Summary
Chemical biology relies on effective synthetic chemistry for building molecules to probe and modulate biological function. Olefin metathesis in organic solvents is a valuable addition to this armamentarium, and developments during the previous decade are enabling metathesis in aqueous solvents for the manipulation of biomolecules. Functional group-tolerant ruthenium metathesis catalysts modified with charged moieties or hydrophilic polymers are soluble and active in water, enabling ring-opening metathesis polymerization, cross metathesis, and ring-closing metathesis. Alternatively, conventional hydrophobic ruthenium complexes catalyze a similar array of metathesis reactions in mixtures of water and organic solvents. This strategy has enabled cross metathesis on the surface of a protein. Continuing developments in catalyst design and methodology will popularize the bioorthogonal reactivity of metathesis.
Introduction
Olefin metathesis is a versatile, chemoselective means to create carbon–carbon bonds in a range of molecules including natural products, polymers, and biomolecules [1–6]. In the metathesis reaction, carbon–carbon multiple bonds are broken and made through the intermediacy of transition metal carbenes. This process requires no additional reagents beyond the alkene starting materials and a metal catalyst, and produces simple alkene byproducts, such as ethylene. Modern ruthenium olefin metathesis initiators, such as complexes 1–4, tolerate many functional groups common in biomolecules, including amides, alcohols, and carboxylic acids (Figure 1). Under some circumstances, metathesis occurs even in the presence of amines and sulfur-containing moieties. Moreover, olefin metathesis is highly chemoselective. Most functional groups other than alkenes and alkynes are not modified by ruthenium metathesis catalysts, and the reaction forms only carbon–carbon bonds. These characteristics suggest that olefin metathesis is a candidate for a “bioorthogonal” reaction [7]. Solvent-exposed alkenyl groups are uncommon in living systems, so biomolecules labeled with alkenes could be modified selectively by olefin metathesis.
Figure 1.
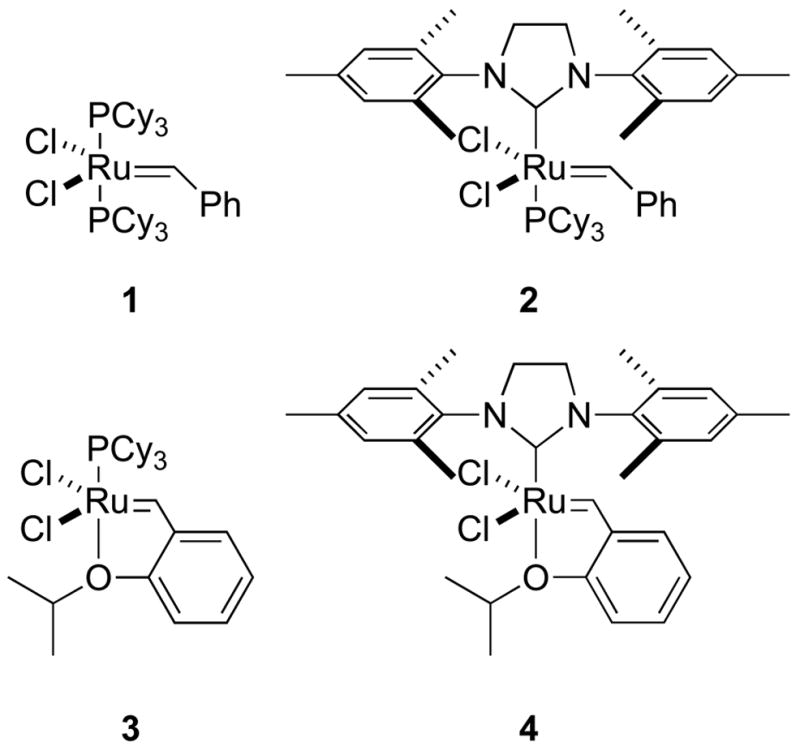
Conventional ruthenium catalysts for olefin metathesis.
