Abstract
Recombinant interleukin-2 (IL-2) therapy for malignancy is associated with a pulmonary vascular leakage syndrome (VLS) similar to that seen in sepsis. We investigated the possibility that the IL-2-induced VLS may be associated with the release of peroxynitrite (ONOO−), and used a model of IL-2-induced VLS in sheep to test the effects of the ONOO− decomposition catalyst WW-85. Eighteen sheep were chronically instrumented and randomly divided into 3 groups (n=6 per group): sham: lactated Ringer’s solution, control: IL-2, and treatment: IL-2 and WW-85. Treatment with WW-85 significantly improved lung transvascular fluid flux, decreased lipid peroxidation, limited iNOS as well as PAR intensity, prevented tachycardia, and attenuated the increase in core body temperature resulting from IL-2 treatment. These findings suggest that ONOO− plays a pivotal role in the pathology of IL-2-induced pulmonary VLS, and that WW-85 may become a useful treatment option.
Keywords: cancer therapy, lung lymph flow, nitric oxide, transvascular fluid flux, vascular leakage
Interleukin-2 (IL-2), is a lymphokine, synthesized by T-helper lymphocytes and activated by interaction of the T-cell receptor complex with antigen/MHC complexes on the surfaces of antigen-presenting cells [1,2]. IL-2 acts as a paracrine factor, influencing the activity of cells within the immune system. B-cells and natural killer (NK)-cells respond, when properly activated, to IL-2 [3]. Lymphocyte activated killer (LAK)-cells, appear to be derived from NK -cells under the influence of IL-2 [4]. IL-2 has shown promise as an anticancer drug by virtue of its ability to stimulate both the proliferation and activity of tumor-attacking LAK and tumor-infiltrating lymphocyte-cells (TIL), and is under investigation for the immunotherapy of metastatic tumors [5]. Objective responses have been reported in patients with malignant melanoma, metastatic renal cell carcinoma (MRCC), or lung adenocarcinoma [6].
However, IL-2-based cancer treatment is limited by a syndrome of life-threatening adverse reactions, such as disseminated vascular leakage with loss of intravascular fluids to interstitial tissues, considerable fluid retention, and non-cardiac pulmonary edema. Vascular leakage after IL-2 therapy may trigger multiple reversible cardiovascular abnormalities that are similar to the hemodynamic changes seen in the presence of systemic inflammatory response syndrome (SIRS). SIRS is associated with an excessive production of reactive nitrogen species such as nitric oxide (NO). Furthermore, it has been reported [7] that IL-2 therapy increases the release of reactive oxygen species (ROS) from macrophages, e.g. superoxide (O2−). As a consequence, peroxynitrite (ONOO−) is formed by a rapid reaction between NO and O2−. ONOO−, in turn, acts as a terminal mediator of cellular injury under various pathophysiologic conditions of oxidative and nitrosative stress, and is able to induce cell necrosis and apotosis [8].
The purpose of this study is to investigate the effect of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 on IL-2-mediated toxicity, and pulmonary vascular leakage, as well as an initial evaluation of the safety of this approach.
Materials and methods
Animal care and use
The Institutional Animal Care and Use Committee at the University of Texas Medical Branch at Galveston approved this study, accordingly to the guidelines of the National Institutes of Health for the care and use of experimental animals. The animals were individually housed in metabolic cages and were studied in the awake state.
Surgical preparation
Eighteen female Merino sheep, weighing 34.5 ± 1.1 kg, were chronically instrumented for hemodynamic monitoring with a femoral artery catheter and a 7-French Swan-Ganz thermodilution catheter, as previously described [9,10]. Through a left thoracotomy at the level of the 5th intercostal space, a silastic catheter was positioned in the left atrium. Through an incision on the right, 6th intercostal space, the efferent lymphatic vessel of the CMN was cannulated with Silastic medical grade tubing by a modification of the technique originally described by Staub et al. [11]. The distal end of the CMN was ligated through the 9th intercostal space and the borders of the diaphragm, and posterior aspects of the right hemithorax were cauterized to avoid any systemic afferent lymphatic duct to enter the CMN. After surgery, animals were given a seven-day recovery period with free access to food and water.
