Abstract
Pavlovian fear conditioning is a model of emotional learning in which a neutral stimulus such as a tone is paired with an aversive stimulus such as a foot shock. Presentation of a tone with a foot shock in a context (test box) elicits a freezing response representative of stereotypic fear behavior. After conditioning has occurred, presentation of the context (test box) or tone in the absence of the unconditioned stimulus (shock) causes extinction of the fear response. Rats chronically exposed to environmentally relevant levels of lead (Pb2+) and controls were tested in a fear-conditioning (FC) paradigm at 50 days of age (PN50). Littermates to FC rats received an immediate shock (IS) when place in the test box with no tone. Blood Pb2+ levels in control and Pb2+-exposed animals were (mean ± sem): 0.76 ± 0.11 (n=15) and 25.8 ± 1.28 μg/dL (n=14). Freezing behavior was recorded during acquisition (day of training) or during 4 consecutive extinction days. Control and Pb2+-exposed FC rats exhibited the same level of freezing time on the acquisition day. No freezing behavior occurred in IS rats regardless of treatment. Presentation of context 24 hrs later produced a freezing response on both control and Pb2+-exposed FC rats but not in IS rats. When tested in the extinction phase, Pb2+-exposed FC rats exhibited deficits in extinction compared to control FC rats. That is, when presented with context on 4 consecutive days after acquisition of the fear response, Pb2+-exposed FC rats exhibited a greater freezing response than control FC rats. These findings indicate that chronic Pb2+ exposure produces a deficit in extinction learning and the animals remain more fearful than controls.
Keywords: Fear Conditioning, Pb2+, NMDA receptors, Anxiety
Introduction
Plasticity of the central nervous system is essential for the acquisition and consolidation of new information. These processes are thought to be dependent upon strengthening of synaptic connections and the N-methyl-d-aspartate subtype (NMDAR) of excitatory amino acid receptors has been shown to play a key role in synaptic plasticity in the mammalian brain (Malenka and Nicoll, 1993). For example, synaptic plasticity in the form of long-term potentiation (LTP) is thought to represent a cellular model of learning and memory and NMDAR activation is essential to this cellular phenomenon (Zalutsky and Nicoll, 1990; Bliss and Collingridge, 1993; Malenka and Nicoll, 1993, 1999).
Previous work from several laboratories has shown that lead (Pb2+) is a potent inhibitor of the NMDAR (Alkondon et al., 1990; Guilarte and Micelli, 1992; Omelchenko et al., 1996; Marchetti and Gavazzo, 2005). Exposure to Pb2+ during brain development impairs spatial learning and LTP in the hippocampus (Altman et al., 1993; Gilbert et al., 1996; Ruan et al., 1998; Nihei et al., 2000), both of which are dependent upon NMDAR activation. Animals exposed to Pb2+ during development exhibit alterations in the expression of NMDAR subunits in the hippocampus (Guilarte and McGlothan, 1998; Nihei and Guilarte, 1999; Guilarte et al., 2000; Zhang et al., 2002), altering their subunit composition and coupling to signaling transduction pathways (Toscano et al., 2002; 2003). We have proposed that the deficits in spatial learning and LTP documented in Pb2+-exposed rats are mediated by Pb2+ inhibition of NMDAR and subsequent changes in their subunit composition and signaling (Toscano and Guilarte, 2005). To further study the effects of Pb2+ on associative learning, we examined the effect of developmental Pb2+ exposure on fear conditioning in young adult rats.
Fear conditioning is a model of emotional learning in which a neutral conditioned stimulus (CS) such as a tone or the context in which the animal was present (test box) when paired with an aversive unconditioned stimulus (US) such as a foot shock elicits a freezing response (or conditioned response) representative of stereotypic fear behavior. The conditioned fear response diminishes if the condition stimulus or the context is presented in the absence of a shock and this process is known as “Extinction”. Extinction of conditioned fear is thought to involve the formation of a “new memory” rather than the erasure of the fear conditioning (Quirk, 2002; Bouton et al., 2006; Myers and Davis, 2007). This is supported by evidence, as with many types of learning, that extinction requires activation of NMDAR (Falls et al., 1992; Baker and Azorlosa, 1996; Santini et al., 2001; Walker et al., 2002) and a switch from long-term depression (LTD) to LTP (Herry and Garcia, 2002; Herry and Mons, 2004). Based on this information, we tested the hypothesis that extinction of fear conditioning may be altered in young adult rats that were exposed to environmentally relevant levels of Pb2+ during gestation and postnatally.