As far back as 1975, olefin metathesis was exploited to create molecules to modulate biological function. The laboratories of Rossi and Streck independently recognized the opportunity of metathesis to create complex olefins and used primitive ill-defined metathesis catalysts to prepare insect pheromones from simple alkene precursors [8,9]. Although these initial examples employed sensitive early-transition metal catalysts, major advances in catalyst development since 1975 have produced well-defined metathesis initiators such as air- and moisture-stable ruthenium complexes 1–4. These user-friendly initiators have popularized metathesis among organic chemists and are being used to address biological questions. Nonetheless, olefin metathesis in biological contexts remains limited because of the difficulty of enacting the reaction in aqueous solvents. Conventional metathesis catalysts are insoluble in water, and metathesis with water-soluble complexes in water tends to be far less efficient than in organic solvents. After briefly surveying applications of metathesis in chemical biology, this review will focus on recent progress toward metathesis in homogenous aqueous solutions in order to modify biomolecules in their native environment.
Applications of olefin metathesis in chemical biology
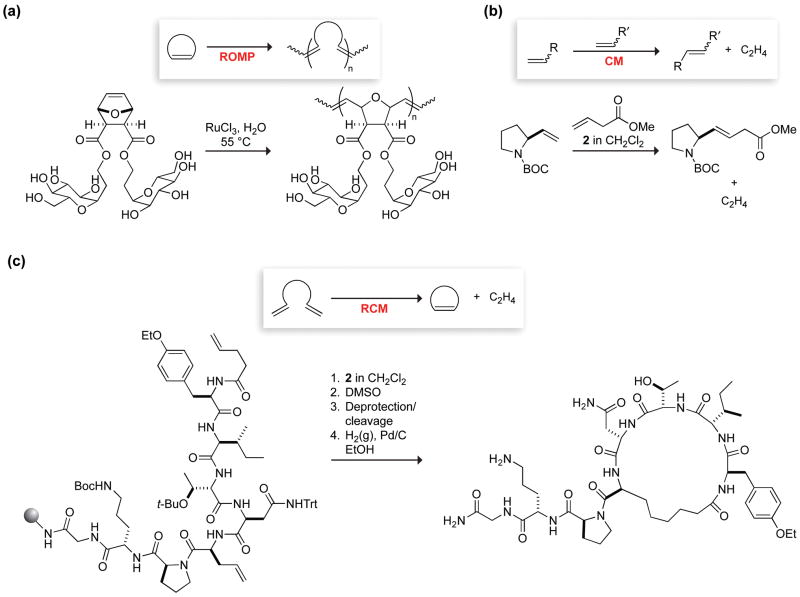
One of the advantages of the metathesis reaction is the wide variety of structures which can be created through its many variations [1,2]. Ring-opening metathesis polymerization (ROMP) converts a cyclic olefin into an unsaturated polymer (Figure 2a). Kiessling and coworkers were the first to use ROMP to synthesize bio-active polymers, including multivalent displays of carbohydrates and other bioactive ligands [10–12]. Such polymers have recently been used to modulate immune responses in vivo [13]. Cross metathesis (CM) exchanges the linear alkene fragments, providing a means to connect two molecules (Figure 2b). The chemoselectivity and functional group tolerance of ruthenium metathesis catalysts enabled Diver and Schreiber to dimerize unprotected FK506 in a single step to form a small-molecule ligand that activated signal transduction and protein expression in vivo [14]. More recently, Raines, Miller, and coworkers examined protein folding using a trans peptide bond mimic made by CM of two alkene-containing fragments (Figure 2b) [15,16]. Ring-closing metathesis (RCM) transforms a diene (or alkene–alkyne) into a cyclic alkene and has proven to be a potent method for creating macrocycles, including bio-active cyclic peptidomimetics (Figure 2c). Demonstrating the robustness of peptide RCM, Liskamp and coworkers have constructed alkene mimics of the five peptide-thioether rings of the lantibiotic nisin [17,18]. Such carba analogs are anticipated to be more stable than native linkages such as disulfide bonds. Vederas and coworkers confirmed this prediction with the disulfide-bridged peptide macrocycle atosiban, finding that a carba-atosiban generated through RCM had a significantly longer half-life in placental tissue and only moderately lower activity as an oxytocin antagonist (Figure 2c) [19,20]. RCM offers an alternative approach to improve peptide stability by creating crosslinks between otherwise flexible portions of a peptide chain. Grubbs and coworkers were the first to demonstrate that RCM could be used to tether residues of helical peptides [21]. From this initial report, the field of peptide “stapling” has blossomed and is reviewed elsewhere in this issue [22]. In one sense, the maturity of metathesis as a tool for chemical biology is evident in Aileron Therapeutics, which is seeking to commercialize bio-active peptides stabilized by RCM-crosslinks [23]. On the other hand, the use of aqueous metathesis for chemical biology is just beginning.