Experimental protocol and measurements
Following a baseline measurement, sheep were randomly allocated to one of the three groups (n=6 each): sham (uninjured, untreated), control (injured (IL-2), untreated), and WW-85 (injured (IL-2), treated (WW-85)). Thereafter, a Foley urinary retention catheter was placed. Directly after baseline (BL), IL-2 was intravenously (300.000 U/kg) given to control and treatment animals in 8h intervals. One hour after the first IL-2 administration, WW-85 (dissolved in saline) was intravenously administered with an initial bolus of 0.1 mg/kg followed by a continuous infusion of 0.02 mg·kg−1·h−1 for the remaining 47h duration of the experiment. The sham and control group received the vehicle. All animals had free access to food but not to water and were fluid resuscitated, initially started on an infusion rate of 2 mL·kg−1·h−1 of lactated Ringer’s solution, to precisely measure the fluid intake. Hemodynamic and oxygenation variables were measured every 6 h during the experimental period. All pressures were determined with disposable pressure transducers. Cardiac output (CO) and core body temperature was determined, using the Swan-Ganz™ catheter. Arterial and mixed venous blood gases were determined using a conventional blood gas analyzer and corrected for core body temperature. While lung lymph flow (QL) was determined with graduated test tubes, blood and lymph samples were collected in heparinized sample tubes. Total protein concentrations in lymph (CL) and plasma (CP) were measured with a refractometer. During the entire 48 h study period, all animals were spontaneously breathing in standing position and not sedated. The concentration of NO and its metabolites in plasma were assessed intermittently as previously reported [12].
Histopathology and molecular biology
After completion of the 48-h experiment, the animals were anesthetized and euthanized by a lethal intravenous injection of saturated potassium chloride. Immediately after sacrifice, several tissue samples were harvested for a) measurement of iNOS mRNA activity, b) immunostaining for iNOS protein, c) immunostaining for poly(ADP-ribose) (PAR), d) quantification of myeloperoxidase (MPO) activity, e) quantification of malondialdehyde (MDA) activity f) quantification of 3-nitrotyrosine (3-NT) concentration, and g) measurement of bloodless lung wet-to-dry weight-ratio, as previously reported [12,13].
Statistical analysis
For statistical analysis, Sigma Stat 2.03 software (SPSS Inc., Chicago, IL) was used. After confirming normal distribution (Kolmogorov-Smirnov test), a two-way analysis of variance (ANOVA) for repeated measurements with appropriate Student-Newman-Keuls post hoc comparisons was used to detect differences within and between groups. Significance was assumed when P-value was less than 0.05. Data are presented as mean ± standard error of mean (S.E.M.).
Results
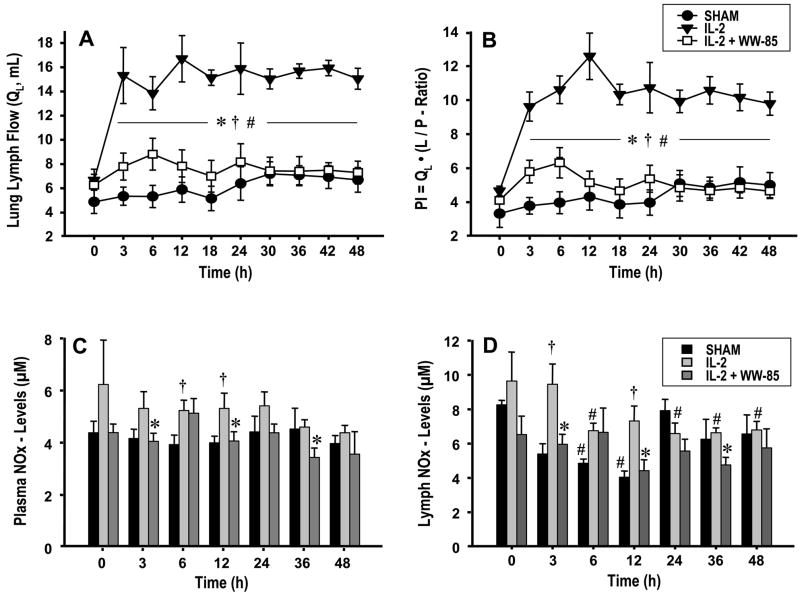
Pulmonary transvascular fluid flux was evaluated by determination of lung lymph flow and the pulmonary permeability index. The injured control group showed significant increases in both, lung lymph flow and the pulmonary permeability index vs. BL and compared to the sham group and animals treated with WW-85 (Fig. 1A/B). There was no statistical difference between the sham and the treatment group.
FIG 1.
Changes in: 1A lung lymph flow (QL, mL), 1B permeability index (PI=QL·(L/P-ratio)), 1C plasma nitrate/nitrite (NOx, μM), and 1D lymph NOx levels over 48 h experimental period. Data are expressed as mean ± S.E.M. of 6 animals per group. Significance p <0.05; # vs. baseline (0 h); † vs. Sham; * IL-2 vs. IL-2 + WW85.
