Abstract
Although both monocytes and macrophages possess essential requirements for HIV-1 entry, peripheral blood monocytes are infrequently infected with HIV-1 in vivo and in vitro. In contrast, tissue macrophages and monocyte-derived macrophages in vitro are highly susceptible to infection with HIV-1 R5 tropic strains. We investigated intracellular anti–HIV-1 factors that contribute to differential susceptibility of monocytes/macrophages to HIV-1 infection. Freshly isolated monocytes from peripheral blood had significantly higher levels of the anti–HIV-1 microRNAs (miRNA, miRNA-28, miRNA-150, miRNA-223, and miRNA-382) than monocyte-derived macrophages. The suppression of these anti–HIV-1 miRNAs in monocytes facilitates HIV-1 infectivity, whereas increase of the anti–HIV-1 miRNA expression in macrophages inhibited HIV-1 replication. These findings provide compelling and direct evidence at the molecular level to support the notion that intracellular anti–HIV-1 miRNA-mediated innate immunity may have a key role in protecting monocytes/macrophages from HIV-1 infection.
Introduction
Cells of the macrophage lineage play a crucial role in initial HIV-1 infection and contribute to the pathogenesis of the disease throughout the course of infection. Both monocytes and macrophages are considered as major cell targets for HIV-1 infection.1–3 However, peripheral blood monocytes are infrequently infected with HIV-1 in vivo and in vitro, and only a very small proportion of them (0.001%-1%) harbor HIV-1 at any given time throughout the course of infection.2–4 In contrast, tissue macrophages are readily susceptible to HIV-1 infection in vivo.3,5–9 These in vivo findings are supported by the in vitro observations, showing that peripheral blood isolated monocytes acquire the ability to support productive HIV-1 infection only after their differentiation to macrophages7,10,11 and that HIV-1 replication in monocytes is generally enhanced by factors that facilitate differentiation.12–14 These studies suggest that host innate immunity plays a key role in governing HIV-1 infection of monocyte/macrophages.
Since HIV-1 R5 strains use CCR5 as a primary coreceptor for entry into macrophages, the demonstration10,15of differential expression of the CCR5 receptor on monocytes and macrophages has been suggested as an explanation for the differential susceptibility of monocytes and macrophages to HIV-1 infection. However, this observation does not account fully for differences in HIV-1 infectivity in monocytes and macrophages.10,15,16 Recent studies16,17 showed that several members of APOBEC family members (APOBEC3G/3F), the newly identified HIV-1 restriction factors,18–21 are differentially expressed in immature monocytes and differentiated macrophages. Although monocytes express high levels of functional APOBEC3G proteins, APOBEC3G in macrophages is sequestered in an inactive ribonucleoprotein complex, which increases macrophages' permissibility to HIV-1 infection.18–21
Among a growing list of innate cellular factors that impair various early steps of the HIV-1 life cycle, several cellular microRNAs (miRNAs) have recently been identified to potentially target a set of accessory genes of HIV-1.22 For instance, human miRNA-28, miRNA-125b, miRNA-150, miRNA-223, and miRNA-382 can target the 3′UTR of HIV-1 transcripts,23 potentially rendering productive infection into latency in resting CD4+ T lymphocytes. In addition to their direct effect on HIV-1 replication, the miRNAs also have crucial roles in regulating the host innate immune defense that relies on phagocytes, such as macrophages. Thus, there is a need to examine the role of the anti–HIV-1 miRNAs in protecting monocytes/macrophages from HIV-1 infection. In this study, we investigated expression patterns of the innate anti–HIV-1 miRNAs in the course of monocyte differentiation to macrophages. We also determined the correlation between the levels of the anti–HIV-1 miRNAs and HIV-1 infectivity in monocytes and macrophages.
