Abstract
Background & Aims
Diabetes mellitus is associated with an increased risk of colorectal cancer. Thiazolidinediones, which are ligands for PPARγ, are widely used to treat patients with diabetes. PPARγ is highly expressed in the colon and exposure to thiazolidinediones has been proposed to affect the risk for colorectal neoplasia. Studies using in vitro models suggest that thiazolidinediones have anti-neoplastic effects, whereas in vivo studies have produced mixed results--some indicate an increased risk for intestinal tumors. This study examined the association between PPARγ-targeted therapies and the risk of colonic neoplasia in patients with diabetes.
Methods
We conducted 3 retrospective case-control studies nested within the cohort of diabetic patients that were cared for within the Kaiser Permanente of Northern California system from 1994 to 2005. Case subjects were those with colonic neoplasia identified at the time of colonoscopy (study-1), sigmoidoscopy (study-2), or at follow-up lower endoscopy (study-3). Controls had no neoplasia identified at the endoscopic exam. A minimum of 1 year of therapy was used to define medication exposure.
Results
14,086 patients were included. Among patients undergoing colonoscopy, there was an inverse association between thiazolidinedione exposure and prevalence of neoplasia (adjusted OR=0.73, 95% CI 0.57–0.92); however, this was not evident among patients without anemia (adjusted OR= 0.97, 95% CI 0.64–1.49). Significant associations between any or long-term thiazolidinedione use and colonic neoplasia were not observed among patients undergoing sigmoidoscopy or serial lower endoscopies.
Conclusions
These results indicate that thiazolidinedione therapy is not associated with an increased risk for colonic neoplasia.
Introduction
Patients with type 2 diabetes mellitus have an increased risk for colorectal neoplasia.1 One hypothesis for the relation between type 2 diabetes mellitus and colon neoplasia is that hyperinsulinemia promotes colon carcinogenesis. High fasting levels of insulin are associated with an increased risk of colon cancer.2 Similarly, among patients with type 2 diabetes mellitus, those treated long-term with exogenous insulin may have a particularly high risk of colon cancer.3 Elevated insulin levels and glucose have also been linked to the risk of colorectal adenomas, the precursor of most colorectal cancers.4
Peroxisome proliferator-activated receptor gamma (PPARγ) is expressed at high levels in both the colonic epithelium and in colon cancer cell lines.5–8 Thiazolidinedione (TZD) ligands for PPARγ are used to treat type 2 diabetes mellitus. Unlike other commonly used therapies for diabetes mellitus, TZDs increase insulin sensitivity, thereby decreasing insulin and insulin-like growth factor levels.9, 10 As such, if hyperinsulinemia directly leads to colonic neoplasia, one would anticipate that long-term therapy with TZDs might decrease the risk. Further, antiproliferative and proapoptotic effects of TZDs have been demonstrated in numerous cancer cell lines, including colorectal cancer.11 However, animal models of colon carcinogenesis have provided mixed results.11 In the azoxymethane model, TZDs have been shown to prevent aberrant crypt foci and tumor formation.12, 13 In addition, partial deficiency of PPARγ receptor leads to increased susceptibility to tumors in this model.14 In contrast, in two studies, C57BL/6J-APCMin/+ mice treated with TZDs had higher rates of intestinal tumor formation6, 7, while in a third study, pioglitazone decreased tumor formation in APC deficient mice.15
Data are limited on the effect of TZD therapy on colon carcinogenesis in humans.16 17 Two prior clinical studies were limited by short follow-up and exposure periods. Because it may take many years to decades to develop colon cancer and TZDs were only first marketed in 1997, studies that focused on cancer as an outcome may have lacked sufficient follow-up time to observe a biologically important effect. Therefore, we investigated whether there is an association between TZD use and development of adenomatous polyps, the precursor lesion to nearly all human colon cancers.
Methods
We conducted three retrospective case-control studies nested within the cohort of diabetic patients cared for within the Kaiser Permanente healthcare system in Northern California (KPNC) during the period 1994 to 1996 and who continue to receive care between 1999 and 2005.
Setting
Kaiser Permanente Northern California provides comprehensive healthcare services to approximately 3.2 million members, representing approximately 25% of the population of the geographic area. The KPNC pharmacy database includes information on each outpatient prescription dispensed at a KPNC pharmacy. Prior research has demonstrated that 80% to 85% of KPNC members fill all of their prescriptions at Kaiser pharmacies; it is approximately 95% for those with a pharmacy benefit18.
Patients with diagnosed diabetes mellitus are identified from several sources (pharmacy data, glycosylated hemoglobin [HbA1c] level >=6.7%, and outpatient, emergency room, and hospitalization records) annually for inclusion in the KPNC Diabetes Registry. As of January 1, 1996, the identification method was estimated to be 96% sensitive19.
Source cohort and selection of cases and controls
To be included in the study cohort, patients were required to have completed a survey of diabetic patients that was administered during the year 1994 to 1996 and to be identified as having type 2 diabetes mellitus. From approximately 85,000 patients with type 1 or type 2 diabetes enrolled in KPNC between 1994 and 1996, 62,465 completed the questionnaire and were identified as having type 2 diabetes mellitus. This cohort has been previously described.20 Patients were excluded from the study cohort if they had a history of inflammatory bowel disease, familial adenomatous polyposis syndrome, or hereditary non-polyposis colon cancer syndrome (n= 237).
