Abstract
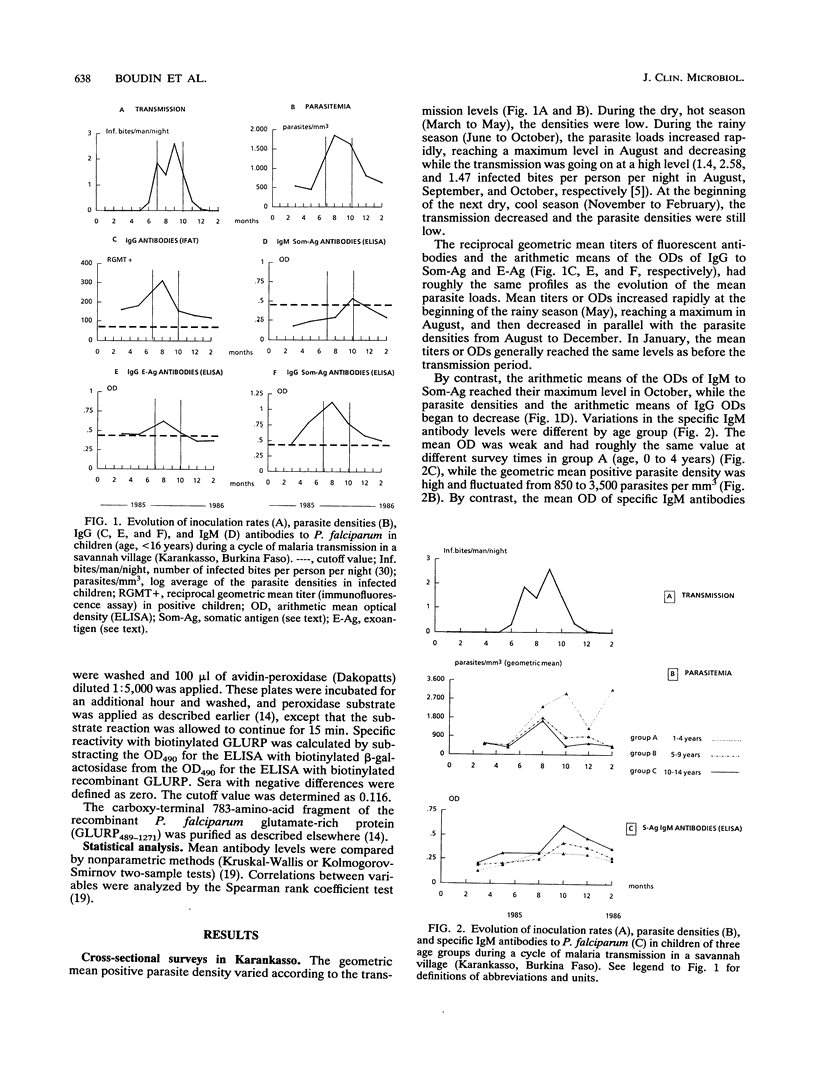
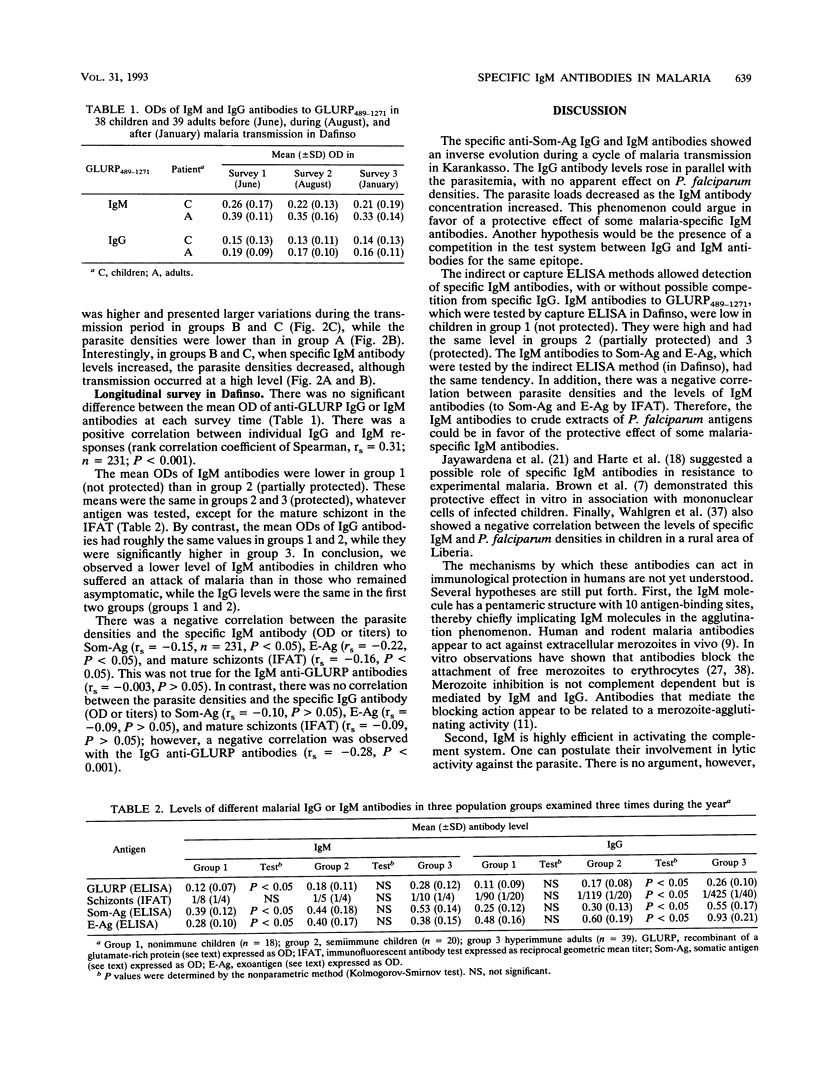
Two seroepidemiological studies were performed in an area of Burkina Faso hyperendemic for malaria to estimate the protective role of immunoglobulin M (IgM) antibodies. Six cross-sectional surveys were carried out on children (ages, < 16 years) in the village of Karankasso. The evolution of antibodies to crude extracts of Plasmodium falciparum (IgG or IgM antisomatic and IgG antiexoantigens) were tested by IFI or enzyme-linked immunosorbent assay and were followed up according to the fluctuations of the parasite densities. Specific IgG antibodies had the same evolution as parasite densities. By contrast, specific IgM antibodies increased when IgG and parasite densities began to decrease (despite a high inoculation rate). A longitudinal survey of 77 children and adults was conducted in another village (Dafinso). In that study, clinical follow-up of the selected individuals allowed us to define three groups in the population. Children in group 1 were considered nonimmune (children with one or more malaria attacks). Group 2 was composed of semiimmune children who did not present with any malarial attack during the survey but who had high levels of parasitemia during the transmission period. Group 3 was composed of immunoprotected adults. Specific IgM and IgG antibodies to crude extracts or a recombinant antigen (glutamate-rich protein) of P. falciparum were tested. Specific IgM antibodies were lower in group 1 (nonimmune) than in groups 2 (semiimmune) and 3 (immunoprotected). Furthermore, there was a negative correlation between parasite densities and the levels of specific IgM antibodies. We discuss the possible role of IgM antibodies in the acquisition of immunity to malaria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bate C. A., Taverne J., Davé A., Playfair J. H. Malaria exoantigens induce T-independent antibody that blocks their ability to induce TNF. Immunology. 1990 Jul;70(3):315–320. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Malarial parasites induce TNF production by macrophages. Immunology. 1988 Jun;64(2):227–231. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunology. 1989 Apr;66(4):600–605. [PMC free article] [PubMed] [Google Scholar]
- Boudin C., Robert V., Carnevale P., Ambroise-Thomas P. Epidemiology of Plasmodium falciparum in a rice field and a savanna area in Burkina Faso. Comparative study on the acquired immunoprotection in native populations. Acta Trop. 1992 Jun;51(2):103–111. doi: 10.1016/0001-706x(92)90052-y. [DOI] [PubMed] [Google Scholar]
- Brown I. N. Immunological aspects of malaria infection. Adv Immunol. 1969;11:267–349. doi: 10.1016/s0065-2776(08)60481-2. [DOI] [PubMed] [Google Scholar]