Methods
Healthy donor and cells culture
Peripheral blood was purchased from University of Pennsylvania, Center for AIDS Research (CFAR). The protocol used to isolate blood from donors, purify the blood components, and distribute this material to the University of Pennsylvania was approved by the IRB of the Center for AIDS Research. The donors gave informed consent in accordance with the Declaration of Helsinki for their blood to be used in this manner. The samples was screened for the common blood-borne pathogens and certified to be pathogen free. Monocytes were purified from peripheral blood of 3 healthy adult donors according to our previously described technique.24 Freshly isolated monocytes were cultured in 96-well culture plates at a density of 5 × 104 cells/well in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum. Macrophages refer to 7-day-cultured monocytes in vitro.
miRNA real-time RT PCR
Total cellular RNA, including miRNA, was extracted from cells using miRNeasy Mini Kit from QIAGEN (Valencia, CA). Total RNA (1 μg) was reverse-transcribed with miScript Reverse Transcription Kit from QIAGEN. The real-time reverse-transcription polymerase chain reaction (RT PCR) for the quantification of a subset of miRNAs (miRNA-28, miRNA-150, miRNA-223, and miRNA-382) was carried out with miScript Primer Assays and miScript SYBR Green PCR Kit from QIAGEN.
Transfection of miRNA mimics and miRNA inhibitors and HIV-1 infection
The miRNA mimics and miRNA inhibitors for miRNA-28, miRNA-150, miRNA-223, or miRNA-382 were chemically synthesized by Dharmacon (Lafayette, CO). The design of miRNA mimic negative control 2 (miRNA Ctl) and miRNA inhibitor negative control 2 (anti-miRNA Ctl) was previously described.23 D0 monocytes or D7 macrophages in 96-well plate were transfected with either 10 pmol mimic miRNA (for monocytes) or 10 pmol miRNA inhibitors (for macrophages) using the transfection reagents (Siport NeoFX [Ambion, Austin, TX] or DharmaFECT 3 [Dharmacon]). At 48 hours after transfection, monocytes or macrophages were infected with HIV-1 R5 strains (Bal or Yu2) at p24 protein concentration (30 ng/106 cells) for 16 hours. Both strains were obtained from the AIDS Research and Reference Reagent Program (National Institutes of Health [NIH]; Germantown, MD). Culture supernatants were collected for HIV-1 RT activity25 at days 6, 8, 10, and 12 after infection.
Results and discussion
HIV-1 replication and the anti–HIV-1 miRNA expression in monocytes/macrophages
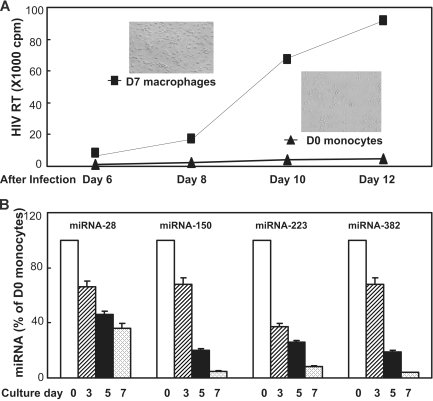
We first compared the susceptibility of D0 monocytes and D7-cultured macrophages to HIV-1 infection. Freshly isolated monocytes were either infected with HIV-1 Bal strain immediately after isolation (day 0) or cultured for 7 days in vitro when the majority of monocytes differentiated to macrophages (Figure 1A inset). Macrophages were infected with HIV-1 Bal at the same titer as for donor-matched monocytes. As anticipated, HIV-1 Bal-infected D0 monocytes exhibited minimal evidence of HIV-1 replication, whereas donor-matched macrophages were highly permissive to HIV-1 infection as evidenced by a steady increase of RT activity during the period of cultures (Figure 1A). In parallel, we also determined the expression of the miRNAs 28, 150, 223, and 382, the newly identified HIV-1 restriction factors, in monocytes/macrophages during the course of differentiation. Because very few monocytes harbor HIV-1 in vivo and monocytes are poorly susceptible to HIV-1 infection in vitro, we hypothesized that monocytes may have high levels of the anti–HIV-1 miRNAs, contributing to their refractory property to HIV-1 infection. This speculation is supported by the observation that D0 monocytes expressed the highest levels of intracellular miRNAs 28, 150, 223, and 382 than the cells cultured at later time points of the in vitro differentiation (Figure 1B). The lowest levels of these miRNAs were observed in the cells cultured for 7 days (Figure 1B) when the majority of monocytes differentiate into macrophages (Figure 1A inset). These observations demonstrated that although both monocytes and macrophages expressed the anti–HIV-1 miRNAs, the levels of these miRNAs in monocytes and macrophages significantly differed, which was correlated with the extent of cell's permissibility to HIV-1 infection.
Figure 1.