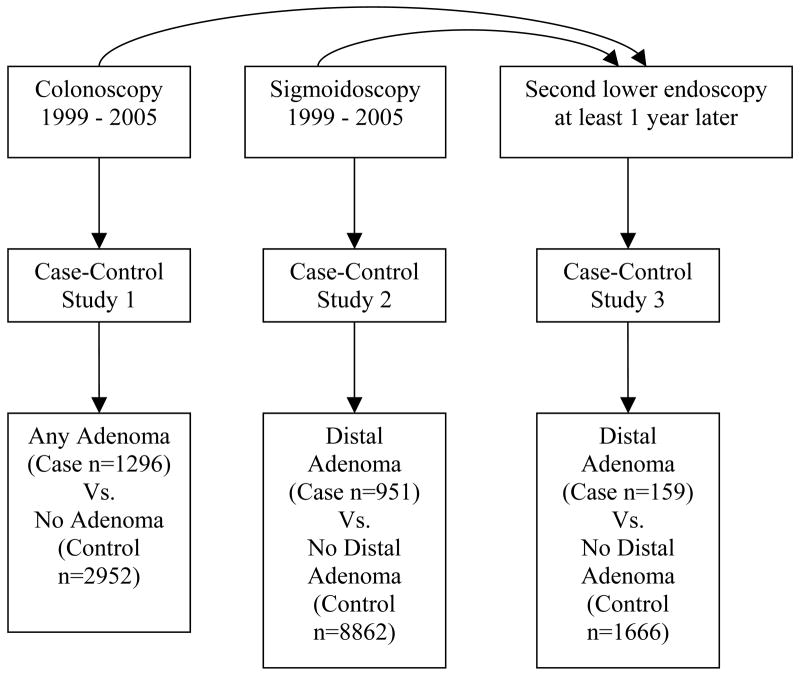
Three case-control studies were conducted within this cohort (Figure 1), one to examine neoplasia in the entire colon, one to focus primarily on patients undergoing colon cancer screening, and one to examine newly formed adenomas. To be included in the first case-control study, patients were required to have undergone at least one colonoscopy between January 1, 1999 and December 31, 2005 (the first colonoscopy being defined as the index endoscopy), to have been at least 50 years old at the time of the index colonoscopy and to have continuous pharmacy benefits from KPNC between January 1, 1997 and the date of the index colonoscopy. For the primary analyses, there could not have been a sigmoidoscopy in the 6 months preceding the colonoscopy. Inclusion criteria for the second case-control study were identical, except that patients must have undergone a sigmoidoscopy as their index endoscopy. Inclusion criteria for the third case-control study required the patient to have undergone two lower endoscopies (colonoscopy or sigmoidoscopy) between January 1, 1999 and December 31, 2005 with the second lower endoscopy being at least one year after the first, regardless of whether adenomatous neoplasia was present at the first exam. The second lower endoscopy was defined as the index endoscopy.
Figure 1.
Schematic representation of the three case-control studies. From the 62,465 Kaiser Permanente members who completed the diabetes survey in 1994–1996, 14,061 were eligible and underwent a colonoscopy or sigmoidoscopy during the study period. Among these, 1,825 underwent a second lower endoscopy that qualified for inclusion in the third case-control study, with case and control status based on the findings at the second lower endoscopy.
In the first case-control study, cases were defined as patients with one or more adenomatous lesions (adenomatous polyps or invasive colorectal cancer) at any location within the colon on the index endoscopy. For patients with a second lower endoscopy within six months of the index endoscopy (such as a colonoscopy to follow-up a polyp found on sigmoidoscopy), we included any adenomatous lesion identified at either procedure (i.e., this was treated as a single procedure). In the second case-control study, the adenomatous polyps or invasive cancer had to be located in the distal colon, defined as any lesion diagnosed at sigmoidoscopy or if diagnosed at follow-up colonoscopy that was located in the rectum or sigmoid colon or located in the distal 40cm if no anatomic segment was noted. Control subjects were defined as patients without adenomatous lesions on the index endoscopy (or follow-up lower endoscopy) using the same anatomic criteria. Similar criteria were used to define cases and controls for the third case-control study, except that the second lower endoscopy (more than 12 months after first endoscopy) was defined as the index exam.
All pathology reports were manually reviewed to determine the presence or absence of adenomatous lesions.
Identification of TZD exposure
Exposure was measured at any time prior to the index endoscopy. The primary exposure definition was cumulative exposure to TZDs for a minimum of one year prior to the date of the index endoscopy. Cumulative exposure was measured from prescription records. If the next prescription was filled within 30 days of the expected end date of the previous prescription, therapy was categorized as uninterrupted. However, if there were no refills within the 30 days after the expected end date of the previous prescription, we assumed a gap in therapy starting 30 days after the date that the previous prescription should have ended. Based on review of refill intervals, the following exception was made for prescriptions with 200 or more days supply. If there were no further refills (i.e. the last or only prescription) and the days supply was for 200 or more days, we assumed a duration of 100 days (the mode) for all oral medications and 30 days (the mode) for insulin. Only prescriptions from 1/1/97 to the index date were included when calculating cumulative exposure.
In secondary analyses, we examined alternative definitions of TZD exposure, including cumulative duration of TZD therapy (categorized as none, less than 1 year, 1 to <2 years, 2 years or longer), time since most recent TZD therapy (categorized no use, current use, last use within the preceding year, last use 1 to less than 2 years prior, and at least 2 years prior), and average dose of TZD during the period of use. Because three different TZDs have been prescribed in the health plan, we standardized average daily dose by expressing it as a percentage of maximum recommended dose (45mg for pioglitazone, 8mg for rosiglitazone, and 600mg for troglitazone).
Confounder variables
Data on potential confounders were extracted from the diabetes survey (diabetes duration, body mass index, and race) and a variety of electronic databases. These databases included registration files (age, sex, and location of residence), laboratory data (baseline HbA1C concentration), pharmacy data (other diabetes medications, acid suppression medications, statins, and nonsteroidal anti-inflammatory drugs [NSAIDs]), and medical encounters (lower endoscopy between January 1, 1995 and January 1, 1999, and diabetic retinopathy). Household income was estimated based on the median income of the census block of residency. For diabetes medications other than TZDs, we used the same definition of exposure (i.e. at least one year of cumulative exposure at any time prior to the index date). However, for other concomitant medications such as aspirin and NSAIDs, we required at least one year of cumulative exposure and use within the year preceding the index endoscopy to be considered exposed.21
To adjust for confounding as a result of prior colon cancer screening with lower endoscopy, we included a variable for the most recent prior lower endoscopy during the period from January 1, 1995 to the index date. We elected to categorize this variable into five levels: no prior lower endoscopy, prior colonoscopy (with or without sigmoidoscopy) in the preceding 3 years, prior sigmoidoscopy without colonoscopy in the preceding 3 years, prior colonoscopy (with or without sigmoidoscopy) more than 3 years prior to the index endoscopy, and prior sigmoidoscopy without colonoscopy more than 3 years prior to the index endoscopy. This categorization was based on the concept that the likelihood of developing new polyps increases with increasing duration from the most recent colonoscopy, that the subclinical period for colonic polyps is likely more than seven years, and that sigmoidoscopy examines only part of the colon22.
