Abstract
Lecithin retinol acyltransferase (LRAT) catalyzes the esterification of retinol (vitamin A). Retinyl esters and LRAT protein levels are reduced in many types of cancer cells. We present data that both the LRAT and retinoic acid receptor β2 (RARβ2) mRNA levels in the human prostate cancer cell line PC-3 are lower than those in cultured normal human prostate epithelial cells (PrEC). The activity of the human LRAT promoter (2.0 kb) driving a luciferase reporter gene in PC-3 cells is less than 40% of that in PrEC cells. Retinoic acid (RA) treatment increased this LRAT promoter-luciferase activity in PrEC cells, but not in PC-3 cells. Deletion of various regions of the human LRAT promoter demonstrated that a 172-bp proximal promoter region is essential for LRAT transcription and confers RA responsiveness in PrEC cells. This 172-bp region, contained within the 186 bp pLRAT/luciferase construct, has five putative GATA binding sites. Co-transfection of RARβ2 or RARγ and the transcription factor GATA-4 increased LRAT (pLRAT186) promoter activity in both PrEC and PC-3 cells. In addition, we found that both retinoic acid and retinol induced transcripts for the STRA6 gene, which encodes a membrane receptor involved in retinol (vitamin A) uptake, in PrEC cells but not in PC-3 cells. In summary, our data show that the transcriptional regulation of the human LRAT gene is aberrant in human prostate cancer cells and that GATA transcription factors are involved in the transcriptional activation of LRAT in PrEC cells.
Keywords: cancer, GATA, LRAT, prostate, retinoic acid, STRA6, retinol, transcription, promoter analysis, retinoic acid receptor, tumor cell
INTRODUCTION
Retinoids, which include retinol (vitamin A) and its natural and synthetic derivatives, are essential for many biological processes such as cell proliferation and cell differentiation (Lotan, 1981, Gudas, 1994, Mongan and Gudas, 2007). Mammals obtain vitamin A from their diet. In the body, retinol binds to the serum retinol binding protein (RBP4) and is delivered to tissues via the blood (Goodman, 1980). Then, RBP4 binds to appropriate cell types via the STRA6 protein (Kawaguchi et al., 2007), allowing entry of retinol. When retinol enters cells, it can be metabolized to the more biologically active all-trans retinoic acid (RA) (Niles, 2004, Bohnsack and Hirschi, 2004). RA exerts its biological functions by activating its nuclear receptors. These nuclear receptors include the retinoic acid receptors (RARs) α, β2, and γ, and the retinoid X receptors (RXRs) α, β, and γ, which act as transcription factors (Mangelsdorf, 1994, Kastner et al., 1994, Mongan and Gudas, 2007).
Although retinol is metabolized to all-trans retinoic acid to regulate gene expression, retinol can also be metabolized to retinyl esters, the major storage form of retinol, in various tissues including liver, prostate, breast, and kidney (Guo et al., 2000, Guo et al., 2001, Guo et al., 2002). Two enzymes, lecithin:retinol acyltransferase (LRAT) and acyl CoA:retinol acyltransferase (ARAT), catalyze retinyl ester synthesis. These two enzymes differ in substrate preferences (Guo et al., 2000, Yost et al., 1988, Ong et al., 1988, Herr, 1991). LRAT is the major enzyme involved in retinol esterfication in most tissues, and LRAT activity is required, along with STRA6 and the RBP4 protein, for uptake of appropriate amounts of retinol into cells (Kawaguchi et al., 2007, Zolfaghari and Ross, 2004, Liu and Gudas, 2005, O’Byrne et al., 2005, Kim et al., 2007, Wongsiriroj et al., 2008, Liu et al., 2008). ARAT has not been cloned.
Retinoids can inhibit the proliferation of normal human prostate epithelial cells and some lines of human prostate cancer cells (Peehl et al., 1993, de Vos et al., 1997, Dahiya et al., 1994, Campbell et al., 1998, Blutt et al., 1997). All-trans-retinoic acid and 13-cis-retinoic acid have been used in conjunction with other therapeutic agents, such as paclitaxel and IFN-α, for the treatment of human prostate cancer (Zheng et al., 2004, Thalasila et al., 2003, DiPaola et al., 1999). These studies suggest that retinoids can be used as drugs in combination therapies to treat human prostate cancer (Nanus and Gudas, 2000). However, some abnormalities in retinoid signaling have been observed in different cancers. These include alterations in the levels of retinoid receptors and in the expression of enzymes involved in retinoid metabolism (Soprano, 2004, Guo et al., 2000, Sun and Lotan, 2002, Boorjian et al., 2004, Mongan and Gudas, 2007, Kim et al., 2005). Such alterations in retinoid signaling in tumor cells may impact the ability of retinoids to be used in cancer treatment.
Aberrant retinol metabolism may also promote the development of various cancers. The defects in retinol esterification in cancer cells interfere with retinol storage and its metabolism to retinoic acid, which can lead to a striking, local retinoid deficiency and tumor progression (Guo et al., 2000, Guo et al., 2002, Chen et al., 1997, Zhan et al., 2003). Our laboratory has demonstrated that retinol esterification is reduced in human cancer cell specimens from various types of cancers, including kidney, prostate, breast, and bladder, as compared to normal human epithelial cell strains from same tissues (Guo et al., 2001, Guo et al., 2002, Boorjian et al., 2004, Zhan et al., 2003, Sheren-Manoff et al., 2006). The levels of LRAT correlate with the ability of cells to esterify retinol. We showed that LRAT protein levels are reduced in kidney and prostate cancer cell lines (Guo et al., 2001, Guo et al., 2002). These data suggest that the reduction or lack of LRAT expression leads to defects in retinol esterification in cancer cells. In this series of experiments, we explored some of the mechanisms by which LRAT gene expression is regulated in normal human prostate epithelial cells and prostate cancer cells. We also investigated whether aberrant expression of the STRA6 gene occurs in cultured prostate cancer cells.