Differential HIV-1 infectivity and anti–HIV-1 miRNAs expression in monocytes and macrophages. (A) HIV-1 infection of monocytes and macrophages. Cells were infected with HIV-1 Bal strain either immediately after isolation (D0 monocytes) or after having been cultured for 7 days (D7 macrophages). Replication kinetics of HIV-1 Bal in cell culture was measured by RT activity in culture supernatants. Culture supernatants were collected at the indicated time points postinfection. The data shown are the mean (± SD) of triplicate cultures, representative of three experiments using cells from three different donors. The inserts shown in A are the morphologies of freshly isolated monocytes (D0 monocytes) and 7-day cultured monocytes (D7 macrophages). (B) Anti-HIV-1 miRNAs expression during monocyte differentiation. Cells collected at the indicated time points were subjected RNA extraction for miRNA expression by real-time RT PCR. The data shown are the mean (± SD) of triplicate cultures representative of 3 experiments using cells from 3 different donors.
Modulation of anti–HIV-1 miRNA expression alters permissibility of cells to HIV-1 infection
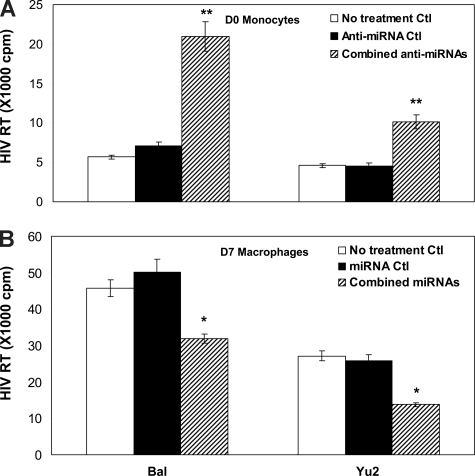
To demonstrate the crucial role of the anti–HIV-1 miRNAs in protecting of monocytes/macrophages from HIV-1 infection, we examined whether the modulation of the anti–HIV-1 miRNA levels in monocytes/macrophages could alter the cell's susceptibility to HIV-1 infection. Monocytes transfected with the combined miRNA inhibitors showed higher levels of HIV-1 RT activity than untreated cells or control inhibitor-transfected monocytes (Figure 2A). We then conducted a second set of experiments to determine whether an increase of the anti–HIV-1 miRNA expression in macrophages could decrease the susceptibility of cells to HIV-1 infection. As shown in Figure 2B, HIV-1 infectivity in macrophages transfected with the combined anti–HIV-1 miRNAs was significantly lower than that in untreated cells or in the cells transfected with the control miRNAs.
Figure 2.
Effect of modulation of the anti-HIV-1 miRNA expression on HIV-1 infection of monocytes and macrophages. (A) D0 monocytes were transfected with the combined microRNA (miRNA-28, 150, 223, and 382) inhibitors or the negative control inhibitor (Anti-miRNA Ctl) for 48 hours, and then infected with the HIV-1 Bal or Yu2 strain. Culture supernatants were collected for HIV-1 RT activity at day 12 postinfection. (B) D7 macrophages were transfected with the combined miRNAs (miRNA-28, 150, 223, and 382) or negative control miRNAs (miRNA Ctl) for 48 hours, and then infected with HIV-1 Bal or Yu2 strain. Culture supernatants were collected for HIV-1 RT activity at day 12 postinfection. The data shown are the mean (± SD) of triplicate culture, representative of 3 experiments using cells from 3 different donors (*P < 0.05, **P < 0.01; combined miRNAs or combined anti-miRNAs vs miRNAs Ctl or anti-miRNA Ctl).
Although other studies have proposed several mechanisms, including the possibility that both extracellular and intracellular innate factors10,15–18 could be linked to myeloid differentiation and susceptibility to HIV-1 infection in vitro, the underlying molecular mechanisms remain to be determined. In an attempt to identify intracellular molecules that contribute to susceptibility of monocytes/macrophages to HIV-1, we examined the expression patterns of several anti–HIV-1 miRNAs in monocytes and macrophages. These miRNAs have been shown to be involved in HIV-1 latency in resting CD4+ T cells.23 Our observation that the levels of intracellular miRNAs were negatively associated with permissiveness of monocytes/macrophages to HIV-1 infection not only provides an additional explanation at the molecular level to address the fundamental question why differentiation of monocytes into macrophages is a prerequisite for productive HIV-1 infection, but also offers insight into the development of intracellular innate immunity-mediated therapy for HIV-1 infection and AIDS. Future studies will have to be done to reveal whether induction of anti–HIV-1 miRNAs in tissue macrophages might have therapeutic potential for purging the HIV-1 reservoir in vivo.