Statistical analyses
In each of the case-control studies, the association between TZD exposure and colonic polyps was assessed using logistic regression. We included age, sex, other categories of diabetes medications and calendar year in all models. A separate indicator variable was created for each of the different classes of diabetes medications. We then added other confounders separately to determine whether inclusion of the variable altered the baseline estimate of the odds ratio by 10% or more. Variables that met this criterion were included in the final multivariable model. Time between lower endoscopies and the presence or absence of adenomatous neoplasia on the first lower endoscopy were tested for confounding and effect modification in the third case-control study using multivariable models and stratified models. For analyses of dose and duration, we repeated this process of confounder selection.
Secondary analyses were performed defining case subjects as having advanced adenomas or early adenomas. We defined advanced adenoma according to the histology and did not include the polyp size since we did not have access to endoscopy reports. Advanced neoplasia was defined as any adenoma with villous feature, high-grade dysplasia or invasive cancer. Separate logistic regression models were used to calculate odds ratios for early adenoma and advanced adenoma relative to no adenoma. In these analyses, patients were categorized according to their most advanced lesion.
In order to assure that we were not including patients with glucose intolerance rather than diabetes mellitus, a sensitivity analysis was conducted in the first case-control study excluding patients without a minimum of one year of cumulative exposure to at least one category of diabetes medications. A set of sensitivity analyses was conducted in the first two case-control studies including all patients who had a sigmoidoscopy prior to their index colonoscopy in the first study and including patients who had a colonoscopy or sigmoidoscopy as their index lower endoscopy in the second study. Another sensitivity analysis included large (>9mm) tubular adenomas based on the size noted on the pathology report as advanced adenomas. An additional sensitivity analysis in the third case-control study only defined exposure based solely on prescriptions filled between the first and second lower endoscopy. Finally, because TZD use can cause anemia that might prompt a lower endoscopy, a final sensitivity analysis excluded patients with a diagnosis of anemia (hemoglobin less than 13.5g/dl in males, 11.5g/dl in females) within the 6 months preceding the index date.
Results
Among the 62,465 patients with type 2 diabetes mellitus, 14,086 had undergone at least one lower endoscopy during the study period and met the other inclusion criteria. Cases and controls were relatively similar in all baseline characteristics and across the three case-control studies (Table 1). In each case-control study, there were slightly lower percentages of case subjects that were women (e.g. 41% cases vs. 49% controls in study-1) or users of prescription NSAIDs (e.g. 12% cases vs. 17% controls in study-1). The median duration of TZD use was 769.5 days (IQR 545 – 1108 days) in study-1, 760.5 days (IQR 545.5 – 1114.5 days) in study-2, and 850.5 days (IQR 590.0 – 1296.0 days) in study-3.
Table 1.
Characteristics of TZD Users and Non-Users
| Study-1 Neoplasia anywhere in the colon
|
Study-2 Distal colon neoplasia
|
Study-3 Distal colon neoplasia on repeat exam
|
||||
|---|---|---|---|---|---|---|
| Cases n=1296 | Controls n=2952 | Cases n=951 | Controls n=8862 | Cases n=159 | Controls n=1666 | |
| Female (%) | 41 | 49 | 37 | 46 | 46 | 47 |
| Age (median years, IQR) | 71 (65–77) | 71 (64–77) | 67 (60–74) | 66 (60–73) | 71 (65–76) | 71 (65–76) |
| Race (%) | ||||||
| White | 60 | 59 | 57 | 52 | 62 | 55 |
| Black | 14 | 12 | 12 | 16 | 11 | 16 |
| Hispanic | 12 | 12 | 12 | 13 | 13 | 13 |
| Asian | 10 | 11 | 14 | 14 | 10 | 12 |
| Other | 2 | 3 | 2 | 3 | 1 | 2 |
| Missing | 2 | 3 | 2 | 3 | 3 | 2 |
| BMI (%) | ||||||
| 25 or less | 17 | 17 | 16 | 17 | 18 | 17 |
| >25 to 30 | 36 | 34 | 38 | 34 | 36 | 33 |
| >30 | 36 | 38 | 36 | 37 | 32 | 39 |
| Missing | 10 | 11 | 10 | 12 | 13 | 12 |
| Socioeconomic status (%) | ||||||
| < median | 49 | 47 | 42 | 45 | 48 | 45 |
| ≥ the median | 49 | 50 | 55 | 52 | 48 | 53 |
| Missing | 3 | 3 | 2 | 3 | 4 | 3 |
| Education (%) | ||||||
| <12 years | 19 | 18 | 14 | 14 | 13 | 15 |
| 12 years | 26 | 28 | 23 | 25 | 33 | 27 |
| > 12 years | 51 | 49 | 57 | 55 | 45 | 52 |
| Missing | 5 | 5 | 6 | 6 | 9 | 6 |
| Diabetes duration (%) | ||||||
| Less than 5 years | 26 | 30 | 34 | 33 | 31 | 31 |
| 5 to <10 years | 25 | 23 | 25 | 24 | 22 | 23 |
| 10 or more years | 39 | 40 | 32 | 35 | 36 | 38 |
| Missing | 10 | 8 | 9 | 9 | 11 | 8 |
| Diabetic retinopathy (%) | 12 | 11 | 11 | 10 | 10 | 10 |
| Hemoglobin A1c percentage* (%) | ||||||
| <7 | 26 | 28 | 23 | 26 | 20 | 28 |
| 7 to <8 | 21 | 20 | 21 | 21 | 17 | 21 |