- Brown J., Greenwood B. M., Terry R. J. Cellular mechanisms involved in recovery from acute malaria in Gambian children. Parasite Immunol. 1986 Nov;8(6):551–564. doi: 10.1111/j.1365-3024.1986.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Celada A., Cruchaud A., Perrin L. H. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin Exp Immunol. 1982 Mar;47(3):635–644. [PMC free article] [PubMed] [Google Scholar]
- Chulay J. D., Aikawa M., Diggs C., Haynes J. D. Inhibitory effects of immune monkey serum on synchronized Plasmodium falciparum cultures. Am J Trop Med Hyg. 1981 Jan;30(1):12–19. doi: 10.4269/ajtmh.1981.30.12. [DOI] [PubMed] [Google Scholar]
- Chumpitazi B. F., Peyron F., Boudin C., Picot S., Oury B., Ambroise-Thomas P. Relationships between circulating S-antigens, naturally acquired antibodies to Plasmodium falciparum exoantigens and malaria attack in a mesoendemic area. Acta Trop. 1992 Apr;50(4):295–304. doi: 10.1016/0001-706x(92)90064-5. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. Properties of protective malarial antibody. Immunology. 1970 Aug;19(2):369–383. [PMC free article] [PubMed] [Google Scholar]
- Cornille-Brögger R., Mathews H. M., Storey J., Ashkar T. S., Brögger S., Molineaux L. Changing patterns in the humoral immune response to malaria before, during, and after the application of control measures: a longitudinal study in the West African savanna. Bull World Health Organ. 1978;56(4):579–600. [PMC free article] [PubMed] [Google Scholar]
- Dziegiel M., Borre M. B., Jepsen S., Hogh B., Petersen E., Vuust J. Recombinant Plasmodium falciparum glutamate rich protein; purification and use in enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1991 Mar;44(3):306–313. doi: 10.4269/ajtmh.1991.44.306. [DOI] [PubMed] [Google Scholar]
- Gabriel J., Berzins K. Specific lysis of Plasmodium yoelii infected mouse erythrocytes with antibody and complement. Clin Exp Immunol. 1983 Apr;52(1):129–134. [PMC free article] [PubMed] [Google Scholar]
- Gravely S. M., Kreier J. P. Adoptive transfer of immunity to Plasmodium berghei with immune T and B lymphocytes. Infect Immun. 1976 Jul;14(1):184–190. doi: 10.1128/iai.14.1.184-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harte P. G., Cooke A., Playfair J. H. Specific monoclonal IgM is a potent adjuvant in murine malaria vaccination. Nature. 1983 Mar 17;302(5905):256–258. doi: 10.1038/302256a0. [DOI] [PubMed] [Google Scholar]
- Jackson S., Sogn J. A., Kindt T. J. Microdetermination of rabbit immunoglobulin allotypes by ELISA using specific antibodies conjugated with peroxidase or with biotin. J Immunol Methods. 1982;48(3):299–309. doi: 10.1016/0022-1759(82)90331-3. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Janeway C. A., Jr, Kemp J. D. Experimental malaria in the CBA/N mouse. J Immunol. 1979 Dec;123(6):2532–2539. [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Davies A. J. The immunological response of CBA mice to P. yoelii. II. The passive transfer of immunity with serum and cells. Immunology. 1978 Jan;34(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Longenecker B. M., Breitenbach R. P., Congdon L. L., Farmer J. N. Natural and acquired antibodies to Plasmodium lophurae in intact and bursaless chickens. I. Agglutination and passive immunity studies. J Parasitol. 1969 Apr;55(2):418–425. [PubMed] [Google Scholar]