Figure 2.
Olefin metathesis reactions and their application to chemical biology. (a) Ring-opening metathesis polymerization can produce highly-functionalized and well-defined macromolecules, such as this carbohydrate-displaying polymer [10]. (b) Cross metathesis links two alkene fragments to create increasingly complex molecules, including this proline–glycine dipeptide mimic [15,16].
(c) Ring-closing metathesis is an effective reaction for producing cyclic molecules with constrained conformations, such as this oxytocin hormone mimic [20].
Water-soluble phosphine complexes
Grubbs and coworkers led the development of ruthenium olefin metathesis catalysts and recognized that the catalytic ruthenium species were actually water-tolerant, in contrast to other metathesis-active metals. In fact, they noted that ill-defined ruthenium ROMP catalysts initiated faster in water than in organic solvents [24]. These observations led to the subsequent development of well-defined ruthenium initiators such as 1. Grubbs and coworkers returned to water by substituting the hydrophobic phosphines in 1 with electron-rich, cationic phosphines to create complexes such as 5, which are capable of catalyzing ROMP and RCM in water (Figure 3). Notably, complex 5 accomplishes living ROMP in water when activated with one equivalent of hydrochloric acid. These phosphine-based catalysts, however, were highly air-sensitive, and required that one of the alkene partners in RCM be substituted with a terminal alkyl group [25–27]. These problems limited the practicality of water-soluble phosphine-based initiators. More recently, Schanz and coworkers reported benzylidene-functionalized catalysts, such as 6, that catalyze ROMP in mixtures of 2,2,2-trifluoroethanol and water, but are also air-sensitive (Figure 3) [28].
Figure 3.
Ruthenium catalysts for aqueous olefin metathesis.
Polymeric water-soluble N-heterocyclic carbene complexes
Application of N-heterocyclic carbene (NHC) ligands to ruthenium olefin metathesis catalysts has led to remarkable increases in stability and activity in both organic solvents and water. The laboratories of Herrmann [29], Nolan [30], and Grubbs [31] nearly concurrently reported ruthenium complexes bearing one tightly-bound NHC ligand and one labile phosphine, such as 2. Although these complexes initiate more slowly than 1, they transform much faster within the metathesis catalytic cycle. In addition, the NHC ligand increases the stability of the ruthenium complex. Hoveyda and coworkers reported another important advance in catalyst design with complexes 3 and 4 [32,33]. In these initiators, a chelating ether replaces the phosphine ligand, further decreasing the air-sensitivity of the catalyst and improving catalyst stability [34,35•].
Many researchers have capitalized on these improvements in organic-soluble complexes to develop more effective metathesis catalysts for aqueous solution, chiefly by substituting complexes with poly(ethylene glycol) (PEG) or quaternary ammonium moieties. In an early development, Connon and Blechert immobilized a complex similar to 4 on hydrophilic PEGA-NH2 resin (amino-functionalized dimethylacrylamide and mono-2-acrylamidoprop-1-yl polyethyleneglycol) [36]. This heterogeneous catalyst was capable of CM of a variety of alcohols in water but was less effective with charged substrates. In contrast, Grubbs and coworkers have developed soluble polymer-supported complexes. Complex 7, in which PEG is attached to an N-benzyl NHC, is more active than 5 for ROMP of hindered cyclic olefins in water (Figure 3). Nonetheless, complex 7 is rather ineffective for RCM of simple unsubstituted dienes in methanol, and was not reported to catalyze RCM in water [37]. Despite these disappointing results with the first PEG-derivatized initiator, minor modifications led to much more efficient water-soluble catalysts. In particular, shifting the point of attachment of the PEG to the NHC backbone and replacing the phosphine with an ether chelate dramatically improved the activity of 8a over that of 7 (Figure 3) [38]. Complex 8a is stable in water at room temperature for more than one week under inert atmosphere and is faster at ROMP than is complex 7. Moreover, the modified complex enables both RCM of water-soluble α, ω-dienes in neat water in good yields and CM of allyl alcohol. Gratifyingly, complex 8a accomplishes the challenging formation of trisubstituted olefins through RCM. Unfortunately, CM using this catalyst fails with some alkenes substituted with carboxylic acids or quaternary ammonium groups. The basis for the improved activity of this PEG-based initiator is likely its maintaining the crucial 1,3-diaryl NHC structure while incorporating an ether chelate. Furthermore, the amphiphilic nature of the PEG-complex conjugate leads to aggregation of the complexes into micelle-like structures. The resulting sequestration in a PEG shell would protect the catalyst from water, but also have the deleterious consequence of preventing metathesis of bulky, water-soluble substrates such as proteins.