| 8 to <9 | 14 | 15 | 16 | 14 | 20 | 15 |
| 9 to <10 | 9 | 9 | 10 | 9 | 9 | 9 |
| 10 or greater | 15 | 14 | 15 | 16 | 18 | 14 |
| Missing | 14 | 14 | 15 | 14 | 16 | 13 |
| Diabetes medications** (%) | ||||||
| TZD | 8 | 11 | 6 | 7 | 11 | 14 |
| Sulfonylurea | 64 | 67 | 63 | 66 | 68 | 70 |
| Metformin | 32 | 38 | 37 | 38 | 45 | 50 |
| Insulin | 35 | 33 | 29 | 29 | 38 | 33 |
| Other | 0 | <1 | <1 | <1 | 0 | <1 |
| Concomitant medications† (%) | ||||||
| NSAID‡ | 12 | 17 | 11 | 15 | 10 | 17 |
| Aspirin‡ | 2 | 2 | 1 | 1 | 6 | 2 |
| Proton pump inhibitor | 4 | 6 | 2 | 3 | 6 | 6 |
| Statin | 38 | 38 | 32 | 34 | 47 | 53 |
| Most recent lower endoscopy during 1995–1998 (%) | ||||||
| Colonoscopy ≤ 3 years prior†† | 5 | 5 | 1 | 1 | 6 | 6 |
| Sigmoidoscopy ≤ 3 years prior†† | 5 | 5 | 1 | 3 | 6 | 7 |
| Colonoscopy > 3 years prior†† | 19 | 19 | 3 | 3 | 11 | 9 |
| Sigmoidoscopy >3 years prior†† | 19 | 22 | 11 | 16 | 11 | 14 |
| None | 53 | 49 | 84 | 78 | 67 | 64 |
| Lower endoscopy with polypectomy 1995–1998 (%) | 18 | 16 | 5 | 4 | 13 | 12 |
| Index endoscopy (%) | ||||||
| Colonoscopy | N/A | N/A | N/A | N/A | 65 | 62 |
| Sigmoidoscopy | N/A | N/A | N/A | N/A | 16 | 35 |
| Sigmoidoscopy followed by colonoscopy | N/A | N/A | N/A | N/A | 19 | 3 |
| Time between lower endoscopies (median years, IQR) | N/A | N/A | N/A | N/A | 2.8 (1.6–4.4) | 3.0 (1.9–4.3) |
HbA1c was measured at baseline entry into the cohort
At least one year of cumulative use prior to the index date
At least one year of cumulative use and use within 1 year of the index date
Based on prescription data. Excludes over-the-counter purchases.
Prior to index date
Study 1 – Adenoma in Any Region of the Colon
During the study period, 4248 patients underwent at least one colonoscopy, of whom 1296 (31%) had at least one adenoma. TZDs were used by 104 (8%) patients with adenomas and 318 (11%) patients without adenomas (unadjusted OR 0.72, 95% CI 0.57–0.91). After adjusting for age, sex, year of the endoscopy and use of diabetes medications other than TZDs, case subjects were less likely to have been exposed to TZDs (adjusted OR 0.73, 95% CI 0.57–0.92). No other variables met our definition of a confounder. We observed stronger associations between TZD use and absence of adenomas with current use than with former use and with use for at least one year than with less than 1 year in duration (Table 2). When we categorized adenomas as early or advanced, the results were similar, although the confidence intervals were wider because of the smaller number of case subjects.
Table 2.
Association Between TZD Use and the Prevalence of Adenomatous Polyps in the Colon (Study-1)
| All Patients
|
Without Anemia
|
With Anemia
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls
|
Any adenoma
|
Early adenoma
|
Advanced adenoma
|
Any adenoma
|
Any adenoma
|
||||
| n | n | Adjusted OR* (95% CI) | n | Adjusted OR* (95% CI) | n | Adjusted OR* (95% CI) | Adjusted OR* (95% CI) | Adjusted OR* (95% CI) | |
| Ever exposed | |||||||||
| No (no TZD use or <1 year of use) | 2634 | 1192 | 1.00 | 753 | 1.00 | 439 | 1.00 | 1.00 | 1.00 |
| Yes (1 year or longer) | 318 | 104 | 0.73 (0.57, 0.92) | 65 | 0.72 (0.54, 0.95) | 39 | 0.74 (0.52, 1.07) | 0.97 (0.64, 1.46) | 0.65 (0.48,0.87) |
| Time since most recent TZD use | |||||||||
| No TZD use or <1 year of use | 2634 | 1192 | 1.00 | 753 | 1.00 | 439 | 1.00 | 1.00 | 1.00 |
| Current user | 206 | 61 | 0.66 (0.49, 0.89) | 39 | 0.66 (0.46, 0.95) | 22 | 0.65 (0.41, 1.03) | 0.73 (0.43, 1.22) | 0.64 (0.44,0.93) |
| Less than 1 year | 65 | 21 | 0.72 (0.44, 1.19) | 12 | 0.65 (0.35, 1.22) | 9 | 0.85 (0.41, 1.73) | 1.33 (0.54, 3.30) | 0.58 (0.31,1.08) |
| 1 to <2 years | 21 | 8 | 0.85 (0.37, 1.95) | 7 | 1.14 (0.48, 2.73) | 1 | 0.30 (0.04, 2.26) | 0.91 (0.17, 4.84) | 0.87 (0.33,2.25) |
| 2 years or longer | 26 | 14 | 1.21 (0.62, 2.35) | 7 | 0.98 (0.42, 2.29) | 7 | 1.58 (0.67, 3.73) | 2.85 (0.95, 8.48) | 0.69 (0.27,1.73) |
| Duration of therapy** | |||||||||
| No TZD use | 2398 | 1098 | 1.00 | 694 | 1.00 | 404 | 1.00 | 1.00 | 1.00 |
| Less than 1year | 236 | 94 | 0.90 (0.69, 1.16) | 59 | 0.91 (0.67, 1.23) | 35 | 0.89 (0.61, 1.30) | 0.84 (0.53, 1.33) | 0.95 (0.70,1.30) |
| 1 to <2 years | 149 | 46 | 0.70 (0.49, 0.98) | 26 | 0.62 (0.40, 0.95) | 20 | 0.83 (0.51, 1.36) | 0.89 (0.51, 1.56) | 0.62 (0.40,0.97) |
| 2 years or longer | 169 | 58 | 0.73 (0.54, 1.01) | 39 | 0.78 (0.54, 1.14) | 19 | 0.65 (0.40, 1.07) | 1.02 (0.58, 1.80) | 0.66 (0.45,0.97) |
| Average daily dose | |||||||||
| No or <1 year of use | 2634 | 1192 | 1.00 | 753 | 1.00 | 439 | 1.00 | 1.00 | 1.00 |
| <0.65 units per day | 156 | 48 | 0.69 (0.49, 0.96) | 30 | 0.69 (0.46, 1.03) | 18 | 0.69 (0.41, 1.15) | 0.99 (0.55, 1.77) | 0.60 (0.39,0.91) |
| 0.65 or greater units per day | 162 | 56 | 0.77 (0.56, 1.05) | 35 | 0.74 (0.51, 1.09) | 21 | 0.80 (0.50, 1.28) | 0.95 (0.55, 1.64) | 0.70 (0.47,1.03) |
Adjusted only for age, sex, calendar year of index date, and use of other diabetes medications. Addition of other variables did not change any of the odds ratios by 10% of more.