MATERIALS AND METHODS
Cell culture and drug treatments
Normal human prostate epithelial cells (PrEC) were purchased from Cambrex and cultured at 37°C, 5% CO2 in PrEGM (Cambrex). PC-3, an androgen-independent human prostate cancer cell line, was grown in RPMI 1640 supplemented with 10% fetal calf serum at 37°C, 10% CO2.
All-trans retinoic acid (RA) and all-trans retinol (ROL) were purchased from Sigma (St. Louis, MO). Stocks of RA and retinol were prepared in 100% ethanol.
Plasmid constructions
The human LRAT promoter sequence was amplified from human genomic DNA by PCR using high-fidelity Pfx DNA polymerase (Invitrogen). The PCR fragment was produced using a sense primer LRAT-L1 (5′-AGC TAG GTA CCA CAA GAG GCT TCG GTA TTG G-3′; the KpnI restriction site is underlined) that starts at -2008 relative to the transcription start site (+1) of the human LRAT gene (Zolfaghari and Ross, 2004) and an antisense primer LRAT-R1 (5′-TGA GTA GAT CTG GCT GGG CAA GTT AAG CTC-3′; the BglII restriction site is underlined) that starts at +272. The PCR fragment was digested with KpnI and BglII, and inserted into the pGL 3-Basic promoterless reporter plasmid (Promega) to create an LRAT promoter-luciferase reporter construct, pLRAT2008.
Deletions of the LRAT promoter were generated by PCR, using the pLRAT2008 plasmid as a template; four constructs of different lengths of LRAT promoter were made. The 5′-deleted PCR fragments were produced with the common anitsense primer LRAT-R1 and four different sense primers that start at -833 (5′-AGC TAG GTA CCG AAA GCA TGG AGG ATT CAG G-3′), -478 (5′-AGC TAG GTA CCT CGG TCA GGA AAG AAT CTG C-3′), -186 (5′-AGC TAG GTA CCA CTG TGC AGA ACC CTG GAG-3′), and -14 (5′–AAA TAG GTA CCA AGC CTG CAC CTC CGA GCA-3′). The amplified promoter fragments were subcloned into the KpnI and BglII restriction sites in the pGL 3-Basic plasmid to generate the constructs pLRAT833, pLRAT478, pLRAT186, pLRAT14. The DNA sequence of each construct was confirmed by sequencing at the Cornell University Biotechnology Core.
Transient transfections, luciferase assays, and β-galactosidase assays
The plasmids were prepared using an EndoFree Plasmid Max Kit (QIAGEN Sciences, Maryland). PrEC or PC-3 cells (4 × 105) were plated in each 60-mm culture dish. Cells were cultured for 24 hours, and then transiently transfected with a total of 3 μg of plasmids and 9 μl of FuGENE 6 Transfection Reagent (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions. The Tk-Renilla luciferase plasmid (pRL-TK) was co-transfected for normalization of the transfection efficiency (Promega). After 24 hours of culture with the FuGENE 6 Reagent-plasmid complex, the medium was changed, and RA, retinol, or 0.1% ethanol (vehicle control) was added. Cells were cultured for an additional 48 hours, and then were lysed and cell extracts were analyzed for firefly luciferase activity using the Dual-Luciferase Reporter Assay System (Promega). The luciferase activity measurements were performed in duplicate or in triplicate according to the manufacturer’s instructions.
Plasmids pSG5-RARα, pSG5-RARβ2, pSG5-RARγ, and pMT2-GATA-4 were used to express RARα, RARβ2, RARγ, and GATA-4 in PrEC and PC-3 cells. pSG5-RARα, pSG5-RARβ2, and pSG5-RARγ were from Dr. Pierre Chambon. pMT2-GATA-4 was from Dr. Stuart Orkin. Empty plasmids pSG5 and pMT2 were used as controls where appropriate.
The β-galactosidase assay was performed as previously described (Sambrook J., 1989). The activity of β-galactosidase (lacZ, β-gal) was calculated as follows: units/μlh = (OD420) (100)/(lysate volume in micro liters) (reaction time in hours). The plasmid pβAc-lacZ (Vasios et al., 1989) was used to express β-galactosidase under the control of the mouse β-actin promoter in PrEC and PC-3 cells. Normalization of data between the PrEC and PC-3 cells was by cell number and by β-actin/lacZ activity.
RNA isolation and real-time quantitative RT-PCR
Total RNA was extracted from PrEC and PC-3 cells using the TRIzol reagent (Invitrogen) as instructed by the supplier. The RNA concentration was determined by measuring the absorbance at 260nm (A260). First-strand cDNA was synthesized from 4 μg of total RNA by reverse transcription with Superscript II Reverse Transcriptase (Invitrogen) at 42°C for 50 minutes. The synthesized cDNA was diluted and amplified by real-time quantitative PCR using a QuantiTect SYBR Green PCR kit (Qiagen). PCR reactions were set up in triplicate for each cDNA sample. Relative quantitation was achieved from standard curves prepared from 3-fold serial dilutions of a cDNA sample. For each sample, the mRNA levels of the target gene and the GAPDH gene were determined individually. After that, the target mRNA level was divided by the GAPDH mRNA level in the same sample. The values obtained from triplicate reactions were averaged. Three independent experiments were performed. The primers used for amplifying the LRAT cDNA were LRAT-RTL1 (5′ GTGGAACAACTGCGAGCAC 3′) and LRAT-RTR1 (5′ TAGACGCCAATCCCAAGACT 3′). The RARβ2 cDNA was amplified using RARβ2-119 (5′GCTCCAGGAGAAAGCTCTCAAAG 3′) and RARβ2-085 (5′ATTTGTCCTGGCAGACGAAGC 3′). A pair of primers, STRA6-RTL2 (5′ CACCACGGATGTCTCCTACC 3′) and STRA6-RTR2 (5′ GGAAGGTGAGTAAGCAGGACA 3′), was used to amplify STRA6. GAPDH-RTL1 (5′ ATCCTGGGCTACACTGAGCA 3′) and GAPDH-RTR1 (5′ TGCTGTAGCCAAATTCGTTG 3′) were used to amplify GAPDH. All primers were designed around introns.