An additional problem with these early water-soluble complexes is that the NHC slows initiation of the complex, preventing the synthesis of well-defined polymers via ROMP. Grubbs and coworkers overcame this problem in organic solvents by creating the fast-initiating bis(3-bromopyridine) complex 9a, and Emrick and coworkers have sought to do the same in water with water-soluble bis(pyridine) complexes such as 9b and 9c (Figure 3) [39,40•]. O-Linked PEG initiator 9b is capable of ROMP at in water low pH, which is presumably required for dissociation of the basic ligands. On the other hand, the phosphorylcholine-based 9c is active under both neutral and acidic conditions, in part because of the increased basicity of the pyridine nitrogen. Unfortunately, these catalysts produce polymers with polydispersity indices (PDI) between 1.3 and 2.4, whereas well-controlled ROMP results in PDI < 1.1. Hence, the production of well-defined ROMP polymers in water remains challenging.
Small-molecule water-soluble N-heterocyclic carbene complexes
Small-molecule aqueous-soluble catalysts avoid the heterogeneity problems of PEG-based catalysts and, in fact, are typically as active as PEG-based catalysts. To date, most of these complexes have been made hydrophilic by the attachment of a quaternary ammonium groups. Taking advantage of the well-documented stability of ruthenium salicylaldimine complexes, we reported complex 10, which is soluble and stable in 2:1 methanol/water (Figure 3) [41]. This robust initiator requires elevated temperatures for activation but enables RCM and ring-closing enyne metathesis of hydrophobic and hydrophilic substrates in methanol/water under air at 55 °C.
The laboratories of both Grubbs [42••] and Grela [43–45,46••] have developed chelating ether complexes functionalized with a range of quaternary ammonium substituents. Jordan and Grubbs reported complex 8b, which bears two cationic centers and is readily soluble in water (Figure 3). This initiator is as active as 8a for aqueous ROMP, RCM, and CM of water-soluble substrates, but does display a greater propensity for promoting isomerization side reactions of challenging substrates. As with 8a, the reported CM substrate scope of 8b is limited to allylic alcohols.
Grela and coworkers examined the activity of complex 8c [43,46••] as well other benzylidene-tagged initiators [44,45] in water and water/alcohol mixtures (Figure 3). This diethylmethylammonium-substituted catalyst is moderately soluble in water and displays a substrate scope similar to that of 8a and 8b for RCM and CM, though this catalyst has not been reported to mediate RCM of water-soluble substituted dienes. Complex 8c also accomplishes RCM and CM of a variety of substrates in homogeneous alcohol/water mixtures. Notably, metathesis by 8c often can be achieved with lower catalyst loadings (1–2.5 mol% versus 5 mol%), a finding Grela and coworkers attribute to the electronic activation provided by the electron-withdrawing quaternary ammonium group. Nonetheless, the multiple water-soluble metathesis catalysts now available all have similar activities and substrate scopes, which, though impressive, have not yet been shown to be sufficient for the synthesis of complex molecules in water.
