties by influencing the passage of cations and—to a lesser extent—anions (Hartsock and Nelson 2008). For instance, high claudin-2 expression confers leakiness to the TJ complex, which becomes less restrictive for ions (Amasheh et al. 2002; Kiuchi-Saishin et al. 2002). Therefore, the observed high claudin-2 expression in the gallbladder may play a role in bile concentration, analogous to its functions in the kidney where it participates in reabsorption in the proximal tubule and in the thin descending limb of Henle's loop (Kiuchi-Saishin et al. 2002). Moreover, alterations in claudin expression may bring about disturbances in cell polarity and thereby modify the distribution of lipid rafts and their protein components (receptors, channels, etc.). The consequent expansion of the signal reception field may have an impact on the responsiveness of epithelial cells (Schneeberger and Lynch 2004; Gonzalez-Mariscal et al. 2007).
This study showed that claudins are differentially expressed in various compartments of the biliary tract (normal intrahepatic and extrahepatic bile ducts and gallbladder); furthermore, carcinomas arising from the various compartments showed divergences from their originating tissue. Discriminant analysis based on all positivity and intensity data of the performed immunoreactions (claudins-1, 2, 3, 4, 7, 8, 10) resulted in a fair separation of the six sample groups (normal intrahepatic and extrahepatic bile ducts, normal gallbladder, and carcinomas in the corresponding locations). Each investigated claudin was expressed in all normal sample groups, with the immunoprofile of normal bile duct samples being similar to that of normal pancreatic tissue (Hewitt et al. 2006; Borka et al. 2007). Significant differences were detected in only claudin-2 and -4; these two showed strong immunoreactions in the gallbladder but not in bile ducts. Laurila et al. (2007) also found the gallbladder epithelium positive for claudin-1, -2, -3, and -4, although they reported claudin-2 staining in only one half of the gallbladder epithelial cells (Laurila et al. 2007).
The claudin expression pattern of carcinomas was remarkably altered in comparison with the originating normal epithelia. The positivity and intensity of claudin-1, -7, -8, and -10 immunoreactions were significantly decreased in most adenocarcinomas relative to their normal counterparts. A significant decrease in claudin-2 was detected only when comparing neoplastic gallbladder samples with the normal gallbladder. Although no similar data are available on the biliary tract, a real-time RT-PCR survey of claudin gene expression by Hewitt et al. (2006) showed decreased expression of several claudins in various tumors compared with the corresponding non-neoplastic tissues, including downregulation of claudin-2 mRNA in the carcinomas of the stomach, kidney, and liver and of claudin-10 mRNA in pancreatic carcinomas.
When comparing the various carcinoma groups, we found stronger claudin-1 and -10 expression in IBDC relative to both EBDC and GBC. On the other hand, claudin-2 immunoreactions were stronger in GBC than in IBDC and EBDC, and the intensity of claudin-4 immunostaining was the highest in EBDC. In other words, all three carcinoma types were distinguished from the others by the relatively strong expression of one or more claudins, the prominent feature being claudin-1 and claudin-10 for IBDC, claudin-2 for GBC, and claudin-4 for EBDC.
Tumor type–specific claudin expression patterns may facilitate differential diagnosis. Claudin-1 was reported to differentiate between a multitude of tumor types such as pancreatic ductal and endocrine tumors (Borka et al. 2007), fetal and embryonic components of human hepatoblastoma (Halasz et al 2006), endometrioid and serous papillary endometrial adenocarcinoma (Sobel et al. 2006), and medullary and poorly differentiated carcinoma vs papillary and follicular carcinoma of the thyroid gland (Tzelepi et al. 2008). Claudin-2, -3, and -4 were proven to clearly distinguish pancreatic tumor types (Borka et al. 2007), and claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas (Lódi et al. 2006). Claudin-3, which in this study yielded uniformly positive reactions across all biliary tumor groups, was consistently negative in ductal pancreatic adenocarcinomas (Borka et al. 2007).