Statistical analyses
All experiments were independently performed, starting with plating of the cells, at least three times. Graphpad Prism software was used to perform statistical calculations. Data were analyzed using the one-tailed, paired t test. Differences were considered to be statistically significant if P < 0.05. Data are expressed as means ± SE.
RESULTS
LRAT mRNA levels are reduced in cultured human prostate cancer cells
We first wanted to ascertain the levels of LRAT mRNA in cultured normal prostate epithelial cells vs. tumor cells. Normal human prostate epithelial cells (PrEC) and the androgen-independent human prostate cancer cell line PC-3 were cultured in medium containing 1μM all-trans retinoic acid (RA), 1μM all-trans retinol, or 0.1% ethanol (control) for 24 or 48 h, and harvested. Total RNA was isolated and used for real-time, quantitative RT-PCR analysis. Under control conditions, the relative abundance of LRAT mRNA in PC-3 cells was < 50% of that in PrEC cells (P < 0.05) (Fig. 1A). These results indicate that LRAT mRNA levels are reduced in the PC-3 prostate cancer cell line.
Figure 1. LRAT mRNA levels in normal human prostate epithelial cells (PrEC) are higher than that in human prostate cancer cells (PC-3).
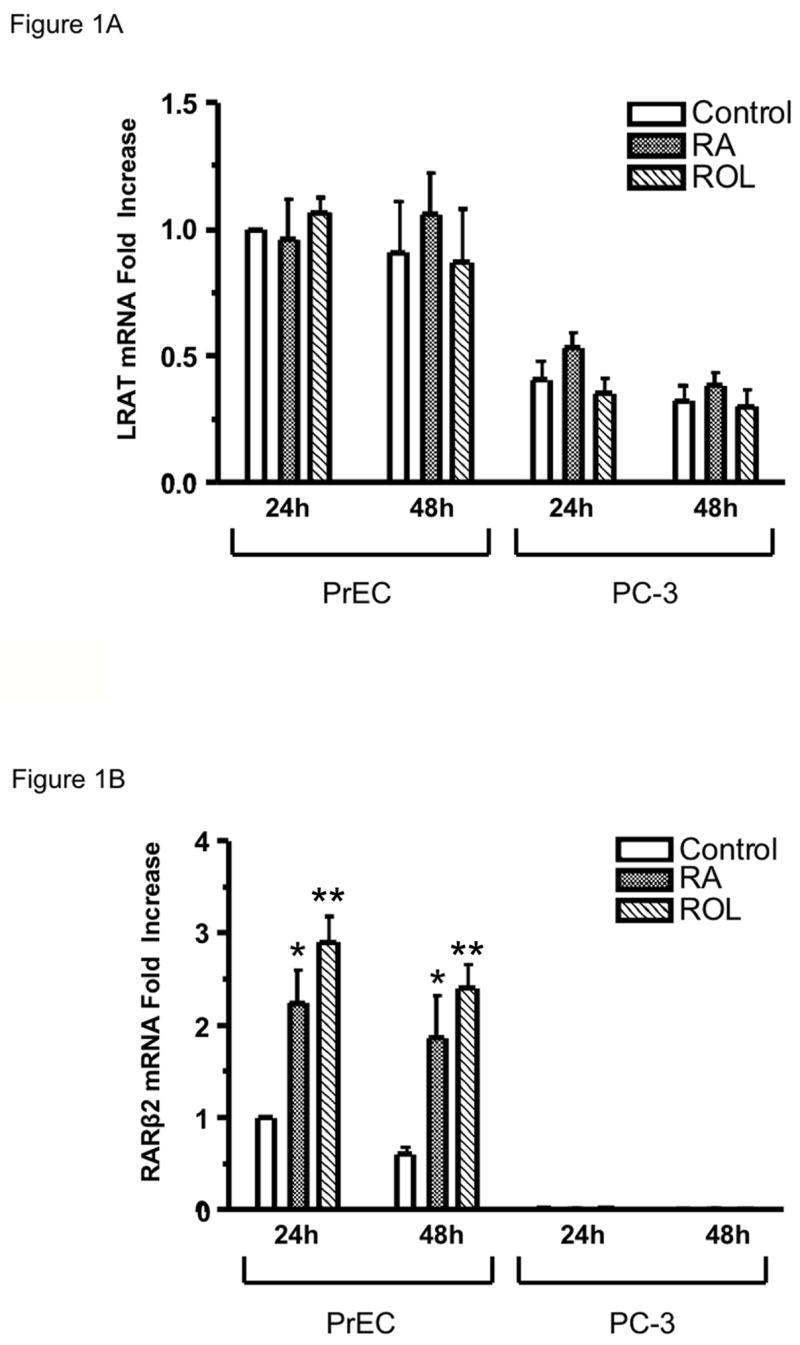
Normal human prostate epithelial cells (PrEC) and human prostate cancer cells (PC-3) were cultured in the medium with 1μM RA, 1μM retinol (ROL), or 0.1% ethanol (control) for 24 h and 48 h. Total RNA was extracted using the TRIzol reagent (Invitrogen). (A) LRAT mRNA levels in PrEC and PC-3 cells. LRAT transcripts were quantified by real-time RT-PCR. The mRNA levels are normalized to GAPDH mRNA and are expressed relative to the value derived from PrEC cells treated with ethanol (control) for 24 h. (B) RARβ2 mRNA levels in PrEC and PC-3 cells (24 hr). RARβ2 transcripts were quantified by real-time RT-PCR. The mRNA levels are normalized to GAPDH mRNA and are expressed relative to the values derived from PrEC cells treated with 0.1% ethanol (control) for 24 h. The values were obtained from three independent experiments. *, p <0.05; * *, p <0.01 (compared to control)
It has been reported that retinol and retinoic acid can regulate LRAT mRNA levels in specific rat tissues (Zolfaghari and Ross, 2002, Zolfaghari and Ross, 2000, Randolph and Ross, 1991). In PrEC and PC-3 cells, 1μM RA or 1μM retinol treatment for 24 and 48 h did not cause any statistically significant increases in LRAT mRNA levels, as assessed by quantitative real-time RT-PCR (Fig. 1A).