Olefin metathesis in homogeneous aqueous solvent mixtures
The intricate synthesis of water-soluble ligands presents a further limitation of specially-designed aqueous metathesis catalysts. The use of conventional hydrophobic ruthenium complexes in mixtures of organic solvents and water avoids this difficulty, yet effective metathesis reactions of water-soluble substrates typically require homogeneous conditions. Blechert and coworkers initially demonstrated the possibility of using chelating ether complexes under these conditions, finding that a complex similar to 4 enabled RCM of a hydrophobic substrate in moderate yields in 3:1 N,N-dimethylformamide/water [47]. We extended these results through a more comprehensive survey of reaction conditions [48•]. By testing a variety of water-miscible aprotic solvents, we found that acetone, 1,2-dimethyoxyethane (DME), and PEG are superior cosolvents for aqueous RCM. In addition, 4 is superior to complexes 1–3 in DME/water, likely for the same reasons that NHC ligands and chelating ethers enhance water-soluble catalyst performance. In DME/water and acetone/water, complex 4 enables RCM of a range of hydrophobic and hydrophilic substrates, as well as CM of allyl alcohol, and its performance in these mixed solvents is similar to that of the best water-soluble complexes in water. Thus, the main advantage of the specialized ligands of water-soluble complexes is their hydrophilicity rather than their effects on stability or activity. The efficiency of both catalyst systems is still far lower than that of conventional complexes in organic solvents. Further advances in aqueous metathesis will likely require ligands that provide even greater water-tolerance.
Olefin metathesis on proteins
Using a similar strategy, Davis and coworkers have further developed aqueous CM and achieved metathesis on a protein [49••]. Screening a variety of alkene partners for CM with allyl alcohol in 1:1 water/t-butyl alcohol, they found that alkenes with allylic heteroatoms are privileged participants in CM and that sulfur substitution is the most effective. The effectiveness of CM diminishes with butenyl and pentenyl sulfides, leading Davis and coworkers to hypothesize that the allylic heteroatom plays a role as a directing group, while the more distant sulfides form unproductive chelates. Incorporation of S-allylcysteine on the surface of a model protein, subtilisin Bacillus lentus, provides a potential protein CM partner [50]. Although CM fails with allyl alcohol and 200 equivalents of 4 in 7:3 phosphate buffer/t-butyl alcohol, the addition of MgCl2 (presumably to mask protein carboxylate groups) enables CM with allyl alcohol and allylic ethers in 50–90% conversion (Figure 4). This methodology allowed Davis and coworkers to functionalize the model protein with carbohydrate and PEG moieties while maintaining the enzymatic activity of subtilisin. It is noteworthy that S-allylcysteine can be incorporated genetically as a methionine surrogate [49••].
Figure 4.
Olefin cross metathesis for site-specific protein modification at allyl sulfide substituents [49]. The allyl group is appended to Cys156 of the S156C variant of subtilisin Bacillus lentus. The protein structure is based on the atomic coordinates in PDB entry 1st3.
Conclusions
The olefin metathesis reaction is a highly versatile tool for synthetic chemical biologists. Modern ruthenium-based catalysts are readily available and can be easily used in organic solution to construct molecules for probing and manipulating biological function. On the other hand, metathesis on biomolecules in water remains challenging. Advances in catalyst design during the past decade have enabled simple ROMP, RCM, and CM in water, but controlled ROMP to create low polydispersity polymers is still difficult and the CM of many substrates remains impossible. Many of the reactions made possible by water-soluble catalysts are also catalyzed by conventional complexes in aqueous–organic solutions amenable to biological substrates. This hybrid approach has enabled the first CM on the surface of a protein using complex 4, a success that heralds olefin metathesis in water as a method for chemical biology. Nonetheless, more complex applications of metathesis, such as demanding ROMP or CM in water, will likely require the endowment of ruthenium catalysts with additional stability and activity in aqueous environments.