Duration of therapy with TZDs prior to the index date.
Sensitivity analyses for study 1 provided similar results with one exception. Among the 554 case subjects and 1095 control subjects without a history of anemia, there was no evidence of an inverse association between TZD exposure and adenomatous neoplasia at colonoscopy. In this subgroup, TZD exposure for 1 year or longer [39 cases (7%) and 84 controls (8%)] had an adjusted odds ratio of 0.97 (95% CI 0.64–1.46). Similarly, the inverse associations for current use and longer term therapy observed in the overall cohort were not observed after excluding patients with anemia (Table 2). When we examined the subjects who had a history of anemia, the results were similar to the overall analyses. In this subgroup, TZD exposure was less common among patients with adenomas, particularly among patients who had taken TZDs for at least 1 year and who were currently being treated with a TZD (Table 2). A test for statistical interaction based on the presence or absence of anemia was not significant (p=0.39).
Post hoc stratified analyses on selected clinical characteristics (HBA1c, diabetes duration, insulin therapy, and race) are included in supplemental Table 1 available at www.gastrojournal.org. These analyses generally provided similar results to the primary analyses.
Study 2 – Adenoma in the Distal Colon
During the study period, 9813 patients underwent at least one lower endoscopy, of whom 951 (10%) had at least one distal adenoma. TZDs were used by 60 (6%) patients with distal adenomas and by 656 (7%) of patients without distal adenomas. After adjusting for age, sex, year of the endoscopy and use of diabetes medications other than TZDs, use of TZDs was not associated with the presence of distal adenomas (adjusted OR 0.86, 95% CI 0.65–1.14) (Table 3). Likewise, there was little evidence of strengthening the association with more recent use (current use adjusted OR 0.86, 95% CI 0.61–1.20), increased duration of use (≥2 years adjusted OR 1.08, 95% CI 0.76–1.54), or higher average daily dose (≥0.65 units adjusted OR 0.96, 95% CI 0.67–1.38) (Table 3). When we categorized distal adenomas as early or advanced, the results were similar. Exposure to TZDs was not associated with early adenoma (adjusted OR 0.83, 95% CI 0.59–1.17) or advanced adenomas (adjusted OR 0.93, 95% CI 0.58–1.50). The sensitivity and stratified analyses produced nearly identical results to the primary analyses (sensitivity analyses data not shown, stratified analyses available in supplemental Table 2 at www.gastrojournal.org).
Table 3.
Association Between TZD Use and Prevalence of Distal Adenomatous Polyps in the Colon (Study-2)
| Controls
|
Any adenoma
|
Early adenoma
|
Advanced adenoma
|
||||
|---|---|---|---|---|---|---|---|
| n | n | Adjusted OR* (95% CI) | n | Adjusted OR* (95% CI | n | Adjusted OR* (95% CI) | |
| Ever exposed | |||||||
| No (no TZD use or <1 year of use) | 8206 | 891 | 1.00 | 622 | 1.00 | 269 | 1.00 |
| Yes (1 year or longer) | 656 | 60 | 0.86 (0.65, 1.14) | 40 | 0.83 (0.59, 1.17) | 20 | 0.93 (0.58, 1.50) |
| Time since most recent TZD use | |||||||
| No TZD use or <1 year of use | 8206 | 891 | 1.00 | 622 | 1.00 | 269 | 1.00 |
| Current user | 437 | 40 | 0.86 (0.61, 1.20) | 30 | 0.92 (0.62, 1.35) | 10 | 0.70 (0.37, 1.34) |
| Less than 1 year | 107 | 10 | 0.86 (0.45, 1.66)† | 6 | 0.75 (0.33, 1.73) | 4 | 1.1 2 (0.41, 3.08) |
| 1 to <2 years | 40 | 5 | 1.22 (0.48, 3.13) | 2 | 0.69 (0.16, 2.87) | 3 | 2.58 (0.78, 8.51) |
| 2 years or longer | 72 | 5 | 0.70 (0.28, 1.74) | 2 | 0.42 (0.10, 1.73) | 3 | 1.22 (0.37, 3.99) |
| Duration of therapy** | |||||||
| No TZD use | 7650 | 826 | 1.00 | 575 | 1.00 | 251 | 1.00 |
| Less than 1year | 556 | 65 | 1.12 (0.86, 1.48) | 47 | 1.17 (0.85, 1.60) | 18 | 1.02 (0.62, 1.67) |
| 1 to <2 years | 316 | 22 | 0.66 (0.42, 1.03) | 13 | 0.57 (0.32, 1.01) | 9 | 0.85 (0.43, 1.68) |
| 2 years or longer | 340 | 38 | 1.08 (0.76, 1.54) | 27 | 1.11 (0.73, 1.67) | 11 | 1.02 (0.54, 1.92) |
| Average daily dose | |||||||
| No or <1 year of use | 8206 | 891 | 1.00 | 622 | 1.00 | 269 | 1.00 |
| <0.65 units per day | 328 | 26 | 0.76 (0.50, 1.15) | 17 | 0.72 (0.44, 1.20) | 9 | 0.83 (0.42, 1.66) |
| 0.65 or greater units per day | 328 | 34 | 0.96 (0.67, 1.38) | 23 | 0.93 (0.60, 1.44) | 11 | 1.03 (0.55, 1.91) |
Adjusted only for age, sex, calendar year of index date, and use of other diabetes medications.