The RARβ2 mRNA level was also measured as a positive control. Both 1μM RA and 1μM retinol treatments caused a 2 to 4 fold increase in the RARβ2 mRNA level in PrEC cells (P < 0.05 and P < 0.01, respectively) (Fig. 1B), which indicates that RA and retinol can signal in the normal prostate epithelial cells. Under control conditions, the RARβ2 mRNA levels in PC-3 cells were < 2% of those in PrEC cells. Moreover, neither RA nor retinol treatment altered the RARβ2 mRNA level in the PC-3 prostate cancer cell line (Fig. 1B).
Human LRAT promoter analysis
To study the transcriptional regulation of the human LRAT gene, we constructed a human LRAT promoter-luciferase reporter plasmid. A 5′-flanking DNA region extending from −2008 through the transcriptional start site (+1) to +272 was amplified from human genomic DNA by PCR and inserted into the pGL 3-Basic promoterless reporter plasmid to create an LRAT promoter-luciferase reporter construct, pLRAT2008 (Fig. 3A). PrEC and PC-3 cells were transiently transfected and then treated with 1μM RA, 1μM retinol, or 0.1% ethanol (vehicle control) for 48 h. Luciferase activity was then fluorometrically measured. In the normal prostate PrEC cells, 1μM RA treatment for 48 h caused a 2.1-fold increase in the luciferase activity (P < 0.01), whereas 1μM retinol treatment for 48 h did not alter the luciferase activity (P > 0.05) (Fig. 2). These results suggest that this 2280-bp 5′-flanking region harbors at least one cis element required for RA responsiveness. Under control conditions (treatment with 0.1% ethanol for 48 h), the luciferase activity in PC-3 cells was < 40% of that in PrEC cells (P < 0.01) (Fig. 2).
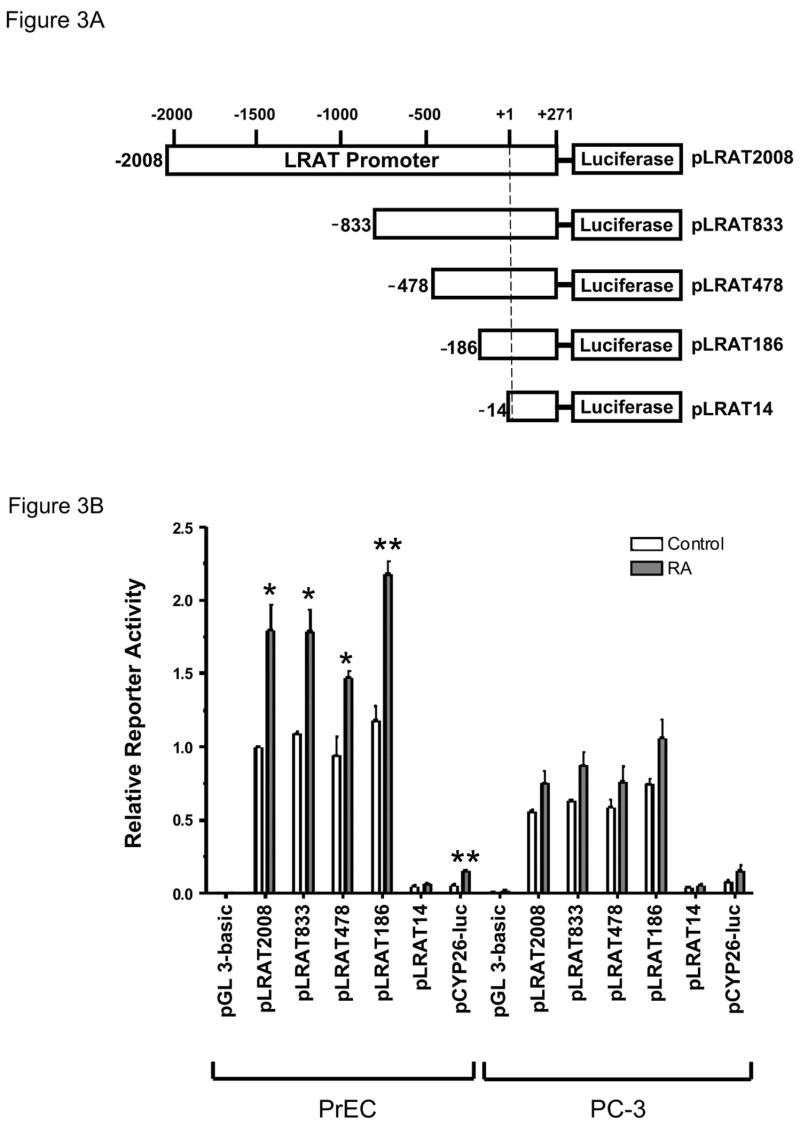
Figure 3. Mapping of the regulatory elements in the LRAT promoter.
(A) Reporter constructs resulting from deletions of the LRAT promoter 5′-flanking region were prepared as described in Materials and Methods. (B) PrEC and PC-3 cells were transiently transfected with pLRAT2008 (3.1 μg), pLRAT833 (2.5 μg), pLRAT478 (2.4 μg), pLRAT186 (2.3 μg), pLRAT14 (2.2 μg), pGL 3-basic plasmid (negative control; 2.2 μg), or pCYP26-Luc (positive control; 2.2 μg), together with pRL-TK (0.8 μg) for normalization of transfection efficiency. Then cells were cultured in the medium with 0.1% ethanol (control), or 1 μM RA for 48 h. Luciferase activities were measured by a dual luciferase reporter assay system (Promega), and expressed relative to the value derived from PrEC cells transiently transfected with the pLRAT2008 and treated with 0.1% ethanol (control) for 48 h. The values were obtained from three independent experiments. *, p <0.05; * *, p <0.01 (compared to control).
Figure 2. The luciferase activity of the human LRAT promoter in human PrEC and PC-3 cells treated with RA or retinol.
