Abstract
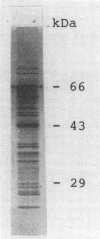
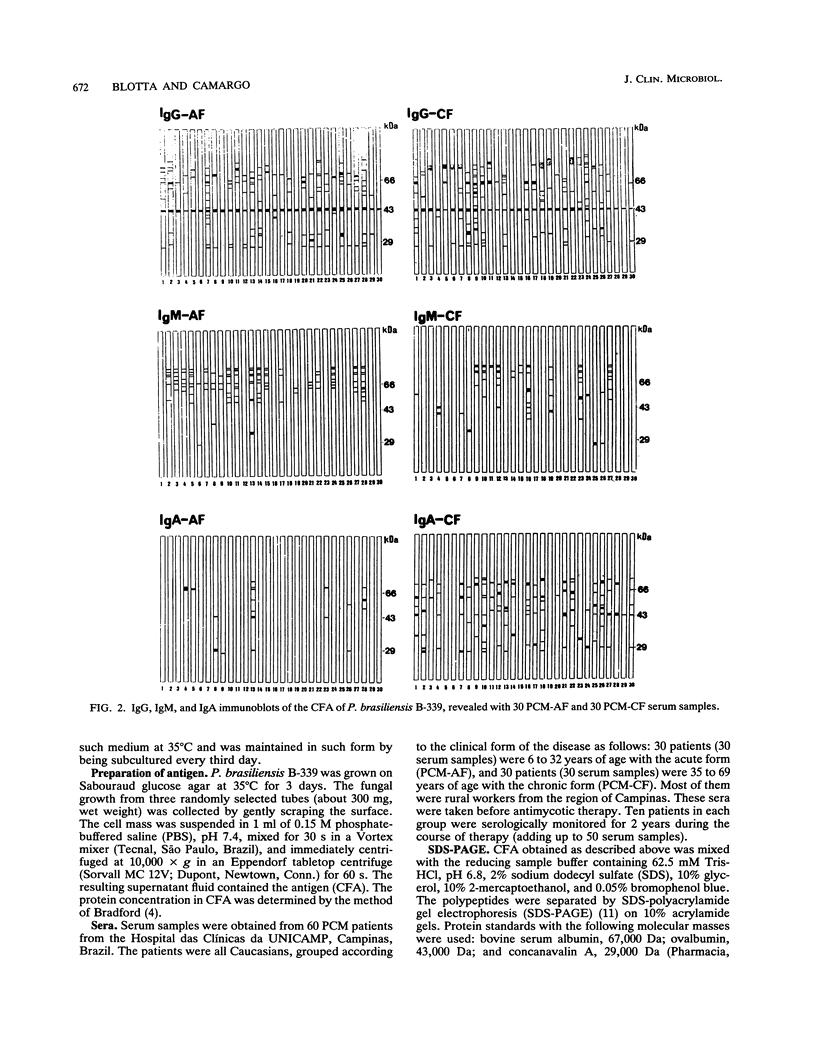
Sera from patients with the acute (AF) and chronic (CF) forms of paracoccidioidomycosis (PCM) were tested against Paracoccidioides brasiliensis cell-free antigens by Western blot (immunoblot). The CFA preparation contained components ranging in molecular mass from 18 to 102 kDa. The immunoglobulin G (IgG) reactivity profiles were similar for patients with both forms of the disease, and the 43-kDa component was recognized by 100% of the sera. IgM antibodies from the AF- and the CF-PCM sera recognized 21 and 20 components, respectively, the AF-PCM sera reacting preferentially with components with molecular masses above 50 kDa. None of the AF-PCM sera (IgM) reacted with the 43-kDa component, and only 10% of the CF-PCM sera recognized this molecule. The IgA response was more significant in the CF-PCM group than in the AF-PCM group, and the 43- and 74-kDa components were the most reactive ones (about 40% each). Our results showed that the cell-free antigen preparation is very appropriate for the immunoblotting analysis of PCM sera, and they also showed that the detection of IgG anti-gp43 is the best marker for the diagnosis and the following up of patients with the acute or the chronic form of the disease.