Finally, a few intriguing findings were made on several samples. In two cases of GBCs, the portion of the tumor infiltrating the liver exhibited higher claudin-1 and -10 expression than the extrahepatic portion of the same tumor. The altered claudin expression may be a sign of dedifferentiation and enhanced invasion potential, because it has been noted in our previous work on claudin-4 in cholangiocarcinoma (Lódi et al. 2006). Furthermore, in contrast to the regular apical appearance, aberrant basal localization of claudin-1, -3, -8, and -10 was occasionally seen in glandular structures. Abnormal targeting of TJ components may simply reflect a disturbance in cell polarity; alternatively, the elaboration of a basal barrier may confer protection to tumor cells against potentially cytotoxic compounds.
In summary, this is the first study to compare the protein expression of claudin-1, -2, -3, -7, -8, and -10 in human normal and neoplastic bile ducts. Specific claudin expression patterns identify the various compartments of the healthy biliary tract and also the carcinomas originating from these locations. Future studies on the observed carcinoma-associated expression changes may give insight into the role of claudins in the biological behavior of these cancers.
Acknowledgments
This work was supported by Grant 75468 from the Hungarian Scientific Research Fund (OTKA).
We thank Mrs Zoltán Pekár and Mrs Francis Azumah for preparing the TMA-s and the IHC reactions and Mrs Elvira Rigó for careful reading and correction of the manuscript.
References
- Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976 [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM (1995) Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol 269:G467–475 [DOI] [PubMed] [Google Scholar]
- Bajor J, Bero T, Garamszegi M, Grexa E, Anga B, Szilágyi K (2001) Common bile duct tumor in a young woman with ulcerative colitis. Orv Hetil 142:1231–1234 [PubMed] [Google Scholar]
- Balda MS, Matter K (1998) Tight junctions. J Cell Sci 111:541–547 [DOI] [PubMed] [Google Scholar]
- Blechacz B, Gores GJ (2008) Tumor-specific marker genes for intrahepatic cholangiocarcinoma: utility and mechanistic insight. J Hepatol 49:160–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borka K, Kaliszky P, Szabo E, Lotz G, Kupcsulik P, Schaff Zs, Kiss A (2007) Claudin expression in pancreatic endocrine tumors as compared with ductal adenocarcinomas. Virchows Arch 450:549–557 [DOI] [PubMed] [Google Scholar]
- Cheung ST, Leung KL, Ip YC, Chen X, Fong DY, Ng IO, Fan ST, et al. (2005) Claudin-10 expression level is associated with recurrence of primary hepatocellular carcinoma. Clin Cancer Res 11:551–556 [PubMed] [Google Scholar]
- Chiba H, Osanai M, Murata M, Kojima T, Sawada N (2008) Transmembrane proteins of tight junctions. Biochim Biophys Acta 1778:588–600 [DOI] [PubMed] [Google Scholar]
- Chuang SC, Lee KT, Tsai KB, Sheen PC, Nagai E, Mizumoto K, Tanaka M (2004) Immunohistochemical study of DPC4 and p53 proteins in gallbladder and bile duct cancers. World J Surg 28:995–1000 [DOI] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S (1998) Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141:1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Lechuga S, Garay E (2007) Role of tight junctions in cell proliferation and cancer. Prog Histochem Cytochem 42:1–57 [DOI] [PubMed] [Google Scholar]
- Gumbiner BM (1993) Breaking through the tight junction barrier. J Cell Biol 123:1631–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman G, Chejfec G (2007) Tumors of the digestive system. In Damjanov I, Fan F, eds. Cancer Grading Manual. New York, Springer, 43–44
- Halasz J, Holczbauer A, Paska C, Kovacs M, Benyo G, Verebely T, Schaff Zs, et al. (2006) Claudin-1 and claudin-2 differentiate fetal and embryonal components in human hepatoblastoma. Hum Pathol 37:555–561 [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ (2008) Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt KJ, Agarwal R, Morin PJ (2006) The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer 6:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y, Suzuki S, Sakaguchi T, Nakamura T, Baba S, Reinecker HC, Nakamura S, et al. (2007) Loss of claudin-1 expression correlates with malignancy of hepatocellular carcinoma. J Surg Res 139:68–76 [DOI] [PubMed] [Google Scholar]