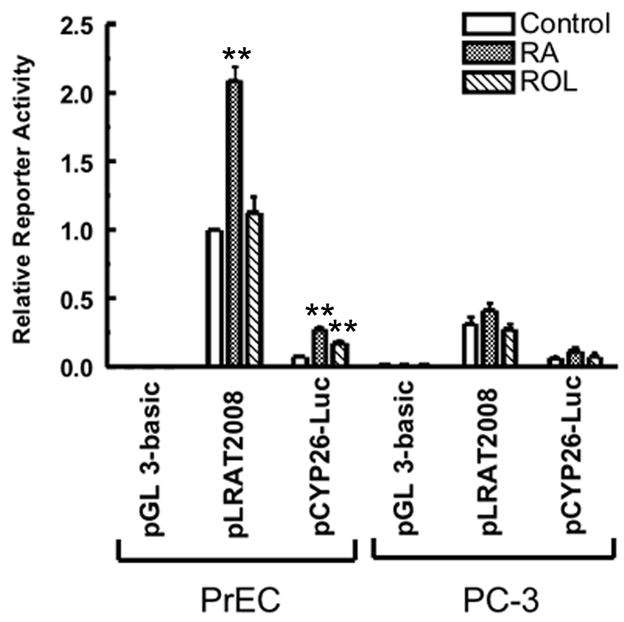
PrEC and PC-3 cells were transiently transfected with the pLRAT2008 (2.2 μg), pGL 3-basic plasmid (negative control; 2.2 μg), or pCYP26-Luc (positive control; 2.2 μg), together with pRL-TK (0.8 μg) for normalization of transfection efficiency. Then cells were cultured in the medium supplemented with 0.1% ethanol (control), 1 μM RA, or 1 μM retinol (ROL) for 48 h. Luciferase activites were measured by a dual luciferase reporter assay system (Promega), and expressed relative to the value derived from PrEC cells transiently transfected with the pLRATP2008 and treated with ethanol for 48 h. The values are from three independent experiments. * *, p <0.01 (compared to control)
PrEC and PC-3 cells were also transiently transfected with the pGL 3-Basic plasmid as a negative control and the pCYP26A1-Luc plasmid as a positive control (Lane et al., 2008). When the cells were transiently transfected with the pGL 3-Basic plasmid, the luciferase activity was at background level (Fig. 2), which indicates that the original pGL 3-Basic plasmid contains no promoter. The 2.5kb CYP26A1 promoter fragment in the pCYP26A1-Luc plasmid contains two identified retinoic acid response elements (RAREs) (Loudig et al., 2005, Loudig et al., 2000). In PrEC cells transiently transfected with pCYP26A1-Luc, 1μM RA treatment for 48 hours caused a 3.7-fold increase in luciferase activity (P < 0.005), while 1μM retinol treatment for 48 hours caused a 2.3-fold increase in the luciferase activity (P < 0.01) (Fig. 2). In PC-3 cells transiently transfected with pCYP26-Luc, 1μM RA and 1μM retinol treatment for 48 hours did not cause a statistically significant increase in the luciferase activity (Fig. 2). Thus, the PC-3 tumor cells are not retinoid responsive in this assay.
A 172-bp sequence contains essential regulatory elements required for LRAT expression and RA inducibility
The various 5′ deletion mutants of the human LRAT promoter (Fig. 3A) constructs were created by PCR. PrEC and PC-3 cells were transiently transfected with LRAT promoter-luciferase constructs (pLRAT2008, pLRAT833, pLRAT478, pLRAT186, and pLRAT14) and then treated with 1μM RA, or 0.1% ethanol (control) for 48 h. Sequential removal of promoter regions from −2008 to −186 caused no change in reporter gene expression (Fig. 3B). However, deletion of the promoter region from −186 to −14 caused a 96% loss of luciferase expression under control conditions in PrEC cells. These data indicate that the 172-bp sequence from −186 to −14 contains essential regulatory elements required for LRAT transcription.
The RA-induced level of the pLRAT186 luciferase construct in PrEC cells was double that in the non-RA-treated cells (P < 0.01). However, the RA-induced expression level of pLRAT14 in PrEC cells was similar to the control level of the pLRAT14 (P > 0.05). These results indicate that the 172-bp sequence contains a RA responsive element. However, deletion of the sequence within 50–60 bases of the transcription start site in the pLRAT14 construct most likely interfered with the basal transcription.
The activity of the construct pLRAT186 in PC-3 tumor cells under control conditions (treatment with vehicle for 48 h) was 63% of the activity in the normal PrEC cells (P < 0.05), whereas the luciferase activity of the pLRAT14 in PC-3 cells was similar to the activity in PrEC cells (P > 0.05). These results suggest that there are differences in the transcription factors on the LRAT promoter region from −186 to −14 in PC-3 vs. PrEC cells, and that these differences account for the greater transcription in the PrEC cells.
Retinoic acid receptors and GATA transcription factors cooperate to increase LRAT transcription
As mentioned above, RA exerts its functions by activating its nuclear receptors, including RARs and RXRs. RARs and RXRs form heterodimers which bind to retinoic acid response elements (RAREs) in the promoters of primary target genes. RAREs generally consist of two direct repeats of the consensus sequence AGGTCA with a space between them, most commonly five nucleotides (DR5) (Umesono et al., 1991, Mader et al., 1993, Langston et al., 1997, Huang et al., 1998). To pursue the possibility of a direct activation of the LRAT promoter by RARs and RXRs, we searched for RAREs in the 172-bp human LRAT promoter region (from −186 to −14) using the MatInspector computer program (www.genomatix.de; matrix library 6.1; core similarity = 0.75; matrix similarity = 0.75) (Quandt et al., 1995). However, we did not find any consensus RAR-RXR binding sites. It has been reported that RARα is able to bind GATA-2 and thus, the transcriptional activity of GATA-2 becomes RA responsive (Tsuzuki et al., 2004). Using the MatInspector computer program (www.genomatix.de; matrix library 6.1; core similarity = 0.75; matrix similarity = 0.75) (Quandt et al., 1995), we identified five putative GATA binding sites in this 172-bp promoter region (Fig. 4A).