NOx plasma and lymph levels
While NOx plasma levels remained unchanged in sham animals, IL-2 infusion markedly increased NOx concentrations in the control group during the entire experiment. The treatment group showed a trend of decreased NOx levels during the 48 h experiment, with a significant decrease at 3, 12, and 36 hours after injury compared to controls. The lymph NOx levels also displayed significant differences between the WW-85 and control group at 3,12, and 36 h post injury (p<0.05, Fig. 1C/D).
Gas exchange and pulmonary shunt fraction
PaO2/FiO2 -ratio remained stable throughout the experiment in all groups (sham BL: 529±28, 48h: 556±29; control BL: 535±27, 48h: 493±39; treatment BL: 612±20, 48h: 543±30) and showed no statistical differences between groups (p>0.05). The pulmonary shunt fraction (Qs/Qt) showed a significant increase in the IL-2 group at six and 12 hours post injury compared to BL (Table 1, p<0.05). There was no statistical difference between the groups.
Table 1.
Cardiopulmonary variables
| Table 1.1 | ||||||
|---|---|---|---|---|---|---|
| Parameter | Group | BL | 3 h | 6 h | 12 h | 18 h |
| Temp (°C) | Sham | 39.4 ± 0.1 | 39.4 ± 0.1 | 39.4 ± 0.1 | 39.3 ± 0.1 | 39.2 ± 0.1 |
| IL-2 | 39.5 ± 0.1 | 40.5 ± 0.2#† | 40.0 ± 0.3† | 39.6 ± 0.1 | 40.4 ± 0.2#† | |
| IL-2 + WW-85 | 39.3 ± 0.1 | 39.6 ± 0.1* | 39.5 ± 0.2* | 39.7 ± 0.2 | 39.9 ± 0.1#* | |
| HR (bpm) | Sham | 97 ± 5 | 92 ± 5 | 98 ± 7 | 101 ± 7 | 96 ± 5 |
| IL-2 | 103 ± 5 | 129 ± 8#† | 137 ± 7#† | 125 ± 6#† | 114 ± 4 | |
| IL-2 + WW-85 | 97 ± 3 | 102 ± 2* | 110 ± 3* | 108 ± 3* | 103 ± 3 | |
| CO (L/min) | Sham | 5.28 ± 0.28 | 5.35 ± 0.31 | 5.13 ± 0.20 | 5.15 ± 0.28 | 5.04 ± 0.37 |
| IL-2 | 6.01 ± 0.24 | 5.63 ± 0.29 | 5.36 ± 0.44 | 6.59 ± 0.49† | 5.64 ± 0.30 | |
| IL-2 + WW-85 | 5.55 ± 0.10 | 4.96 ± 0.16 | 5.39 ± 0.22 | 5.27 ± 0.14* | 5.15 ± 0.17 | |
| MPAP (mm Hg) | Sham | 21 ± 1 | 23 ± 1 | 23 ± 1 | 24 ± 1 | 22 ± 1 |
| IL-2 | 20 ± 1 | 28 ± 1#† | 25 ± 2# | 24 ± 0# | 25 ± 1# | |
| IL-2 + WW-85 | 20 ± 1 | 22 ± 1* | 23 ± 2 | 22 ± 1 | 22 ± 1 | |
| Qs/Qt (Ratio) | Sham | 0.17 ± 0.03 | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.15 ± 0.03 | 0.18 ± 0.03 |
| IL-2 | 0.13 ± 0.02 | 0.20 ± 0.03 | 0.23 ± 0.06# | 0.21 ± 0.02# | 0.19 ± 0.02 | |