Materials and Methods
Animal Husbandry
Adult female Long-Evans rats were purchased from Charles River, Inc. (Wilmington, MA) and fed 0 (control) or 1500 ppm lead acetate (PbAc) in the diet (Dyets, Bethlehem, PA). The level of Pb2+ in each diet was confirmed by analysis from an independent laboratory (ESA Laboratories, Inc., Chelmsford, MA). Female rats were bred to normal Long-Evans males following an acclimation period of 10 days. Litters were culled to 10 on postnatal day 1 (PN1). Pups were weaned on PN21 and fed the same diet as their respective dams. Rats were individually housed in plastic cages at 22 ± 2°C on a 12/12 light:dark cycle, and food and water were allowed ad libitum. At PN50, two male rats from each control and Pb2+-exposed litter were randomly assigned to two behavioral experimental groups: fear conditioning (FC) or immediate shock (IS). Blood Pb2+ analysis was conducted using the LeadCare system (ESA Laboratories, Inc, Chelmsford, MA). Litters of rats were considered a single experimental unit for statistical purposes so that for each experiment, one animal was used from a single litter for one data point. All animal studies were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee.
Conditioning Chambers
Operant test boxes were used for the fear conditioning and freezing behavior observations. Rat Test Cages (Coulbourn Instruments, Allentown, PA) were fitted with grid shock floors (Modular Shock Floor, Coulbourn Instruments) containing 18 stainless steel bars connected to a shock generator (Precision Programmable Animal Shocker, Coulbourn Instruments). These cages were placed inside of light- and sound-proof Isolation Cubicles with a white noise generator, light, tone generator, and a monochrome minivideo camera mounted in the center of one side wall of the test box (Coulbourn Instruments). The delivery of the footshock was controlled by the H-Series Habitest Control System (Graphic State Software, Coulbourn Instruments). Cameras were connected to monitors with VHS digital tracking recorders so that all experiments were recorded. Both waste trays and grid floors were washed with soap and water between each experimental animal.
Acquisition and extinction of fear conditioning protocols
On the training day, FC rats were transported to the experimental room and individually placed in the conditioning chambers. After 3 minutes of adaptation, a 30 second tone of 85 dB was presented with a 1 mA foot shock delivered during the last 2 seconds of the tone. Each FC experimental animal remained in the chamber for 2 additional minutes and then were removed and returned to their home cages. The IS rats were transported to the experimental room and individually placed into the conditioning chambers where they immediately received a 2 second 1 mA footshock. Each IS experimental animal remained in the chamber for 2 additional minutes and then were removed and returned to their home cages.
Extinction trials were performed 24 hrs after the conditioning and consisted of non-reinforced exposure to context. Freezing behavior was measured for 3 minutes during 4 consecutive extinction days. Freezing was defined as the absence of all visible movements of the body and vibrissae aside from respiration and heartbeat. Only animals that exhibited freezing behavior greater than 50% of the time on extinction day 1 were included in the analysis. All procedures were monitored and recorded using VHS tapes.
Data Analysis
Freezing data from each day were collected for each animal and converted to a percentage of total observation time. Extinction data were analyzed by two-factor repeated measures ANOVA. Data are represented as the mean ± sem.
Results
Blood lead levels and body weight of animals
Table 1 shows that blood Pb2+ levels measured at the termination of the study in the Pb2+ exposed group are environmentally relevant since they range from 18.1–35.1 μg/dL with a mean of 25.8 μg/dL. There were no significant differences in body weight between the experimental groups (Table 2).
Table 1.