Acknowledgments
Work in our laboratory on olefin metathesis is supported by grant GM044783 (NIH). J.B.B. is supported by an NSF Graduate Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Grubbs RH, editor. Handbook of Metathesis. Weinheim, Germany: Wiley-VCH; 2003. [Google Scholar]
- 2.Nicolaou KC, Bulger PG, Sarlah D. Metathesis reactions in total synthesis. Angew Chem, Int Ed. 2005;44:4490–4527. doi: 10.1002/anie.200500369. [DOI] [PubMed] [Google Scholar]
- 3.Schrock RR. Multiple metal-carbon bonds for catalytic metathesis reactions (Nobel Lecture) Angew Chem, Int Ed. 2006;45:3748–3759. doi: 10.1002/anie.200600085. [DOI] [PubMed] [Google Scholar]
- 4.Grubbs RH. Olefin-metathesis catalysts for the preparation of molecules and materials (Nobel Lecture) Angew Chem, Int Ed. 2006;45:3760–3765. doi: 10.1002/anie.200600680. [DOI] [PubMed] [Google Scholar]
- 5.Schrodi Y, Pederson RL. Evolution and applications of second-generation ruthenium olefin metathesis catalysts. Aldrichimica Acta. 2007;40:45–52. [Google Scholar]
- 6.Hoveyda AH, Zhugralin AR. The remarkable metal-catalysed olefin metathesis reaction. Nature. 2007;450:243–251. doi: 10.1038/nature06351. [DOI] [PubMed] [Google Scholar]
- 7.Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 8.Rossi R. Simple synthesis of sex pheromones of the housefly and tiger moths by transition metal-catalyzed olefin cross-metathesis reactions. Chim Ind (Milano) 1975;57:242–243. [Google Scholar]
- 9.Kuepper FW, Streck R. Synthesis of insect pheromones using metathesis catalysts. Chem-Ztg. 1975;99:464–465. [Google Scholar]
- 10.Mortell KH, Gingras M, Kiessling LL. Synthesis of cell agglutination inhibitors by aqueous ring-opening metathesis polymerization. J Am Chem Soc. 1994;116:12053–12054. [Google Scholar]
- 11.Kiessling LL, Strong LE. Bioactive polymers. Top Organomet Chem. 1998;1:199–231. [Google Scholar]
- 12.Lee Y, Sampson NS. Romping the cellular landscape: Linear scaffolds for molecular recognition. Curr Opin Struct Biol. 2006;16:544–550. doi: 10.1016/j.sbi.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Puffer EB, Pontrello JK, Hollenbeck JJ, Kink JA, Kiessling LL. Activating B cell signaling with defined multivalent ligands. ACS Chem Biol. 2007;2:252–262. doi: 10.1021/cb600489g. [DOI] [PubMed] [Google Scholar]
- 14.Diver ST, Schreiber SL. Single-step synthesis of cell-permeable protein dimerizers that activate signal transduction and gene expression. J Am Chem Soc. 1997;119:5106–5109. [Google Scholar]
- 15.Vasbinder MM, Miller SJ. Synthesis of the Pro–Gly dipeptide alkene isostere using olefin cross-metathesis. J Org Chem. 2002;67:6240–6242. doi: 10.1021/jo025888j. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins CL, Vasbinder MM, Miller SJ, Raines RT. Peptide bond isosteres: Ester or (E)-alkene in the backbone of the collagen triple helix. Org Lett. 2005;7:2619–2622. doi: 10.1021/ol050780m. [DOI] [PubMed] [Google Scholar]
- 17.Ghalit N, Rijkers DTS, Liskamp RMJ. Alkene- and alkyne-bridged mimics of nisin as potential peptide-based antibiotics. J Mol Catal A: Chem. 2006;254:68–77. [Google Scholar]
- 18.Ghalit N, Reichwein JF, Hilbers HW, Breukink E, Rijkers DTS, Liskamp RMJ. Synthesis of bicyclic alkene-/alkane-bridged nisin mimics by ring-closing metathesis and their biochemical evaluation as lipid II binders: Toward the design of potential novel antibiotics. ChemBioChem. 2007;8:1540–1554. doi: 10.1002/cbic.200700244. [DOI] [PubMed] [Google Scholar]
- 19.Stymiest JL, Mitchell BF, Wong S, Vederas JC. Synthesis of biologically active dicarba analogues of the peptide hormone oxytocin using ring-closing metathesis. Org Lett. 2003;5:47–49. doi: 10.1021/ol027160v. [DOI] [PubMed] [Google Scholar]
- 20.Stymiest JL, Mitchell BF, Wong S, Vederas JC. Synthesis of oxytocin analogues with replacement of sulfur by carbon gives potent antagonists with increased stability. J Org Chem. 2005;70:7799–7809. doi: 10.1021/jo050539l. [DOI] [PubMed] [Google Scholar]