| IL-2 + WW-85 | 0.16 ± 0.03 | 0.15 ± 0.02 | 0.17 ± 0.02 | 0.18 ± 0.02 | 0.16 ± 0.01 | |
| Table 1.2 | ||||||
|---|---|---|---|---|---|---|
| Parameter | Group | 24 h | 30 h | 36 h | 42 h | 48 h |
| Temp (°C) | Sham | 39.3 ± 0.1 | 39.2 ± 0.1 | 39.3 ± 0.1 | 39.4 ± 0.1 | 39.3 ± 0.1 |
| IL-2 | 39.6 ± 0.1 | 39.4 ± 0.1 | 39.8 ±0.2† | 40.3 ± 0.1#† | 39.5 ± 0.1 | |
| IL-2 + WW-85 | 39.3 ± 0.1 | 39.3 ± 0.1 | 39.6 ± 0.1 | 39.9 ± 0.3#† | 39.4 ±0.1 | |
| HR (bpm) | Sham | 102 ± 6 | 104 ± 6 | 106 ± 5 | 104 ± 7 | 106 ± 7 |
| IL-2 | 126 ± 9#† | 114 ± 5 | 116 ± 5 | 105 ± 6 | 111 ± 3 | |
| IL-2 + WW-85 | 100 ± 2* | 100 ± 4 | 99 ± 3 | 101 ± 3 | 94 ± 3 | |
| CO (L/min) | Sham | 5.39 ± 0.45 | 5.56 ± 0.46 | 5.09 ± 0.37 | 5.71 ± 0.45 | 5.35 ± 0.32 |
| IL-2 | 6.18 ± 0.44 | 6.45 ± 0.37 | 6.04 ± 0.29 | 5.27 ± 0.21 | 5.55 ± 0.28 | |
| IL-2 + WW-85 | 5.13 ± 0.15 | 5.15 ± 0.23* | 5.27 ± 0.33 | 4.91 ± 0.17 | 4.84 ± 0.24 | |
| MPAP (mm Hg) | Sham | 23 ± 1 | 23 ± 1 | 23 ± 1 | 22 ± 1 | 23 ± 1 |
| IL-2 | 23 ± 2 | 23 ± 1 | 22 ± 1 | 22 ± 1 | 20 ± 1 | |
| IL-2 + WW-85 | 21 ± 1 | 22 ± 2 | 22 ± 2 | 22 ± 1 | 20 ± 1 | |
| Qs/Qt (Ratio) | Sham | 0.17 ± 0.02 | 0.13 ± 0.02 | 0.18 ± 0.04 | 0.16 ± 0.03 | 0.15 ± 0.02 |
| IL-2 | 0.18 ± 0.02 | 0.16 ± 0.01 | 0.17 ± 0.02 | 0.20 ± 0.02 | 0.18 ± 0.04 | |
| IL-2 + WW-85 | 0.14 ± 0.01 | 0.15 ± 0.02 | 0.17 ± 0.02 | 0.14 ± 0.01 | 0.15 ± 0.01 | |
Legend: Temp, Temperature; HR, Heart Rate; CO, Cardiac Output; MPAP, Mean Pulmonary Artery Pressure; Qs/Qt, Pulmonary Shunt Fraction; Mean ± SEM; Significance was assumed when p was less than 0.05;
vs. baseline,
vs. IL-2,
vs. Sham
Lung MPO activity and MDA formation
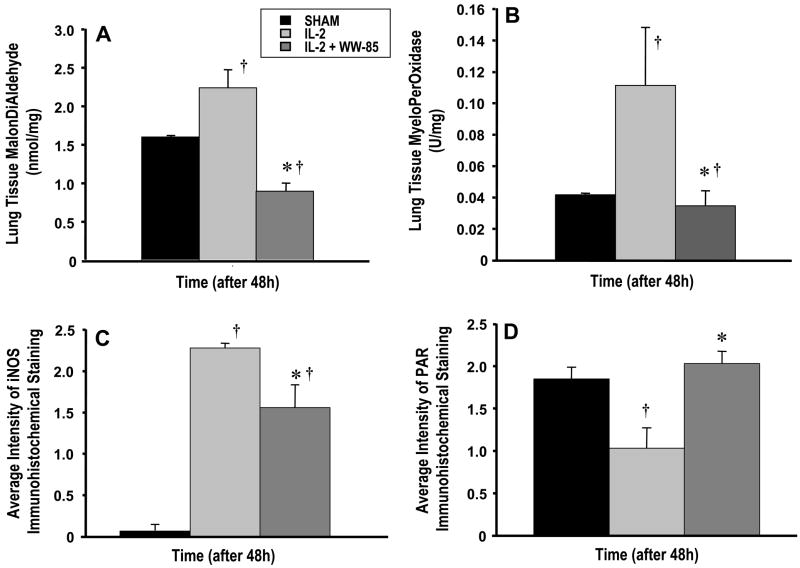
MPO and MDA in lung homogenates was significantly higher in the injured control group than in the sham and treatment groups (p<0.05, Fig. 2A/B). MPO activity in the WW-85-treated group was comparable to the sham group (n.s.).
FIG 2.
Changes in: 2A lung malondialdehyde levels (MDA, nmol/mg), 2B lung myeloperoxidase levels (MPO, U/mg), 2C average intensity of iNOS Immunohistochemical staining, and 2D PAR Immunohistochemical staining 48 h post injury. Data are expressed as mean ± S.E.M. of 6 animals per group. Significance p <0.05; † vs. Sham; * IL-2 vs. IL-2 + WW85.