Blood Pb2+ levels in control and Pb2+-exposed rats measured immediately after Extinction Day 4 observations (PN54).
| Blood Pb2+ Values (μg/dL) | ||
|---|---|---|
| Control (n=15) | 0.75 ± 0.11 | Range: 0.1 – 1.5 |
| Pb2+-exposed (n=14) | 25.8 ± 1.28* | Range: 18.1 – 35.1 |
Data from each behavioral group are combined and expressed as means ± s.e.m. and as the range of values for each exposure group.
p<0.05 relative to control values.
Table 2.
Body weights for control and Pb2+-exposed rats in each behavioral group measured on the Conditioning Day (PN50) and on Extinction Day 4 (PN54).
| Body Weight (g) | ||
|---|---|---|
| Conditioning Day | Extinction Day 4 | |
| CFC-Control (n=10) | 273.9 ± 7.3 | 308.8 ± 8.0 |
| CFC-Pb (n=8) | 245.0 ± 10.5 | 275.5 ± 12.1 |
| IS-Control (n=9) | 254.3 ± 9.2 | 285.1 ± 9.7 |
| IS-Pb (n=11) | 264.8 ± 7.1 | 297.6 ± 7.3 |
Data are expressed as means ± s.e.m. No differences were observed in body weights amongst the groups.
Acquisition of fear conditioning and extinction
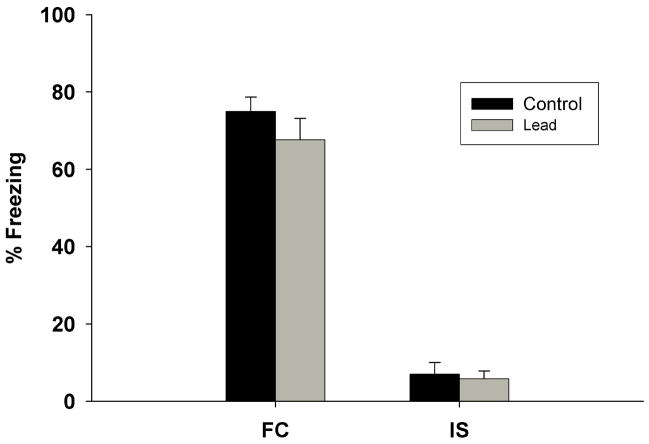
There were no significant differences between control and Pb2+-exposed groups in the percentage of total rats tested in the FC protocol that acquired fear conditioning (data not shown). There was a significant increase in the freezing time of the FC rats on conditioning day after the presentation of the CS when compared to the IS (Figure 1). There were no significant differences in the degree of freezing between control and Pb2+-exposed rats. Therefore, chronic Pb2+ exposure had no effect on the acquisition of FC when compared to controls (Figure 1).
Figure 1.
Percentage of freezing behavior observed after the tone/shock on conditioning day in Control and Pb2+-exposed rats in both the Immediate Shock (IS) and Fear Conditioning (FC) behavioral groups. There were no differences in the observed freezing time between Control and Pb2+-exposed groups. There was an obvious increase in the freezing time for the FC group (allowed exploration of the test box and received a CS and US) when compared to the IS group (no exploration of the test box and only US). Data are expressed as mean ± s.e.m.
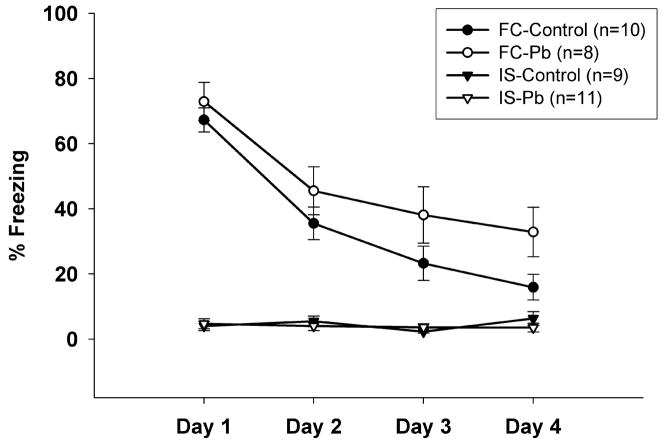
During the Extinction Phase, the control and Pb2+-exposed IS groups froze less than 10% of the observation time on all 4 days. The percent freezing time of the control FC group steadily decreased from approximately 65% to less than 15% by day 4 of extinction (Figure 2). The percent freezing time of the Pb2+-exposed FC group remained significantly higher than the control FC group, decreasing from approximately 75% to only about 40% by day 4 of extinction (Figure 2) at which time the differences between Pb2+-exposed and control rats was significantly different (p < 0.05). These findings suggest that young adult rats chronically exposed to Pb2+ have the ability to acquire a normal fear response, but have deficits during the extinction of the learned response.