- 21.Blackwell HE, Grubbs RH. Highly efficient synthesis of covalently cross-linked peptide helices by ring-closing metathesis. Angew Chem, Int Ed. 1998;37:3281–3284. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Henchey LK, Jochim AL, Arora PS. Contemporary strategies for the stabilization of peptides in the α-helical conformation. Curr Opin Chem Biol. 2008;12:xxx–xxx. doi: 10.1016/j.cbpa.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drahl C. Harnessing helices. Chem Eng News. 2008;86:18–23. [Google Scholar]
- 24.Novak BM, Grubbs RH. Catalytic organometallic chemistry in water: The aqueous ring-opening metathesis polymerization of 7-oxanorbornene derivatives. J Am Chem Soc. 1988;110:7542–7543. [Google Scholar]
- 25.Mohr B, Lynn DM, Grubbs RH. Synthesis of water-soluble, aliphatic phosphines and their application to well-defined ruthenium olefin metathesis catalysts. Organometallics. 1996;15:4317–4325. [Google Scholar]
- 26.Kirkland TA, Lynn DM, Grubbs RH. Ring-closing metathesis in methanol and water. J Org Chem. 1998;63:9904–9909. [Google Scholar]
- 27.Lynn DM, Mohr B, Grubbs RH, Henling LM, Day MW. Water-soluble ruthenium alkylidenes: Synthesis, characterization, and application to olefin metathesis polymerization in protic solvents. J Am Chem Soc. 2000;122:6601–6609. [Google Scholar]
- 28.Roberts AN, Cochran AC, Rankin DA, Lowe AB, Schanz H-J. Benzylidene-functionalized ruthenium-based olefin metathesis catalysts for ring-opening metathesis polymerization in organic and aqueous media. Organometallics. 2007;26:6515–6518. [Google Scholar]
- 29.Weskamp T, Kohl FJ, Hieringer W, Gleich D, Herrmann WA. Highly active ruthenium catalysts for olefin metathesis: The synergy of N-heterocyclic carbenes and coordinatively labile ligands. Angew Chem, Int Ed. 1999;38:2416–2419. doi: 10.1002/(sici)1521-3773(19990816)38:16<2416::aid-anie2416>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Stevens ED, Nolan SP, Petersen JL. Olefin metathesis-active ruthenium complexes bearing a nucleophilic carbene ligand. J Am Chem Soc. 1999;121:2674–2678. [Google Scholar]
- 31.Scholl M, Trnka TM, Morgan JP, Grubbs RH. Increased ring closing metathesis activity of ruthenium-based olefin metathesis catalysts coordinated with imidazolin-2-ylidene ligands. Tetrahedron Lett. 1999;40:2247–2250. [Google Scholar]
- 32.Kingsbury JS, Harrity JPA, Bonitatebus PJ, Jr, Hoveyda AH. A recyclable Ru-based metathesis catalyst. J Am Chem Soc. 1999;121:791–799. [Google Scholar]
- 33.Garber SB, Kingsbury JS, Gray BL, Hoveyda AH. Efficient and recyclable monomeric and dendritic Ru-based metathesis catalysts. J Am Chem Soc. 2000;122:8168–8179. [Google Scholar]
- 34.Maechling S, Zaja M, Blechert S. Unexpected results of a turnover number (TON) study utilizing ruthenium-based olefin metathesis catalysts. Adv Synth Catal. 2005;347:1413–1422. [Google Scholar]
- 35•.Hong SH, Wenzel AG, Salguero TT, Day MW, Grubbs RH. Decomposition of ruthenium olefin metathesis catalysts. J Am Chem Soc. 2007;129:7961–7968. doi: 10.1021/ja0713577. The authors present a highly active water-soluble metathesis catalyst that is capable of ROMP, RCM, and simple CM in water. The complex is solubilized and protected by a pendant PEG. [DOI] [PubMed] [Google Scholar]
- 36.Connon SJ, Blechert S. A solid-supported phosphine-free ruthenium alkylidene for olefin metathesis in methanol and water. Bioorg Med Chem Lett. 2002;12:1873–1876. doi: 10.1016/s0960-894x(02)00260-3. [DOI] [PubMed] [Google Scholar]
- 37.Gallivan JP, Jordan JP, Grubbs RH. A neutral, water-soluble olefin metathesis catalyst based on an N-heterocyclic carbene ligand. Tetrahedron Lett. 2005;46:2577–2580. [Google Scholar]
- 38.Hong SH, Grubbs RH. Highly active water-soluble olefin metathesis catalyst. J Am Chem Soc. 2006;128:3508–3509. doi: 10.1021/ja058451c. [DOI] [PubMed] [Google Scholar]