3-NT content in lung tissue was determined by ELISA after 48h. The concentration in lung homogenates of the control group (1.5±0.5 nM) was significantly higher than in the sham group (0.4± 0.2 nM, p<0.05). Lung tissue 3-NT concentrations in the WW-85 group (0.8±0.4 nM) showed a trend to be lower than in controls.
iNOS and PARS expression
iNOS Immunostaining of the lungs indicated a significant upregulation of iNOS protein in control and WW-85-treated animals as compared to the sham group (p<0.05). The treatment group showed a significant lower iNOS expression than the control group. (p<0.05, Fig. 2C/D). PAR immunostaining of the lungs showed no differences between the sham and the treatment group (p>0.05). The control group, however, showed a significant decrease of the PAR product as compared to the sham and the treatment group (each p<0.05).
Global cardiopulmonary hemodynamics
Heart rate significantly increased in control animals during the first 24 h of the experiment compared to BL, sham, and WW-85 treated animals (p<0.05, Table 1). The WW-85 group displayed no statistical difference to the sham group or BL. Core body temperature significantly increased in response to IL-2 injections in the control group, peaking at 3, 18, and 42 hrs post injury. This temperature increase was significantly attenuated in the WW-85 group as compared to the control groups (p<0.05, Table 1). The central venous pressure (CVP), pulmonary artery occlusion pressure (PAOP) and hematocrit (Hct) remained stable on baseline levels and displayed no statistical difference (n.s., data not shown), indicating adequate fluid resuscitation throughout the 48h experimental period. The mean pulmonary artery pressure (MPAP) significantly increased in IL-2 treated sheep in the first 18h of the experiment compared to BL (p<0.05, Table 1).
Bloodless lung wet/dry weight ratio
The bloodless wet/dry weight ratio of the lung was 4.8±0.4 in sham, 5.0±0.1 in control, and 5.2±0.4 in WW-85 treated animals, respectively. There was no statistical difference between groups.
Discussion
IL-2 is known to play a central role in cell-mediated immune responses directed against cancer cells [1,2,4], and has demonstrated anticancer activity in preclinical and clinical trials. During IL-2 treatment, pulmonary complications are frequently observed, many patients also suffer hypotension, and IL-2 administration is often discontinued because of these side effects [6].
This investigation was designed to test whether the novel peroxynitrite decomposition catalyst WW-85 is useful to attenuate such IL-2-dose-limiting adverse effects on the pulmonary vasculature, since it is known that ONOO− is capable of inducing endothelial dysfunction and vascular hyporeactivity, as well as inactivation of alpha-1 adrenoreceptors and norepinephrine [14]. The present study showed that treatment with WW-85 significantly improved pulmonary transvascular fluid flux, decreased lipid peroxidation, limited iNOS as well as PAR intensity, prevented tachycardia, and attenuated the increase in core body temperature during IL-2 treatment. These findings provide evidence that ONOO− plays a crucial role in the pathology of IL-2 induced toxicity.
Our study suggests that downstream actions of iNOS-dependent ONOO− might explain some of the effects previously attributed to NO. Peroxynitrite is a potent nitrating species and initiator of lipid peroxidation and apoptosis. Traditionally, immunodetection of nitrotyrosine has been used as evidence of peroxynitrite formation in biological tissue [15]. More recently, it has been shown that protein nitration may, under some conditions, arise independently of peroxynitrite via the action of MPO and nitrite [16–18]. While the precise pathways contributing to nitration in SIRS has not yet been determined with certainty, a role of iNOS in nitrotyrosine formation has been confirmed [19]. However, we could show a tendency of reduced 3-NT levels in the present study, the lack of significance might be related to interference with different pathways and the fact, that plasma NOx levels were reduced at certain time points, depending on IL-2 injections, and the 3-NT measurement was performed at the end of the experiment as a total measurement of all time points. Studies using a selective iNOS dimerization inhibitor showed decreased nitrotyrosine levels and apoptosis [20]. Since this intervention also decreased iNOS expression, it is possible that decreased nitration resulted secondarily from decreased production of NO, a substrate for peroxynitrite formation. Other studies using iNOS knockout mice showed decreased apoptosis and lack of nitrotyrosine formation when iNOS−/− donor and recipients were used compared to presence of nitrotyrosine when iNOS+/+ allografts were used [21]. These findings are complemented by studies of Pieper et al. [22] showing limitation of NO by two mechanisms a) by inhibiting iNOS activity but not expression, and b) decreasing iNOS expression by immunosuppressant therapy in rat cardiac transplants, both decreased protein nitration. These findings support a role of iNOS in nitrotyrosine formation. On the other hand, one cannot exclude the possible nitration of proteins via iNOS-dependent but peroxynitrite-independent pathways. Indeed, myeloperoxidase in the presence of nitrite and H2O2 can cause nitration of proteins [16], what may be excluded in this study, since MPO was significantly reduced by WW-85. Based upon our current knowledge, it cannot be excluded that WW-85 may also contribute to peroxynitrite-independent pathways of protein nitration. Our results, however, are in line with previous findings using iron-based metalloporphyrinic agents as peroxynitrite decomposition catalysts which resulted in decreased myocardial myeloperoxidase activity in septic rats [23] and decreased myeloperoxidase activity in lung reperfusion injury [24]. Nevertheless, our findings using the peroxynitrite decomposition catalyst WW-85 are significant in providing evidence that myeloperoxidase-derived nitration is probably not a major source of nitrotyrosine formation in this model of IL-2 induced pulmonary toxicity.