- Hong SM, Kim MJ, Pi DY, Jo D, Cho HJ, Yu E, Ro JY (2005) Analysis of extrahepatic bile duct carcinomas according to the New American Joint Committee on Cancer staging system focused on tumor classification problems in 222 patients. Cancer 104:802–810 [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, Parkos CA (2005) Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays 27:356–365 [DOI] [PubMed] [Google Scholar]
- Kakar S, Ferrell LD (2007) Tumors of the liver, gallbladder and biliary tree. In Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd ed. Philadelphia, Elsevier, 417–462
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S (2002) Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13:875–886 [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ohnuma N, Yoshida H, Ohtsuka Y, Terui K, Asano T, Ryu M, et al. (2006) Preferable operative age of choledochal dilation types to prevent patients with pancreaticobiliary maljunction from developing biliary tract carcinogenesis. Surgery 139:33–38 [DOI] [PubMed] [Google Scholar]
- Kominsky SL (2006) Claudins: emerging targets for cancer therapy. Expert Rev Mol Med 8:1–11 [DOI] [PubMed] [Google Scholar]
- Laurila JJ, Karttunen T, Koivukangas V, Laurila P, Syrjälä H, Saarnio J, Soini Y, et al. (2007) Tight junction proteins in gallbladder epithelium: different expression in acute acalculous and calculous cholecystitis. J Histochem Cytochem 55:567–573 [DOI] [PubMed] [Google Scholar]
- Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC (2007) Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol 31:907–913 [DOI] [PubMed] [Google Scholar]
- Lódi C, Szabó E, Holczbauer A, Batmunkh E, Szíjártó A, Kupcsulik P, Kovalszky I, et al. (2006) Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod Pathol 19:460–469 [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y (2005) Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev 57:815–855 [DOI] [PubMed] [Google Scholar]
- Morin PJ (2005) Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res 65:9603–9606 [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S (1999) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA 96:511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino R, Honda M, Yamashita T, Takatori H, Minato H, Zen Y, Sasaki M, et al. (2008) Identification of novel candidate tumour marker genes for intrahepatic cholangiocarcinoma. J Hepatol 49:207–216 [DOI] [PubMed] [Google Scholar]
- Obama K, Ura K, Li M, Katagiri T, Tsunoda T, Nomura A, Satoh S, et al. (2005) Genome-wide analysis of gene expression in human intrahepatic cholangiocarcinoma. Hepatology 41:1339–1348 [DOI] [PubMed] [Google Scholar]
- Oliveira SS, Morgado-Diaz JA (2007) Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cell Mol Life Sci 64:17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ (1989) Morphogenesis of the polarized epithelial cell phenotype. Science 245:718–725 [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD (2004) The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286:C1213–1228 [DOI] [PubMed] [Google Scholar]
- Sobel G, Nemeth J, Kiss A, Lotz G, Szabó I, Udvarhelyi N, Schaff ZS, et al. (2006) Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol Oncol 103:591–598 [DOI] [PubMed] [Google Scholar]
- Soini Y (2005) Expression of claudins 1, 2, 3, 4, 5 and 7 in various types of tumors. Histopathology 46:551–560 [DOI] [PubMed] [Google Scholar]
- Soini Y, Kinnula V, Kahlos K, Pääkkö P (2006) Claudins in differential diagnosis between mesothelioma and metastatic adenocarcinoma of the pleura. J Clin Pathol 59:250–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soini Y, Talvensaari-Mattila A (2006) Expression of claudin 1, 4, 5, and 7 in ovarian tumors of diverse types. Int J Gynecol Pathol 25:330–335 [DOI] [PubMed] [Google Scholar]
- Swisshelm K, Macek R, Kubbies M (2005) Role of claudins in tumorigenesis. Adv Drug Deliv Rev 57:919–928 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M (1999) Structural and signalling molecules come together at tight junctions. Curr Opin Cell Biol 11:628–633 [DOI] [PubMed] [Google Scholar]
- Tzelepi VN, Tsamandas AC, Vlotinou HD, Vagianos CE, Scopa CD (2008) Tight junctions in thyroid carcinogenesis: diverse expression of claudin-1, claudin-4, claudin-7 and occludin in thyroid neoplasms. Mod Pathol 21:22–30 [DOI] [PubMed] [Google Scholar]