Figure 2.
Percentage of freezing behavior observed on 4 days of extinction observations in Control and Pb2+-exposed rats in each of the behavioral groups. There were low levels of freezing behavior observed in the Control and Pb2+-exposed IS group on all 4 Extinction Days. The Control FC group had a steady decrease in freezing time over the 4 days until they nearly reached the levels of the IS group. However, the freezing time of the Pb2+-exposed FC group remained significantly higher (F3,102=34.7; p=0.0001) than all other groups on day 4. A repeated measures ANOVA showed significant differences across the days of extinction observations. Data are expressed as mean ± s.e.m.
Discussion
In the present study we show that chronic Pb2+ exposure during brain development has no significant effect on the acquisition of fear conditioning but it impairs the extinction of fear. Our findings are consistent with a previous study (Salinas and Huff, 2002) that showed that chronic exposure to Pb2+ that resulted in blood Pb2+ levels higher than those in our present study did not alter the acquisition of contextual or cue conditioning but inhibited the extinction phase. Taken together, these findings suggest that chronic Pb2+ exposure impairs the extinction of fear conditioning.
Extinction of conditioned fear is believed to involve the formation of a new memory rather than the erasure of the conditioned response. This is supported by studies showing that extinction of conditioned fear depends upon NMDA receptor-mediated plasticity. Thus, as with many types of learning, long-term retention of extinction to fear requires activation of NMDA receptors. This is based on studies in which systemic administration of NMDA receptor antagonists or their direct administration into the amygdala (Falls et al., 1992) prevents extinction of conditioned fear. Conversely, facilitation of NMDA receptor activation by direct injection of the partial agonist D-serine into the amygdala, strengthens extinction of fear-potentiated startle in rats (Walker et al., 2002).
Activation of NMDA receptors has also been shown to be essential in the medial prefrontal cortex (mPFC) during consolidation of extinction learning but not during the acquisition of fear extinction (Santini et al., 2001). Other studies have shown that in the prefrontal cortex a switch from LTD to LTP is also required for consolidation of long-term extinction memory (Herry and Garcia, 2002; Herry and Mons, 2004). Our current findings indicate that chronic developmental Pb2+ exposure produces a deficit in extinction learning and the animals remain “more fearful” than controls. Since long-term retention of extinction involves a transfer from NMDA receptor-independent to NMDA receptor-dependent memory (Santini et al., 2001), our present findings provide additional evidence that Pb2+-exposed rats have deficits in NMDA receptor-dependent learning.
Our present findings show that chronic exposure to Pb2+ during brain development leads to the dysfunction of neuronal systems associated with extinction of conditioned fear, leaving the animals resistant to extinction of fear. These findings could have important implications to the treatment of anxiety disorders such as panic disorders and post-traumatic stress.