PARP is a nuclear enzyme that is activated by single strand DNA breakage. This enzyme is increased in tissue injury and catalyzes the transfer of ADP-ribose subunits to proteins associated with DNA damage. Peroxynitrite is a potent activator of DNA strand breaks and PARP activation [25,26]. Detection of poly (ADP-ribose) is used to indicate PARP activation. PARP activation has been shown after reperfusion injury in rat cardiac transplants [27,28] following alloimmune activation and rejection in rat cardiac allografts [29], as well as in systemic inflammatory response to burn and smoke inhalation injury [13]. The observation that 5- aminoisoquinoline, an inhibitor of PARP, attenuated rejection scores and improved graft survival in a rodent model of cardiac allograft rejection suggests the importance of PARP activation [29]. In our study, we found that WW85 given alone, normalized PAR staining in IL-2 treated animals. What is shown in figure 2D is not the PARP enzyme, but the product, displaying the degree of PARP activation. Low levels of PARP could be expected in controls, however, if cells with high PARP activation die, they get off the map of observation, and healthy cells remain. This data suggests that poly (ADP-ribose) acted, in part, by decreasing PARP activation in WW-85 treated animals, inhibiting the feedback loop to iNOS and NO formation. Pacher et al. [30] have shown that pharmacological inhibition of PARP with INO-1001 improved cardiac contractility. Furthermore, INO-1001 also significantly improved acetylcholine-induced, nitric-oxide mediated, vascular relaxation of isolated aortic rings. These data are similar to the effect of a phenanthridinone-based PARP inhibitor PJ34, which also improves endothelium-dependent relaxations in the thoracic aorta of aging animals [31–34]. The current findings are in line with previous studies demonstrating that PARP inhibition improves cardio-pulmonary function in various models.
A limitation of our study is that we cannot guarantee that our findings can be transferred to human beings, and that the animals were initially healthy and not compromised by cancer.
Taken together, the results of the present study suggest that the novel peroxynitrite catalyst WW-85 is a useful agent to attenuate IL-2-associated deterioration in pulmonary microvascular permeability. WW-85 is a newer generation of the recently used FP15 compound [35]. It is an antioxidant that should be able to protect against the side effects of IL-2, while it catalyzes the breakdown of ONOO−, and reduces MPO and MDA activity. Further investigations are necessary to transfer these results on human beings.