Full text
PDF
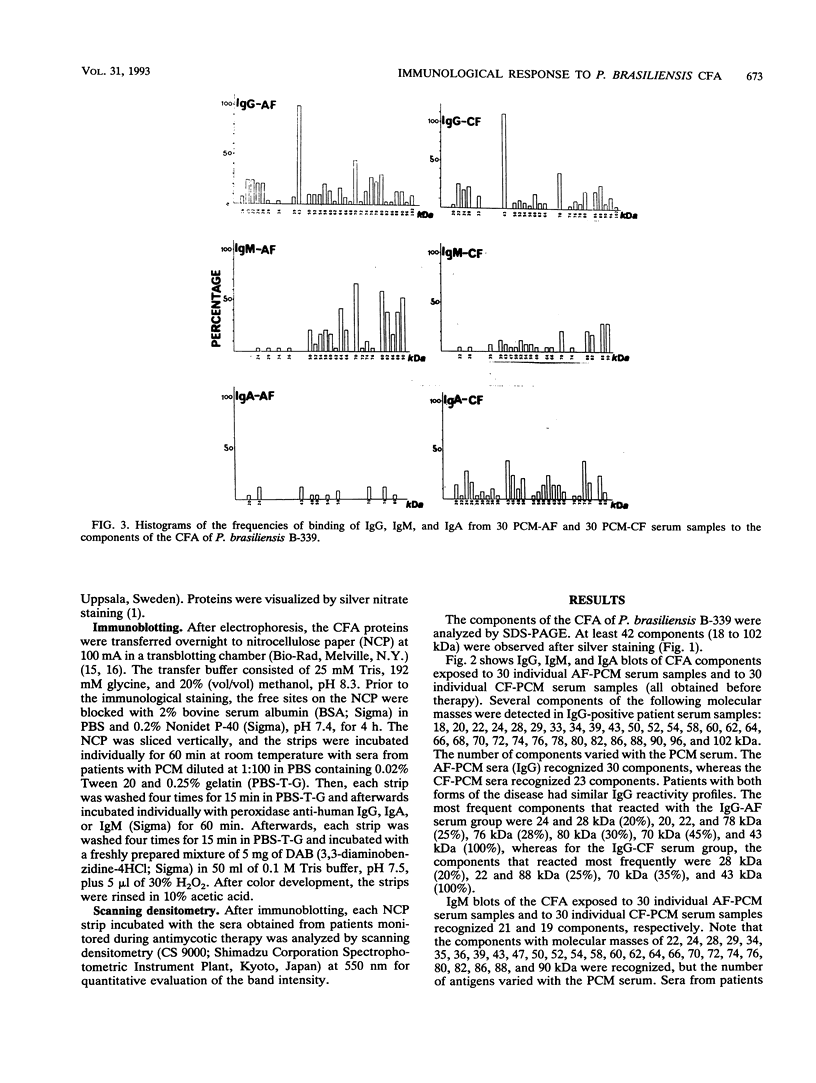
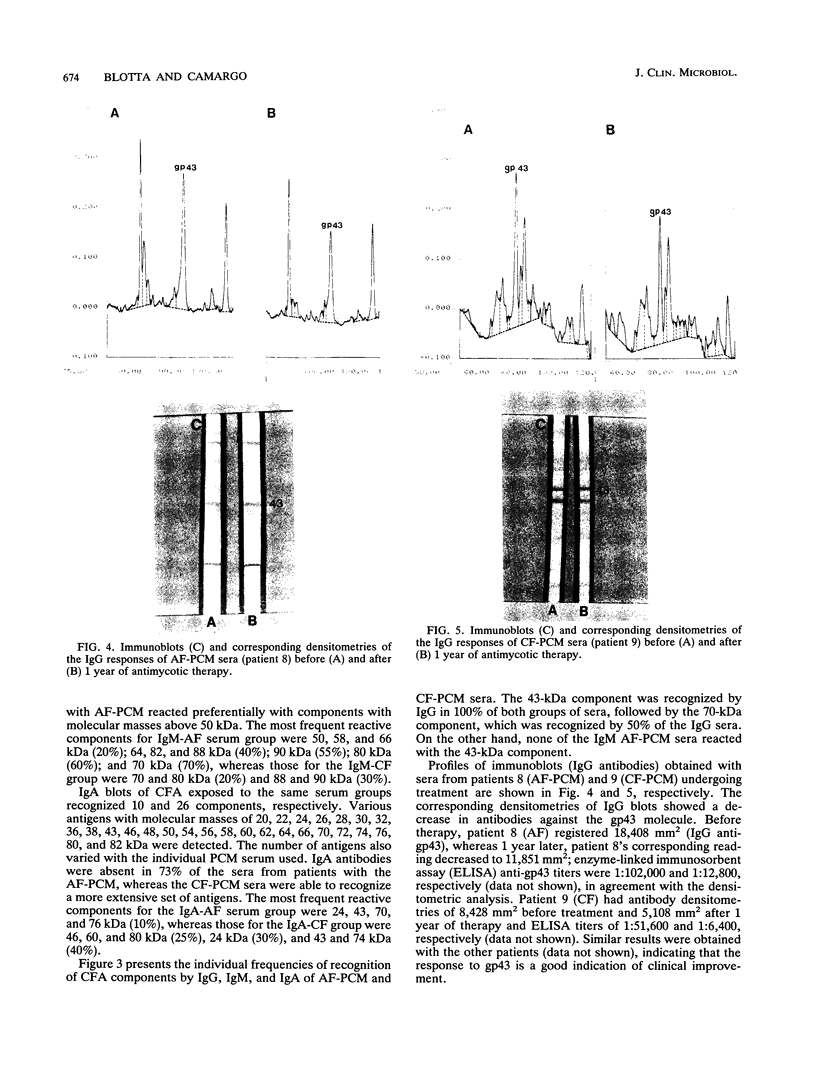


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumer S. O., Jalbert M., Kaufman L. Rapid and reliable method for production of a specific Paracoccidioides brasiliensis immunodiffusion test antigen. J Clin Microbiol. 1984 Mar;19(3):404–407. doi: 10.1128/jcm.19.3.404-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Camargo Z. P., Taborda C. P., Rodrigues E. G., Travassos L. R. The use of cell-free antigens of Paracoccidioides brasiliensis in serological tests. J Med Vet Mycol. 1991;29(1):31–38. [PubMed] [Google Scholar]
- Camargo Z. P., Unterkircher C., Travassos L. R. Identification of antigenic polypeptides of Paracoccidioides brasiliensis by immunoblotting. J Med Vet Mycol. 1989;27(6):407–412. [PubMed] [Google Scholar]
- Casotto M., Paris S., Camargo Z. P. Antigens of diagnostic value in three isolates of Paracoccidioides brasiliensis. J Med Vet Mycol. 1991;29(4):243–253. doi: 10.1080/02681219180000361. [DOI] [PubMed] [Google Scholar]
- De Camargo Z., Unterkircher C., Campoy S. P., Travassos L. R. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J Clin Microbiol. 1988 Oct;26(10):2147–2151. doi: 10.1128/jcm.26.10.2147-2151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M. Host-parasite relationships in paracoccidioidomycosis. J Med Vet Mycol. 1987 Feb;25(1):5–18. doi: 10.1080/02681218780000021. [DOI] [PubMed] [Google Scholar]