Adjusting for race, this OR = 0.78. None of the other odds ratios changed by 10% or more with the addition of race or any other variable to the model.
Duration of therapy with TZDs prior to the index date
Study 3 – New or Missed Lesions in the Distal Colon
During the study period, 1825 patients underwent two lower endoscopies at least 1 year apart, of whom 159 (9%) had at least one distal adenoma on the second endoscopy. TZDs were used by 11% of these patients and 14% of patients without distal adenomas on the second endoscopy. After adjusting for age, sex, year of the endoscopy and use of diabetes medications other than TZDs, use of TZDs was not significantly associated with the presence of adenomatous polyps on the second lower endoscopy (adjusted OR 0.75, 95% CI 0.44, 1.28). There was little evidence of a dose or duration relationship (Table 4). Because only two patients with 1 year or longer exposure to TZDs had advanced adenomas on repeat colonoscopy, subgroup analyses of advanced adenomas were not informative. Sensitivity analyses, including an analysis where exposure definition was limited to the time period between the two endoscopies, provide similar results to the primary analyses (data not shown).
Table 4.
Association Between TZD Use and Prevalence of New or Missed Adenomatous Polyps in the Distal Colon (Study-3)
| Controls
|
Any adenoma
|
Early adenoma
|
|||
|---|---|---|---|---|---|
| n | n | Adjusted OR* (95% CI) | n | Adjusted OR*† (95% CI) | |
| Ever exposed | |||||
| No (no TZD use or <1 year of use) | 1431 | 142 | 1.00 | 100 | 1.00 |
| Yes (1 year or longer) | 235 | 17 | 0.75 (0.44, 1.28) | 15 | 0.87 (0.49, 1.54) |
| Time since most recent TZD use | |||||
| No TZD use or <1 year of use | 1431 | 142 | 1.00 | 100 | 1.00 |
| Current user | 145 | 10 | 0.75 (0.38, 1.47) | 9 | 0.87 (0.43, 1.78) |
| Less than 1 year | 37 | 3 | 0.85 (0.26, 2.82) | 3 | 1.14 (0.34, 3.78) |
| 1 to <2 years | 29 | 2 | 0.65 (0.15, 2.77) | 1 | 0.44 (0.06, 3.29) |
| 2 years or longer | 24 | 2 | 0.79 (0.18, 3.43) | 2 | 1.01 (0.23, 4.45) |
| Duration of therapy** | |||||
| No TZD use | 1287 | 126 | 1.00 | 93 | 1.00 |
| Less than 1year | 144 | 16 | 1.16 (0.66, 2.03) | 7 | 0.64 (0.29, 1.44) |
| 1 to <2 years | 95 | 8 | 0.92 (0.43, 1.97) | 6 | 0.84 (0.35, 2.01) |
| 2 years or longer | 140 | 9 | 0.67 (0.33, 1.36) | 9 | 0.82 (0.40, 1.68) |
| Average daily dose | |||||
| No or <1 year of use | 1431 | 142 | 1.00 | 100 | 1.00 |
| <0.65 units per day | 115 | 9 | 0.87 (0.43, 1.77) | 8 | 0.97 (0.46, 2.07) |
| 0.65 or greater units per day | 120 | 8 | 0.66 (0.31, 1.39) | 7 | 0.78 (0.35, 1.73) |
Adjusted only for age, sex, calendar year of index date, and use of other diabetes medications.
Analyses for advanced adenomas were not performed since only two patients with 1 year or longer TZD exposure had advanced adenomas at follow-up lower endoscopy.
Duration of therapy with TZDs prior to the index date
Neoplasia in the Proximal Colon
To explore the differences in results between studies-1, -2 and -3, a post hoc exploratory analysis of neoplasia in the proximal colon was conducted using data from patients in study-1. This analysis included 964 case subjects (82 exposed to TZDs) and 3284 controls (340 exposed to TZDs) and demonstrated results similar to the primary analysis, with an inverse association between TZD exposure and the prevalence of colonic neoplasia that was nearly statistically significant (adjusted OR 0.78, 95% CI 0.60–1.01). Interestingly, stratification based on the presence or absence of anemia did not influence these results as it did in the analysis of the entire colon, albeit the confidence intervals were wider due to the reduced sample size (with anemia adjusted OR 0.78, 95% CI 0.57–1.07; without anemia adjusted OR 0.79, 95% CI 0.50–1.27).
Discussion
Previous animal studies have suggested that exposure to TZDs can increase or decrease the risk of colonic neoplasia. Because patients with type 2 diabetes mellitus have an increased risk of colorectal cancer, it is important to determine whether the phenomena in observed animal models apply to humans. Two prior studies addressed this question in patients with diabetes. Koro et al. did not observe an association between TZD therapy and colon cancer risk, but lacked sufficient data to conduct a duration response analysis.16 Govindarajan et al. observed a decreased incidence of colon cancer among African American, but not among white patients.17 Both studies had only short-term follow-up periods (median 18 months of total follow-up in the Koro study16 and 12 months of TZD exposure in the Govindarajan study17). As a result, the follow-up and exposure periods were potentially too short to observe a biological effect, particularly if the TZD exposure increases the risk of colon cancer. Because it will take decades to sufficiently follow large cohorts to address the risk of colon cancer following TZD exposure, we studied development of adenomatous polyps. We did not obtain evidence to indicate that exposure to TZDs increases the risk of colonic neoplasia. Although in our primary analysis for neoplasia throughout the colon we observed an inverse association between TZD exposure and colonic neoplasia, this was not observed when we limited the analysis to patients without a history of anemia. Furthermore, when we examined patients undergoing sigmoidoscopy or serial lower endoscopies, we did not observe a significant association between TZD use and adenomatous polyps in the distal colon. However, there was evidence of a reduced prevalence of polyps in the proximal colon among users of TZDs, regardless of presence or absence of anemia. Thus, we conclude that treatment of type 2 diabetes mellitus with TZDs does not increase the risk of colonic neoplasia; whether TZD use decreases the risk of proximal colon neoplasia is less clear.