Figure 4. GATA4 and RARβ2 or RARγ increase LRAT promoter-luciferase activity.
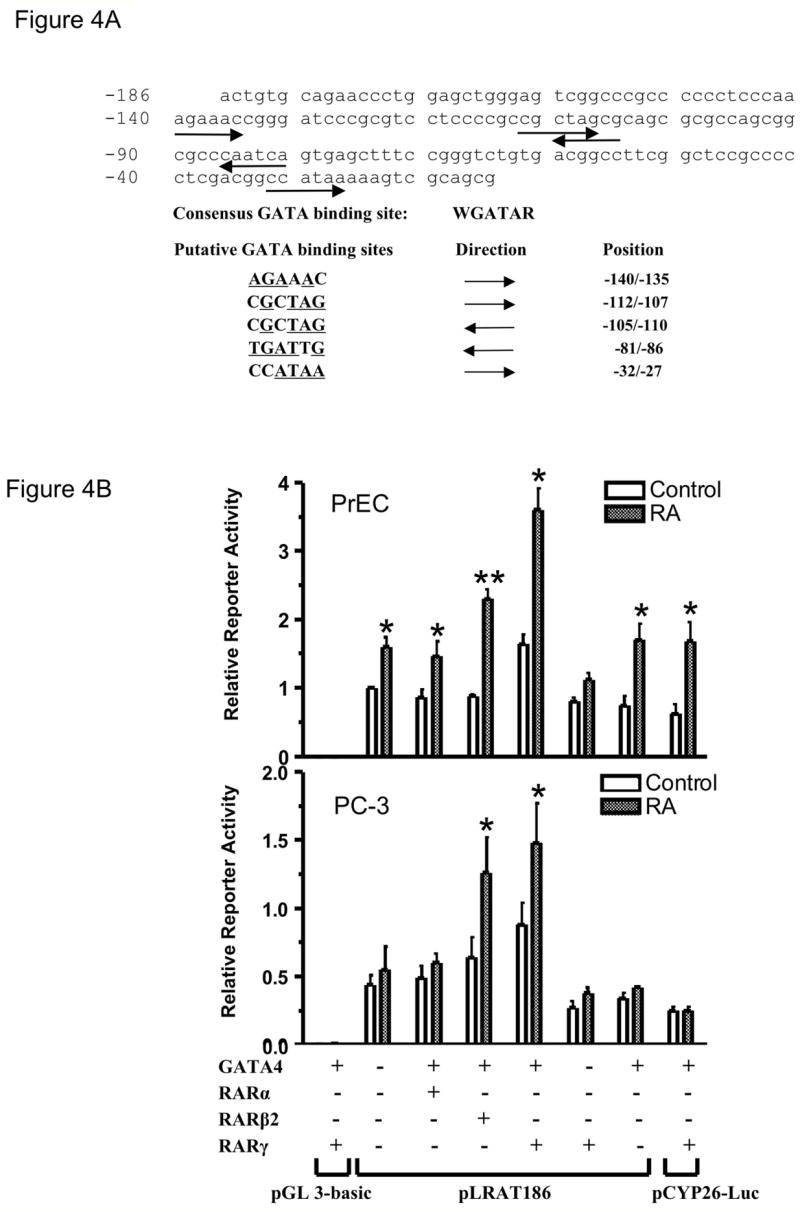
(A) Putative GATA sites in the 172-bp LRAT promoter region. The sequence of the human LRAT promoter (−186 to −15) and the consensus GATA binding site are shown. Five putative GATA binding sites were identified using MatInspector (matrix library 6.1; Core similarity = 0.75; matrix similarity = 0.75). The orientations are indicated by arrows. The positions are indicated by distance upstream from the major transcription start site of LRAT gene (Zolfaghari and Ross, 2004). (B) PrEC and PC-3 cells were transiently transfected with luciferase reporter plasmids pLRAT186 (0.9 μg), pGL 3-basic plasmid (negative control; 0.9 μg), or pCYP26-Luc (positive control; 0.9 μg), together with expression plasmids for GATA4 (0.9 μg) (or an empty vector; 0.9 μg) and RARα (0.9 μg), RARβ2 (0.9 μg), RARγ(0.9 μg), or an empty vector (0.9 μg). pRL-TK (0.3 μg) was used to normalize the transfection efficiencies. Cells were cultured in media with 0.1% ethanol (control), or 1 μM RA for 48 h. Luciferase activities were measured by a dual luciferase reporter assay system (Promega), and expressed relative to the value derived from PrEC cells transiently transfected with the pLRAT186 and empty vectors and treated with 0.1% ethanol (control) for 48 h. PrEC and PC-3 cells were also transiently transfected with pβAc-lacZ (0.9 μg), pSG5 (empty vector; 0.9 μg), pMT2 (empty vector; 0.9 μg), and pRL-TK (0.3 μg). The β-galactosidase activities were measured and used to normalize for any differences in transfection efficiency between PrEC and PC-3 cells (not shown). The values were from three independent experiments. Note the difference in the y-axis scale between PrEC and PC-3. *, p <0.05; * *, p <0.01 (RA treatment compared to control).
To assess the direct activation of the LRAT promoter by GATA transcription factors, we used the pLRAT186 human LRAT promoter-reporter construct in luciferase assays and cotransfected cells with RARs and GATA-4, a retinoic acid-inducible GATA transcription factor (Arceci et al., 1993). PrEC and PC-3 cells were cotransfected with the pLRAT186 plasmid, the pMT2-GATA-4 plasmid, and each of the three different isoforms of RAR (α, β2, and γ). The PrEC cells exhibited a statistically significant increase in luciferase activity after RA, even without cotransfection of RARs or GATA-4, whereas the PC-3 cells did not (Fig. 4B). Compared to the controls, in which the cells were transfected with the pLRAT186 plasmid and appropriate “empty” vectors, cotransfection of pLRAT186 with RARα and GATA-4 did not lead to a statistically significant increase in luciferase activity in PrEC and PC-3 cells (Fig. 4B). However, cotransfection of either RARβ2 or RARγ and pMT2-GATA-4 with pLRAT186 increased the luciferase activity in both PrEC and PC-3 cells treated with 1 μM RA, compared to their control levels (P < 0.05). Notably, cotransfection of RARγ with GATA-4 resulted in the highest promoter transcriptional activity after RA treatment. RA treatment did not alter the luciferase activity in PC-3 cells cotransfected with empty vectors, but increased the luciferase activity when PC-3 cells were cotransfected with GATA-4 and RARβ2 or RARγ. In addition, transfection of RARγ or GATA-4 alone did not increase the promoter activity relative to the controls in PrEC or PC-3 cells (Fig. 4B). These data suggest that retinoic acid receptors and GATA transcription factors are able to cooperate in response to RA and upregulate LRAT transcription in both PrEC and PC-3 cells.