Acknowledgments
We thank the Center for AIDS Research Immunology Core at the University of Pennsylvania for providing fresh peripheral blood monocytes (PBMCs).
This investigation was supported by grants from NIH (DA-012815, and DA-022177 to W.-Z.H.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: X.W. carried out the experiments of the real-time PCR, HIV-1 infection, and RT assay with the support of L.Y., Y.Z., and W.H.; Y.-J.W. performed the experiments with monocyte isolation and cell culture; W.-Z.H. and D.S.M directed and designed the entire study; and all authors critically reviewed the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wen-Zhe Ho, Division of Allergy and Immunology, Abramson Research Center, Room 1202B, The Children's Hospital of Philadelphia, 34th Street & Civic Center Boulevard, Philadelphia, PA 19104; e-mail: ho@email.chop.edu.
References
- 1.Kedzierska K, Azzam R, Ellery P, Mak J, Jaworowski A, Crowe SM. Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy. J Clin Virol. 2003;26:247–263. doi: 10.1016/s1386-6532(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 2.Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem. 2002;9:1893–1903. doi: 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- 3.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 4.Zhu T. HIV-1 in peripheral blood monocytes: an underrated viral source. J Antimicrob Chemother. 2002;50:309–311. doi: 10.1093/jac/dkf143. [DOI] [PubMed] [Google Scholar]
- 5.Lewin SR, Kirihara J, Sonza S, Irving L, Mills J, Crowe SM. HIV-1 DNA and mRNA concentrations are similar in peripheral blood monocytes and alveolar macrophages in HIV-1-infected individuals. AIDS. 1998;12:719–727. doi: 10.1097/00002030-199807000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Sharova N, Swingler C, Sharkey M, Stevenson M. Macrophages archive HIV-1 virions for dissemination in trans. EMBO J. 2005;24:2481–2489. doi: 10.1038/sj.emboj.7600707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu T. HIV-1 genotypes in peripheral blood monocytes. J Leukoc Biol. 2000;68:338–344. [PubMed] [Google Scholar]
- 9.Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 10.Naif HM, Li S, Alali M, et al. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalter DC, Nakamura M, Turpin JA, et al. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J Immunol. 1991;146:298–306. [PubMed] [Google Scholar]
- 13.Koyanagi Y, O'Brien WA, Zhao JQ, Golde DW, Gasson JC, Chen IS. Cytokines alter production of HIV-1 from primary mononuclear phagocytes. Science. 1988;241:1673–1675. doi: 10.1126/science.241.4873.1673. [DOI] [PubMed] [Google Scholar]
- 14.Schrier RD, McCutchan JA, Wiley CA. Mechanisms of immune activation of human immunodeficiency virus in monocytes/macrophages. J Virol. 1993;67:5713–5720. doi: 10.1128/jvi.67.10.5713-5720.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Marzio P, Tse J, Landau NR. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses. 1998;14:129–138. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 16.Chiu YL, Soros VB, Kreisberg JF, Stopak K, Yonemoto W, Greene WC. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature. 2005;435:108–114. doi: 10.1038/nature03493. [DOI] [PubMed] [Google Scholar]
- 17.Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doehle BP, Schafer A, Cullen BR. Human APOBEC3B is a potent inhibitor of HIV-1 infectivity and is resistant to HIV-1 Vif. Virology. 2005;339:281–288. doi: 10.1016/j.virol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Rose KM, Marin M, Kozak SL, Kabat D. Regulated production and anti-HIV type 1 activities of cytidine deaminases APOBEC3B, 3F, and 3G. AIDS Res Hum Retroviruses. 2005;21:611–619. doi: 10.1089/aid.2005.21.611. [DOI] [PubMed] [Google Scholar]
- 20.Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62:957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–2458. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hariharan M, Scaria V, Pillai B, Brahmachari SK. Targets for human encoded microRNAs in HIV genes. Biochem Biophys Res Commun. 2005;337:1214–1218. doi: 10.1016/j.bbrc.2005.09.183. [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Wang F, Argyris E, et al. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13:1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- 24.Hassan NF, Campbell DE, Douglas SD. Purification of human monocytes on gelatin-coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 25.Willey RL, Smith DH, Lasky LA, et al. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988;62:139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]