Like Govindarajan et al. 17, who observed a decreased incidence of colon cancer among African Americans treated with TZDs, we observed a decreased incidence of colonic neoplasia among patients treated with TZDs in study-1 (patients who underwent colonoscopy). However, these findings might have been artifacts of the indication for colonoscopy. Specifically, patients who are treated with TZDs are more likely to develop anemia, a symptom of colorectal cancer. However, the anemia that accompanies TZD therapy is not a consequence of gastrointestinal bleeding, or more specifically bleeding from colonic neoplasia. As a result, among those patients who underwent colonoscopy to evaluate anemia, patients treated with TZDs would be less likely to have experienced anemia as a consequence of colonic neoplasia. This would bias the observed association between TZD exposure and colonic neoplasia toward an inverse association. Govindarajan et al. did not analyze data excluding patients with anemia. However, when we restricted our analyses to African Americans without anemia, we did not observe an inverse association between TZD exposure and colonic neoplasia (data not shown).
The subgroup analysis of patients with anemia who underwent sigmoidoscopy (study-2) or serial lower endoscopies (study-3) yielded similar results to the primary analysis (data not shown). So why didn’t the presence or absence of anemia have the same effects in the case-control studies focusing on the distal colon? One possibility is that sigmoidoscopy is less sensitive than colonoscopy and resulting misclassifications could have masked a true association. Alternatively, because anemia is a sign of possible colon cancer, patients with anemia are generally referred for colonoscopy rather than sigmoidoscopy. In fact, we designed study-2 to primarily capture patients undergoing the lower endoscopy (sigmoidoscopy) for colon cancer screening rather than to evaluate symptoms. Curiously however, when we examined the relationship between TZD exposure and the prevalence of neoplasia in the proximal colon, the results were similar regardless of the presence of anemia, suggesting an inverse relationship between TZD exposure and prevalence of proximal colon neoplasia.
Taken together, these observations indicate that despite conflicting clinical data16, 17 and data from animal models, there is no evidence that TZDs increase the risk of colonic neoplasia. It is possible that that TZD exposure decreases the risk of neoplasia in the proximal colon, compared with the distal colon. However, our study was not designed to test this hypothesis directly, and had limited power for this analysis as only a small number of adenomas were detected in the proximal colon Also, although some studies have reported a differential effect of insulin, insulin like growth factor 1 (IGF-1), and insulin like growth factor binding protein 1 (IGFBP-1) on proximal and distal colon neoplasia (a potential mechanism for this observation), this has not been confirmed in other studies23–26. Future studies should evaluate the differential effects of TZDs in the colon and rectum or the proximal and distal colon.
This study had a longer follow-up period than previous clinical studies, allowing us to examine cumulative dose and duration of TZD exposure. Approximately 30% of the patients treated with TZDs had more than 2 years of exposure. If a positive association between TZD exposure and colonic neoplasia exists, we would have expected to observe a higher incidence of polyp formation among patients with the longest duration of TZD exposure. We did not observe a duration-response or dose-response relationship between TZDs and colon neoplasia in our data sets.
It is possible that our follow-up periods were still too short to observe effects of TZDs on polyp incidence or prevalence. In our longest duration category (at least 2 years), the median durations of exposure were 2.9 years (IQR 2.4 – 4.0 years) and 3.0 years (IQR 2.4 – 3.9 years) in studies-1 and -2, respectively (data not shown). Previous studies have demonstrated that aspirin, COX-2 inhibitors and calcium can reduce the recurrence and/or lead to regression of adenomatous polyps within one year of therapy initiation27–29, presumably by initiating apoptosis and decreasing proliferation, which are proposed mechanisms of PPARγ ligands. Because many of the patients in studies-1 and -2 would have had prevalent polyps at the time that TZD therapy was initiated, were TZDs to have a pro- or anti-carcinogenic effect late in the process of tumor progression, we anticipated seeing an increased or decreased prevalence of advanced polyps among TZD users following even relatively short durations of therapy. If TZDs have a pro- or anti-carcinogenic effect in the early phase of colon tumor progression, these would have been observed in study-3.
Studies-1 and -2 probably included some patients whose polyps formed before TZD therapy was initiated. Study-3 was designed specifically to address this issue. By focusing on the second exam, we minimized the risk of bias from prevalent colonic neoplasia.
In designing the study, concerns were raised about differential patterns of use of lower endoscopies in patients treated with different diabetes therapies. However, we previously demonstrated in this same population that rates of lower endoscopy were generally similar among patients treated with different diabetes medications, but that patients treated with metformin were slightly more likely to undergo lower endoscopy, perhaps because this drug often induces diarrhea.30 However, diarrhea is not a typical symptom of colonic neoplasia, and would therefore not bias the results. Furthermore, all analyses were all adjusted for metformin use.
Our study lacked data on family histories of colon cancer, although it is unlikely that these differed between TZD-exposed and unexposed patients. It should be noted that patients in study-2 were more likely to have undergone screening and may be less likely to have a family history of colon cancer.
This study also had incomplete data on use of aspirin and over-the-counter NSAIDs. Prescription NSAID use was more common among patients without colonic neoplasia, although it did not prove to be a confounding factor in statistical models. Furthermore, although over-the-counter NSAID use is more common than prescription use, among frequent NSAID consumers, prescription use is more common.28; misclassification of NSAID use due to missing over-the-counter data has been demonstrated to have only a minimal effect on the observed relationship between NSAIDs and colonic neoplasia.32
In focusing on adenomatous polyps, the precursor to colon cancer, and including dose and duration analyses, this study provides strong evidence against a clinically significant increase in risk of colonic neoplasia among patients with diabetes mellitus treated for up to several years with TZDs.