Acknowledgments
This work was supported in part by grant GM066312 from the National Institutes of Health, by grants 8820 and 8450 from the Shriners of North America, and the Inotek Pharmaceuticals Corporation, Beverly, MA, U.S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herskind C, Fleckenstein K, Lohr J, Li CY, Wenz F, Lohr F. Antitumoral action of interferons and interleukins in combination with radiotherapy. Part I: immunologic basis. Strahlenther Onkol. 2004;180:187–193. doi: 10.1007/s00066-004-9119-x. [DOI] [PubMed] [Google Scholar]
- 2.Herskind C, Fleckenstein K, Lohr J, Li CY, Wenz F, Lohr F. Antitumoral action of interferons and interleukins in combination with radiotherapy. Part II: radiobiological and immunologic strategies. Strahlenther Onkol. 2004;180:331–339. doi: 10.1007/s00066-004-8119-1. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA, Goldman CK, Robb RJ, Depper JM, Leonard WJ, Sharrow SO, Bongiovanni KF, Korsmeyer SJ, Greene WC. Expression of interleukin 2 receptors on activated human B cells. J Exp Med. 1984;160:1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimm EA, Wilson DJ. The human lymphokine-activated killer cell system. V. Purified recombinant interleukin 2 activates cytotoxic lymphocytes which lyse both natural killer-resistant autologous and allogeneic tumors and trinitrophenyl-modified autologous peripheral blood lymphocytes. Cell Immunol. 1985;94:568–578. doi: 10.1016/0008-8749(85)90280-1. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA. The development of new immunotherapies for the treatment of cancer using interleukin-2. Ann Surg. 1988;208:121–135. doi: 10.1097/00000658-198808000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libra M, Talamini R, Crivellari D, Buonadonna A, Freschi A, Stefanovski P, Berretta M, De Cicco M, Balestreri L, Merlo A, Volpe R, Galligioni E, Sorio R. Long-term survival in patients with metastatic renal cell carcinoma treated with continuous intravenous infusion of recombinant interleukin-2: the experience of a single institution. Tumori. 2003;89:400–404. doi: 10.1177/030089160308900410. [DOI] [PubMed] [Google Scholar]
- 7.Murakami K, Privalle C, Enkhbaatar P, Shimoda K, Schmalstieg FC, Deangelo J, Lee S, Traber LD, Traber DL. Pyridoxalated haemoglobin polyoxyethylene conjugate, a nitric oxide scavenger, decreases dose-limiting hypotension associated with interleukin-2 (IL-2) therapy. Clin Sci (Lond) 2003;105:629–635. doi: 10.1042/CS20030164. [DOI] [PubMed] [Google Scholar]
- 8.Szabo C. The pathophysiological role of peroxynitrite in shock, inflammation, and ischemia-reperfusion injury. Shock. 1996;6:79–88. doi: 10.1097/00024382-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Westphal M, Morita N, Enkhbaatar P, Murakami K, Traber L, Traber DL. Carboxyhemoglobin formation following smoke inhalation injury in sheep is interrelated with pulmonary shunt fraction. Biochem Biophys Res Commun. 2003;311:754–758. doi: 10.1016/j.bbrc.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 10.Westphal M, Morita N, Enkhbaatar P, Murakami K, Traber L, Traber DL. Acute effects of combined burn and smoke inhalation injury on carboxyhemoglobin formation, tissue oxygenation, and cardiac performance. Biochem Biophys Res Commun. 2004;317:945–949. doi: 10.1016/j.bbrc.2004.03.135. [DOI] [PubMed] [Google Scholar]
- 11.Staub NC, Bland RD, Brigham KL, Demling R, Erdmann AJ, III, Woolverton WC. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975;19:315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- 12.Maybauer MO, Maybauer DM, Fraser JF, Traber LD, Westphal M, Enkhbaatar P, Cox RA, Huda R, Hawkins HK, Morita N, Murakami K, Mizutani A, Herndon DN, Traber DL. Recombinant human activated protein C improves pulmonary function in ovine acute lung injury following smoke inhalation injury and sepsis. Crit Care Med. 2006;34:2432–2438. doi: 10.1097/01.CCM.0000230384.61350.FA. [DOI] [PubMed] [Google Scholar]
- 13.Shimoda K, Murakami K, Enkhbaatar P, Traber LD, Cox RA, Hawkins HK, Schmalstieg FC, Komjati K, Mabley JG, Szabo C, Salzman AL, Traber DL. Effect of poly(ADP ribose) synthetase inhibition on burn and smoke inhalation injury in sheep. Am J Physiol Lung Cell Mol Physiol. 2003;285:L240–L249. doi: 10.1152/ajplung.00319.2002. [DOI] [PubMed] [Google Scholar]