- Franco M., Montenegro M. R., Mendes R. P., Marques S. A., Dillon N. L., Mota N. G. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev Soc Bras Med Trop. 1987 Apr-Jun;20(2):129–132. doi: 10.1590/s0037-86821987000200012. [DOI] [PubMed] [Google Scholar]
- Giannini M. J., Bueno J. P., Shikanai-Yasuda M. A., Stolf A. M., Masuda A., Amato Neto V., Ferreira A. W. Antibody response to the 43 kDa glycoprotein of Paracoccidioides brasiliensis as a marker for the evaluation of patients under treatment. Am J Trop Med Hyg. 1990 Aug;43(2):200–206. doi: 10.4269/ajtmh.1990.43.200. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Puccia R., Schenkman S., Gorin P. A., Travassos L. R. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect Immun. 1986 Jul;53(1):199–206. doi: 10.1128/iai.53.1.199-206.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambuk B. U., Puccia R., de Almeida M. L., Travassos L. R., Schenkman S. Secretion of the 43 kDa glycoprotein antigen by Paracoccidioides brasiliensis. J Med Vet Mycol. 1988;26(6):367–373. doi: 10.1080/02681218880000521. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz C. A., Mackenzie D. W., Hearn V. M., Camargo Z. P., Singer-Vermes L. M., Burger E., Calich V. L. Specific recognition pattern of IgM and IgG antibodies produced in the course of experimental paracoccidioidomycosis. Clin Exp Immunol. 1992 Apr;88(1):119–123. doi: 10.1111/j.1365-2249.1992.tb03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]