RA and retinol-induced STRA6 expression is defective in PC-3 cells
STRA6 is a membrane receptor for RBP4 which mediates cellular retinol uptake and is involved in retinyl ester accumulation. The expression of STRA6 mRNA in various types of cancer cells has not been carefully analyzed. To determine if STRA6 mRNA levels are altered in PC-3 cells, PrEC and PC-3 cells were treated with 1μM RA, 1μM retinol, or ethanol (control) for 24 or 48 h. STRA6 mRNA levels were analyzed using real-time quantitative RT-PCR. Under control conditions, STRA6 mRNA basal levels in PrEC and PC-3 cells were the same (Fig. 5). In PrEC cells, 1μM RA or retinol treatment for 24 hours caused a 9 to 10 fold increase of STRA6 mRNA, and treatment for 48 hours resulted in a 15 – 20 fold increase, compared to control conditions (P < 0.05). However, 1μM RA and 1μM retinol treatments for 24 and 48 h did not result in a statistically significant increase in STRA6 mRNA levels in PC-3 cells. These data indicate that RA and retinol-induced STRA6 mRNA expression is defective in PC-3 cells.
Figure 5. The levels of STRA6 mRNA do not increase in PC-3 cells after RA or retinol treatment.
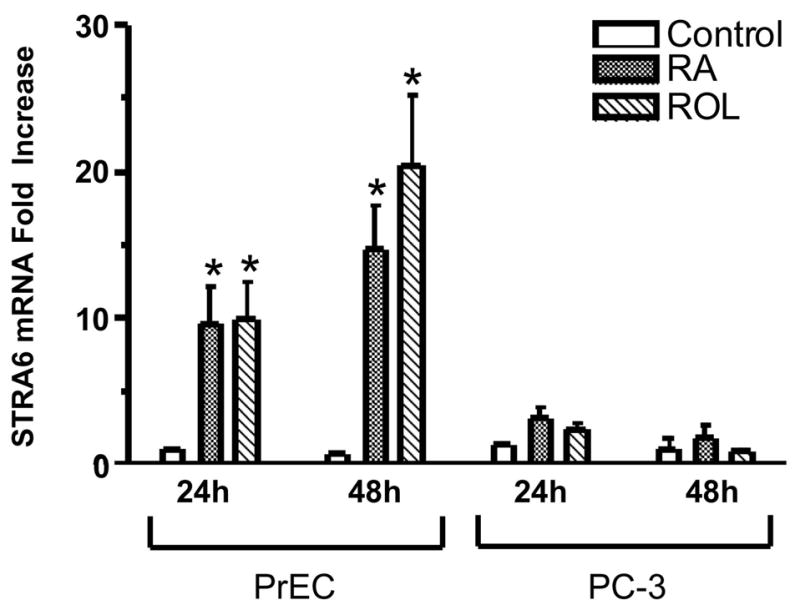
PrEC and PC-3 cells were cultured in media with 1μM RA, 1μM retinol (ROL), or 0.1% ethanol (control) for 24 h or 48 h. Total RNA was extracted using the TRIzol reagent (Invitrogen). STRA6 transcripts were quantified by real-time RT-PCR. The mRNA levels are normalized to GAPDH mRNA and are expressed relative to the value derived from PrEC cells treated with ethanol (control) for 24 h. The values were obtained from three independent experiments. *, p <0.05 (RA or ROL treatment compared to control).
DISCUSSION
Whereas retinoids have been used in conjunction with other therapeutic agents for the treatment of prostate cancer, retinoid metabolism often can become abnormal and retinoid responsiveness is frequently lost during the process of tumorigenesis (Guo et al., 2002, Lotan et al., 2000, Peehl et al., 1999, Mongan and Gudas, 2007). Previous studies in our laboratory showed that retinol esterification and LRAT protein levels are reduced in human prostate cancer cells (Guo et al., 2002). In this study we demonstrated that LRAT mRNA levels in PC-3 cells are lower than those in PrEC cells (Fig. 1A). Furthermore, we analyzed how LRAT mRNA is regulated in PrEC and PC-3 cells.
It has been shown that RARβ2 expression is low or lost in different cancers (Hu et al., 1991, Wu et al., 1998, Swisshelm et al., 1994). The mechanisms by which RARβ2 transcription is reduced in tumor cells include hypermethylation of the RARβ2 promoter (Zochbauer-Muller et al., 2001, Nakayama et al., 2001, Hayashi et al., 2001, Arapshian et al., 2000), loss of histone H3 acetylation (Suh et al., 2002), and different transcription factors regulating the expression of RARβ2 in tumor cells vs. normal cells (Wu et al., 1997). In our experiments the luciferase activity of the LRAT promoter in PC-3 cells was lower than that in PrEC cells (Fig. 2). Since in transient transfection experiments exogenous promoter DNA is introduced into the cells, methylation of the transfected LRAT promoter or histone H3 hypoacetylation is less likely to be the explanation for the observed differences between the normal and tumor cells (Fig. 2 and Fig. 3B). Our results suggest that various transcription factors are present at different levels on this LRAT promoter in the normal prostate vs. the prostate cancer cells.