- Miller L. H., Aikawa M., Dvorak J. A. Malaria (Plasmodium knowlesi) merozoites: immunity and the surface coat. J Immunol. 1975 Apr;114(4):1237–1242. [PubMed] [Google Scholar]
- Mitchell G. H., Butcher G. A., Voller A., Cohen S. The effect of human immune IgG on the in vitro development of Plasmodium falciparum. Parasitology. 1976 Apr;72(2):149–162. doi: 10.1017/s0031182000048459. [DOI] [PubMed] [Google Scholar]
- Phanuphak P., Hanvanich M., Sakulramrung R., Moollaor P., Sitprija V., Phanthumkosol D. Complement changes in falciparum malaria infection. Clin Exp Immunol. 1985 Mar;59(3):571–576. [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Robert V., Gazin P., Boudin C., Molez J. F., Ouedraogo V., Carnevale P. La transmission du paludisme en zone de savane arborée et en zone rizicole des environs de Bobo Dioulasso (Burkina Faso). Ann Soc Belg Med Trop. 1985;65 (Suppl 2):201–214. [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- Taverne J., Bate C. A., Sarkar D. A., Meager A., Rook G. A., Playfair J. H. Human and murine macrophages produce TNF in response to soluble antigens of Plasmodium falciparum. Parasite Immunol. 1990 Jan;12(1):33–43. doi: 10.1111/j.1365-3024.1990.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Thelu J., Ambroise-Thomas P., Chumpitazi B., Kupka P. Purification and immunochemical study of Plasmodium falciparum exoantigens. J Parasitol. 1985 Oct;71(5):542–546. [PubMed] [Google Scholar]
- Thelu J., Ambroise-Thomas P., Contat M., Kupka P. Antigènes excrétés-sécrétés par Plasmodium falciparum en cultures in vitro. Etude comparée avec les antigènes somatiques et les antigènes figurés. Bull World Health Organ. 1982;60(5):761–766. [PMC free article] [PubMed] [Google Scholar]
- Tosta C. E., Wedderburn N. Immune phagocytosis of Plasmodium yoelii-infected erythrocytes by macrophages and eosinophils. Clin Exp Immunol. 1980 Oct;42(1):114–120. [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Berzins K., Perlmann P., Persson M. Characterization of the humoral immune response in Plasmodium falciparum malaria. II. IgG subclass levels of anti-P. falciparum antibodies in different sera. Clin Exp Immunol. 1983 Oct;54(1):135–142. [PMC free article] [PubMed] [Google Scholar]
- Wahlgren M., Björkman A., Perlmann H., Berzins K., Perlmann P. Anti-Plasmodium falciparum antibodies acquired by residents in a holoendemic area of Liberia during development of clinical immunity. Am J Trop Med Hyg. 1986 Jan;35(1):22–29. doi: 10.4269/ajtmh.1986.35.22. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Phillips R. S. Method to test inhibitory antibodies in human sera to wild populations of Plasmodium falciparum. Nature. 1976 Sep 9;263(5573):132–134. doi: 10.1038/263132a0. [DOI] [PubMed] [Google Scholar]