Acknowledgments
This work was funded by NIH grant CA102599
Drs. Lewis, Habel, Ferrara and Quesenberry have received research funding from Takeda. Dr. Lewis has received research support from GlaxoSmithKline. Dr. Habel and Ferrara have received research support from Eli Lilly. Dr. Habel has also received research support from Merck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 2.Schoen RE, Tangen CM, Kuller LH, Burke GL, Cushman M, Tracy RP, Dobs A, Savage PJ. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–54. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 3.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–50. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Keku TO, Lund PK, Galanko J, Simmons JG, Woosley JT, Sandler RS. Insulin resistance, apoptosis, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2076–81. doi: 10.1158/1055-9965.EPI-05-0239. [DOI] [PubMed] [Google Scholar]
- 5.DuBois RN, Gupta R, Brockman J, Reddy BS, Krakow SL, Lazar MA. The nuclear eicosanoid receptor, PPARgamma, is aberrantly expressed in colonic cancers. Carcinogenesis. 1998;19:49–53. doi: 10.1093/carcin/19.1.49. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre AM, Chen I, Desreumaux P, Najib J, Fruchart JC, Geboes K, Briggs M, Heyman R, Auwerx J. Activation of the peroxisome proliferator-activated receptor gamma promotes the development of colon tumors in C57BL/6J-APCMin/+ mice. Nat Med. 1998;4:1053–7. doi: 10.1038/2036. [DOI] [PubMed] [Google Scholar]
- 7.Saez E, Tontonoz P, Nelson MC, Alvarez JG, Ming UT, Baird SM, Thomazy VA, Evans RM. Activators of the nuclear receptor PPARgamma enhance colon polyp formation. Nat Med. 1998;4:1058–61. doi: 10.1038/2042. [DOI] [PubMed] [Google Scholar]
- 8.Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046–52. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- 9.Rosenblatt S, Miskin B, Glazer NB, Prince MJ, Robertson KE Pioglitazone 026 Study G. The impact of pioglitazone on glycemic control and atherogenic dyslipidemia in patients with type 2 diabetes mellitus. Coron Artery Dis. 2001;12:413–23. doi: 10.1097/00019501-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Gibson JM, Westwood M, Crosby SR, Gordon C, Holly JM, Fraser W, Anderson C, White A, Young RJ. Choice of treatment affects plasma levels of insulin-like growth factor-binding protein-1 in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1995;80:1369–75. doi: 10.1210/jcem.80.4.7536208. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan A, Nair SA, Pillai MR. Biology of PPAR gamma in cancer: a critical review on existing lacunae. Curr Mol Med. 2007;7:532–40. doi: 10.2174/156652407781695765. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Kohno H, Yoshitani S, Takashima S, Okumura A, Murakami A, Hosokawa M. Ligands for peroxisome proliferator-activated receptors alpha and gamma inhibit chemically induced colitis and formation of aberrant crypt foci in rats. Cancer Res. 2001;61:2424–8. [PubMed] [Google Scholar]
- 13.Osawa E, Nakajima A, Koichiro W, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H, Nakagama H. Peroxisome proliferator-actived receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124:361–7. doi: 10.1053/gast.2003.50067. [DOI] [PubMed] [Google Scholar]
- 14.Girnun GD, Smith WM, Drori S, Sarraf P, Mueller E, Eng C, Nambiar P, Rosenberg DW, Bronson RT, Edelmann W, Kucherlapati R, Gonzalez FJ, Spiegelman BM. APC-dependent suppression of colon carcinogenesis by PPARgamma. Proc Natl Acad Sci USA. 2002;99:13771–6. doi: 10.1073/pnas.162480299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niho N, Takahashi M, Kitamura T, Shoji Y, Itoh M, Noda T, Sugimura T, Wakabayashi K. Concomitant suppression of hyperlipidemia and intestinal polyp formation in Apc-deficient mice by peroxisome proliferator-activated receptor ligands. Cancer Res. 2003;63:6090–5. [PubMed] [Google Scholar]
- 16.Koro C, Barrett S, Qizilbash N. Cancer risks in thiazolidinedione users compared to other anti-diabetic agents. Pharmacoepidemiol Drug Saf. 2007;16:485–92. doi: 10.1002/pds.1352. [DOI] [PubMed] [Google Scholar]
- 17.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–81. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 18.Friedman G, Habel L, Boles M, Mcfarland B. Kaiser Permanente Medical Care Program: Division of Research, Northern California, and Center for Health Research, Northwest Division. In: Strom BL, editor. Pharmacoepidemiology. 3. West Sussex: John Wiley & Sons, Ltd; 2000. pp. 263–283. [Google Scholar]
- 19.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 20.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287:2519–27. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Weiss NS. Adjusting for screening history in epidemiologic studies of cancer: why, when, and how to do it. Am J Epidemiol. 2003;157:957–61. doi: 10.1093/aje/kwg062. [DOI] [PubMed] [Google Scholar]
- 23.Palmqvist R, Hallmans G, Rinaldi S, Biessy C, Stenling R, Riboli E, Kaaks R. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–6. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei EK, Ma J, Pollak MN, Rifai N, Fuchs CS, Hankinson SE, Giovannucci E. C-peptide, insulin-like growth factor binding protein-1, glycosylated hemoglobin, and the risk of distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev. 2006;15:750–5. doi: 10.1158/1055-9965.EPI-05-0820. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Pollak MN, Giovannucci E, Chan JM, Tao Y, Hennekens CH, Stampfer MJ. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 26.Probst-Hensch NM, Yuan JM, Stanczyk FZ, Gao YT, Ross RK, Yu MC. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br J Cancer. 2001;85:1695–9. doi: 10.1054/bjoc.2001.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, Petrelli N, Pipas JM, Karp DD, Loprinzi CL, Steinbach G, Schilsky R. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 28.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 29.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Bond JH, Greenberg ER. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 30.Lewis JD, Capra AM, Achacoso NS, Ferrara A, Levin TR, Quesenberry CP, Jr, Habel LA. Medical therapy for diabetes is associated with increased use of lower endoscopy. Pharmacoepidemiol Drug Saf. 2007;16:1195–202. doi: 10.1002/pds.1441. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JD, Strom BL, Kimmel SE, Farrar J, Metz DC, Brensinger C, Nessel L, Localio AR. Predictors of recall of over-the-counter and prescription non-steroidal anti-inflammatory drug exposure. Pharmacoepidemiol Drug Saf. 2006;15:39–45. doi: 10.1002/pds.1134. [DOI] [PubMed] [Google Scholar]
- 32.Yood M, Campbell U, Rothman K, Jick S, Lang J, Wells K, Jick H, Johnson CC. Using prescription claims data for drugs available over-the-counter (OTC) Pharmacoepidemiol Drug Saf. 2007;16:961–968. doi: 10.1002/pds.1454. [DOI] [PubMed] [Google Scholar]