- 14.Asfar P, Hauser B, Radermacher P, Matejovic M. Catecholamines and vasopressin during critical illness. Crit Care Clin. 2006;22:131–138. doi: 10.1016/j.ccc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 16.Sampson JB, Ye Y, Rosen H, Beckman JS. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- 17.Eiserich JP, Patel RP, O’Donnell VB. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol Aspects Med. 1998;19:221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 18.Gaut JP, Byun J, Tran HD, Lauber WM, Carroll JA, Hotchkiss RS, Belaaouaj A, Heinecke JW. Myeloperoxidase produces nitrating oxidants in vivo. J Clin Invest. 2002;109:1311–1319. doi: 10.1172/JCI15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanazawa T, Kharitonov SA, Barnes PJ. Increased nitrotyrosine in exhaled breath condensate of patients with asthma. Am J Respir Crit Care Med. 2000;164:1273–1276. doi: 10.1164/ajrccm.162.4.9912064. [DOI] [PubMed] [Google Scholar]
- 20.Szabolcs MJ, Sun J, Ma N, Albala A, Sciacca RR, Philips GB, Parkinson J, Edwards N, Cannon PJ. Effects of selective inhibitors of nitric oxide synthase-2 dimerization on acute cardiac allograft rejection. Circulation. 2002;106(18):2392–2396. doi: 10.1161/01.cir.0000034719.08848.26. [DOI] [PubMed] [Google Scholar]
- 21.Szabolcs MJ, Ma N, Athan E, Zhong J, Ming M, Sciacca RR, Husemann J, Albala A, Cannon PJ. Acute cardiac allograft rejection in nitric oxide synthase-2(−/−) and nitric oxide synthase-2(+/+) mice: effects of cellular chimeras on myocardial inflammation and cardiomyocyte damage and apoptosis. Circulation. 2001;103:2514–2520. doi: 10.1161/01.cir.103.20.2514. [DOI] [PubMed] [Google Scholar]
- 22.Pieper GM, Nilakantan V, Chen M, Zhou J, Khanna AK, Henderson JD, Jr, Johnson CP, Roza AM, Szabo C. Protective mechanisms of a metalloporphyrinic peroxynitrite decomposition catalyst, WW85, in rat cardiac transplants. J Pharmacol Exp Ther. 2005;314:53–60. doi: 10.1124/jpet.105.083493. [DOI] [PubMed] [Google Scholar]
- 23.Lancel S, Tissier S, Mordon S, Marechal X, Depontieu F, Scherpereel A, Chopin C, Neviere R. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J Am Coll Cardiol. 2004;43:2348–2358. doi: 10.1016/j.jacc.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 24.Naidu BV, Fraga C, Salzman AL, Szabo C, Verrier ED, Mulligan MS. Critical role of reactive nitrogen species in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2003;22:784–793. doi: 10.1016/s1053-2498(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 25.Virag L, Szabo C. The therapeutic potential of poly (ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 26.Szabo C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140–141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 27.Szabo C. PARP as a Drug Target for the Therapy of Diabetic Cardiovascular Dysfunction. Drug News Perspect. 2002;15:197–205. doi: 10.1358/dnp.2002.15.4.840052. [DOI] [PubMed] [Google Scholar]
- 28.Szabo G, Bahrle S, Stumpf N, Sonnenberg K, Szabo EE, Pacher P, Csont T, Schulz R, Dengler TJ, Liaudet L, Jagtap PG, Southan GJ, Vahl CF, Hagl S, Szabo C. Poly (ADP-Ribose) polymerase inhibition reduces reperfusion injury after heart transplantation. Circ Res. 2002;90:100–106. doi: 10.1161/hh0102.102657. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Son NH, Szabolcs MJ, Ma N, Sciacca RR, Albala A, Edwards N, Cannon PJ. Effects of inhibition of poly(adenosine diphosphate-ribose) synthase on acute cardiac allograft rejection. Transplantation. 2004;78:668–674. doi: 10.1097/01.tp.0000131662.01491.2e. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P, Vaslin A, Benko R, Mabley JG, Liaudet L, Hasko G, Marton A, Batkai S, Kollai M, Szabo C. A new, potent poly(ADP-ribose) polymerase inhibitor improves cardiac and vascular dysfunction associated with advanced aging. J Pharmacol Exp Ther. 2004;311:485–491. doi: 10.1124/jpet.104.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacher P, Liaudet L, Mabley J, Komjati K, Szabo C. Pharmacologic inhibition of poly(adenosine diphosphate-ribose) polymerase may represent a novel therapeutic approach in chronic heart failure. J Am Coll Cardiol. 2002;40:1006–1016. doi: 10.1016/s0735-1097(02)02062-4. [DOI] [PubMed] [Google Scholar]
- 32.Pacher P, Mabley JG, Soriano FG, Liaudet L, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to the endothelial dysfunction associated with hypertension and aging. Int J Mol Med. 2002;9:659–664. [PubMed] [Google Scholar]
- 33.Pacher P, Mabley JG, Soriano FG, Liaudet L, Komjati K, Szabo C. Endothelial dysfunction in aging animals: the role of poly(ADP-ribose) polymerase activation. Br J Pharmacol. 2002;135:1347–1350. doi: 10.1038/sj.bjp.0704627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002;300:862–867. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 35.Naidu BV, Farivar AS, Woolley SM, Fraga C, Salzman AL, Szabo C, Groves JT, Mulligan MS. Enhanced peroxynitrite decomposition protects against experimental obliterative bronchiolitis. Exp Mol Pathol. 2003;75:12–17. doi: 10.1016/s0014-4800(03)00015-7. [DOI] [PubMed] [Google Scholar]