Acknowledgments
This work was supported by NIEHS grant number ES06189.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alkondon M, Costa AC, Radhakrishnan V, Aronstam RS, Albuquerque EX. Selective blockade of NMDA-activated channel currents may be implicated in learning deficits caused by lead. FEBS Lett. 1990;261:124–130. doi: 10.1016/0014-5793(90)80652-y. [DOI] [PubMed] [Google Scholar]
- Altman L, Weinsberg F, Sveinsson K, Lilienthal H, Wiegand H, Winneke G. Impairment of long-term potentiation and learning following chronic lead exposure. Toxicol Lett. 1993;66:105–112. doi: 10.1016/0378-4274(93)90085-c. [DOI] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, Mack CM, Lasley SM. Chronic developmental lead exposure increases the threshold for long-term potentiation in rat dentate gyrus in vivo. Brain Res. 1996;736:118–124. doi: 10.1016/0006-8993(96)00665-8. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Miceli RC. Age-dependent effects of lead on [3H]MK-801 binding to the NMDA receptor-gated ionophore: in vitro and in vivo studies. Neurosci Lett. 1992;148:27–30. doi: 10.1016/0304-3940(92)90796-a. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL. Hippocampal NMDA receptor mRNA undergoes subunit specific changes during developmental lead exposure. Brain Res. 1998;790:98–107. doi: 10.1016/s0006-8993(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, McGlothan JL, Nihei MK. Hippocampal expression of N-methyl-d-aspartate receptor (NMDAR1) subunit splice variant mRNA is altered by developmental exposure to Pb(2+) Brain Res Mol Brain Res. 2000;76:299–305. doi: 10.1016/s0169-328x(00)00010-3. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdale. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Marchetti C, Gavazzo P. NMDA receptors as targets of heavy metal interaction and toxicity. Neurotoxicity Res. 2005;8:245–258. doi: 10.1007/BF03033978. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Guilarte TR. NMDAR-2A subunit protein expression is reduced in the hippocampus of rats exposed to Pb2+ during development. Brain Res Mol Brain Res. 1999;66:42–49. doi: 10.1016/s0169-328x(99)00005-4. [DOI] [PubMed] [Google Scholar]
- Nihei MK, Desmond NL, McGlothan JL, Kuhlmann AC, Guilarte TR. N-methyl-d-aspartate receptor subunit changes are associated with lead-induced deficits of long-term potentiation and spatial learning. Neuroscience. 2000;99:233–242. doi: 10.1016/s0306-4522(00)00192-5. [DOI] [PubMed] [Google Scholar]
- Omelchenko IA, Nelson CS, Marino JL, Allen CN. The sensitivity of N-methyl-d-aspartate receptors to lead inhibition is dependent on the receptor subunit composition. J Pharmacol Exp Ther. 1996;278:15–20. [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan DY, Chen JT, Zhao C, Xu YZ, Wang M, Zhao WF. Impairment of long-term potentiation and paired-pulse facilitation in rat hippocampal dentate gyrus following developmental lead exposure in vivo. Brain Res. 1998;806:196–201. doi: 10.1016/s0006-8993(98)00739-2. [DOI] [PubMed] [Google Scholar]
- Salinas JA, Huff NC. Lead and conditioned fear to contextual and discrete cues. Neurotoxicol Teratol. 2002;24:541–550. doi: 10.1016/s0892-0362(02)00265-9. [DOI] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano CD, Hashemzadeh-Gargari H, McGlothan JL, Guilarte TR. Developmental lead exposure alters NMDAR subtypes and reduces CREB phosphorylation in the rat brain. Dev Brain Res. 2002;139:217–226. doi: 10.1016/s0165-3806(02)00569-2. [DOI] [PubMed] [Google Scholar]
- Toscano CD, McGlothan JL, Guilarte TR. Lead exposure alters cyclic-AMP response element binding (CREB) protein phosphorylation and binding activity in the developing rat brain. Dev Brain Res. 2003;145:219–228. doi: 10.1016/j.devbrainres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Guilarte TR. Lead neurotoxicity: from exposure to molecular effects. Brain Res Brain Res Rev. 2005;49:529–554. doi: 10.1016/j.brainresrev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Liu AP, Ruan DY, Liu J. Effect of developmental lead exposure on the expression of specific NMDA receptor subunit mRNAs in the hippocampus of neonatal rats by digoxigenin-labeled in situ hybridization histochemistry. Neurotox Teratol. 2002;24:149–160. doi: 10.1016/s0892-0362(01)00210-0. [DOI] [PubMed] [Google Scholar]