- 39.Breitenkamp K, Emrick T. Amphiphilic ruthenium benzylidene metathesis catalyst with PEG-substituted pyridine ligands. J Polym Sci, Part A: Polym Chem. 2005;43:5715–5721. [Google Scholar]
- 40•.Samanta D, Kratz K, Zhang X, Emrick T. A synthesis of PEG- and phosphorylcholine-substituted pyridines to afford water-soluble ruthenium benzylidene metathesis catalysts. Macromolecules. 2008;41:530–532. This paper reports on water-soluble ROMP catalysts functionalized with PEG and phosphorylcholine moieties. [Google Scholar]
- 41.Binder JB, Guzei IA, Raines RT. Salicylaldimine ruthenium alkylidene complexes: Metathesis catalysts tuned for protic solvents. Adv Synth Catal. 2007;349:395–404. doi: 10.1002/adsc.200600264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Jordan JP, Grubbs RH. Small-molecule N-heterocyclic-carbene-containing olefin-metathesis catalysts for use in water. Angew Chem, Int Ed. 2007;46:5152–5155. doi: 10.1002/anie.200701258. This study reports on small-molecule water-soluble metathesis catalysts bearing remote quaternary ammonium groups. These complexes mediate ROMP, RCM, and simple CM. [DOI] [PubMed] [Google Scholar]
- 43.Michrowska A, Gulajski L, Kaczmarska Z, Mennecke K, Kirschning A, Grela K. A green catalyst for green chemistry: Synthesis and application of an olefin metathesis catalyst bearing a quaternary ammonium group. Green Chem. 2006;8:685–688. [Google Scholar]
- 44.Rix D, Clavier H, Coutard Y, Gulajski L, Grela K, Mauduit M. Activated pyridinium-tagged ruthenium complexes as efficient catalysts for ring-closing metathesis. J Organomet Chem. 2006;691:5397–5405. [Google Scholar]
- 45.Rix D, Caijo F, Laurent I, Gulajski L, Grela K, Mauduit M. Highly recoverable pyridinium-tagged Hoveyda–Grubbs pre-catalyst for olefin metathesis. Design of the boomerang ligand toward the optimal compromise between activity and reusability. Chem Commun. 2007:3771–3773. doi: 10.1039/b705451c. [DOI] [PubMed] [Google Scholar]
- 46••.Gulajski L, Michrowska A, Naroznik J, Kaczmarska Z, Rupnicki L, Grela K. A highly active aqueous olefin metathesis catalyst bearing a quaternary ammonium group. ChemSusChem. 2008;1:103–109. doi: 10.1002/cssc.200700111. The authors describe a water-soluble ruthenium complex that is activated by an electron-withdrawing quaternary ammonium group. The complex enables RCM as well as CM of allyl alcohol in water and aqueous mixtures. [DOI] [PubMed] [Google Scholar]
- 47.Connon SJ, Rivard M, Zaja M, Blechert S. Practical olefin metathesis in protic media under an air atmosphere. Adv Synth Catal. 2003;345:572–575. [Google Scholar]
- 48•.Binder JB, Blank JJ, Raines RT. Olefin metathesis in homogeneous aqueous media catalyzed by conventional ruthenium catalysts. Org Lett. 2007;9:4885–4888. doi: 10.1021/ol7022505. This study explores the activity of conventional olefin metathesis catalysts in homogenous mixtures of water and organic solvents. The combination of the Hoveyda–Grubbs II complex and either aqueous DME or acetone facilitates RCM and simple CM, even in the presence of a protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Lin YA, Chalker JM, Floyd N, Bernardes GJL, Davis BG. Allyl sulfides are privileged substrates in aqueous cross-metathesis: Application to site-selective protein modification. J Am Chem Soc. 2008;130:9642–9643. doi: 10.1021/ja8026168. The authors report cross metathesis of allylic alcohols and ethers with an allyl sulfide on the surface of a protein. Their data reveal that allyl sulfides are particularly effective when compared to other CM partners. [DOI] [PubMed] [Google Scholar]
- 50.Bernardes GJL, Chalker JM, Errey JC, Davis BG. Facile conversion of cysteine and alkyl cysteines to dehydroalanine on protein surfaces: Versatile and switchable access to functionalized proteins. J Am Chem Soc. 2008;130:5052–5053. doi: 10.1021/ja800800p. [DOI] [PubMed] [Google Scholar]