Retinol and retinoic acid regulate LRAT mRNA expression in specific organs of rat (Zolfaghari and Ross, 2002, Zolfaghari and Ross, 2000, Randolph and Ross, 1991). Using promoter deletion assays, we demonstrated that a 172-bp proximal LRAT promoter region confers at least some of the RA responsiveness (Fig. 3B). However, we did not find a consensus RARE in this region, suggesting that the LRAT gene may not be a direct target of the retinoic acid receptors. Instead, we identified putative GATA binding sites (Fig. 4A). The GATA family of transcription factors is subdivided into two subfamilies based on their expression patterns and amino acid conservation. GATA-1, -2, and -3 are expressed primarily in hematopoietic cells (Martin et al., 1990, Zon et al., 1991, Mouthon et al., 1993, Leonard et al., 1993). GATA-4, -5, and -6 are expressed in a diverse array of tissues and could be critical in regulating cell-specific gene expression through interactions with other transcription factors (Sumi et al., 2007, Liu et al., 2002, van Wering et al., 2002). Furthermore, GATA-4 is a retinoic acid-inducible transcription factor (Arceci et al., 1993, Su and Gudas, 2008b, Su and Gudas, 2008a). RARα is able to associate with GATA-2, and thus RA can bind and activate RARα and then activate transcription through GATA binding sites (Tsuzuki et al., 2004). Therefore, we reasoned that co-transfection of RARs and GATA transcription factors might activate LRAT transcription. We showed that cotransfection of either RARβ2 or RARγ with GATA-4 increased the LRAT promoter activity after RA treatment (Fig. 4B). This result suggests that GATA-4 is able to recruit RARβ2 or RARγ, which allows RA to activate transcription through GATA binding sites. Interestingly, cotransfection of RARα with GATA-4 did not activate LRAT transcription (Fig. 4B). One explanation for this could be that GATA-4 is unable to bind RARα. It is possible that different GATA transcription factors (GATA-1, -2, -3, -4, -5, and -6) bind to different RAR receptors (RARα, β, and γ). We know that different RARs can recruit different coregulators to RAREs (Gillespie and Gudas, 2007).
The mechanisms by which LRAT gene expression is reduced in PC-3 cells are still not fully elucidated. We showed that RARβ2 mRNA levels in PC-3 cells are much lower than those in PrEC cells (Fig. 1B). It has been reported that CAPE (normal canine prostate epithelium) and PC-3 cells express RARα and RARγ (Jones et al., 1997). However, the expression of RARα, RARγ, and GATA transcription factors in PC-3 vs. PrEC cells is not known. We hypothesize that the reduction in LRAT mRNA levels in PC-3 cells is caused by the low expression of endogenous RARs and GATA transcription factors.
The expression of STRA6 in cancer is far from clear. The only report to date about STRA6 expression in cancer cells is that STRA6 mRNA levels are increased in human tumors that harbor defects in Wnt-1 signaling (Szeto et al., 2001). We showed that the RA and retinol-induction of STRA6 transcripts is defective in PC-3 cells (Fig. 5). This decrease in STRA6 mRNA levels might contribute to the lack of retinyl esters seen in PC-3 cells (Guo et al., 2002). Further experiments are needed to determine if STRA6 levels are reduced in various tumors. STRA6 is probably a direct target of the retinoid receptors (Bouillet et al., 1997, Chiba et al., 1997, Taneja et al., 1995). It has been reported that the RA induction of STRA6 is preferentially mediated by RARγ/RXRα heterodimers (Chiba et al., 1997). Further studies are necessary to clarify the mechanisms by which RA-induced STRA6 mRNA expression is defective in PC-3 cells.
One objective of our research is to devise new methods to increase retinol uptake and esterification in cells. We previously reported that RA, in combination with histone deacetylase inhibitors and/or DNA methyltransferase inhibitors, can restore RARβ2 expression in several types of cancer cells (Mongan and Gudas, 2005, Touma et al., 2005). How to increase the expression of GATA transcription factors in cancer cells is not clear at the present time. Our data suggest that combination therapies which increase the expression of both RARs and GATA transcription factors could increase LRAT expression and potentially reduce prostate tumor progression.
Acknowledgments
We thank N. Mongan for help with real-time RT-PCR, X.H. Tang for the PC-3 line, and K. Scotland for the PrEC cells. R. Sylvester and M. Ng are thanked for technical assistance. We thank members of the Gudas laboratory for helpful discussions and N. Mongan for critically reading this manuscript. K. Ecklund and C. Kelly are thanked for editorial assistance. This research was supported by NIH R01 CA097543 to LJG.
Abbreviations
- ARAT
Acyl CoA, retinol acyltransferase
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- LRAT, Lecithin
retinol acyltransferase
- PCR
Polymerase chain reaction
- PrEC
Prostate epithelial cell
- RA
all-trans retinoic acid
- RAR
Retinoic acid receptor
- RARE
Retinoic acid response element
- RBP
Retinol binding protein
- ROL
retinol
- RT
Reverse transcription
- RXR
retinoid X receptor
References
- Arapshian A, Kuppumbatti YS, Mira-y-Lopez R. Methylation of conserved CpG sites neighboring the beta retinoic acid response element may mediate retinoic acid receptor beta gene silencing in MCF-7 breast cancer cells. Oncogene. 2000;19:4066–4070. doi: 10.1038/sj.onc.1203734. [DOI] [PubMed] [Google Scholar]
- Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutt SE, Allegretto EA, Pike JW, Weigel NL. 1,25-dihydroxyvitamin D3 and 9-cis-retinoic acid act synergistically to inhibit the growth of LNCaP prostate cells and cause accumulation of cells in G1. Endocrinology. 1997;138:1491–1497. doi: 10.1210/endo.138.4.5063. [DOI] [PubMed] [Google Scholar]
- Bohnsack BL, Hirschi KK. Nutrient regulation of cell cycle progression. Annual Review of Nutrition. 2004;24:433–453. doi: 10.1146/annurev.nutr.23.011702.073203. [DOI] [PubMed] [Google Scholar]
- Boorjian S, Tickoo SK, Mongan NP, Yu H, Bok D, Rando RR, Nanus DM, Scherr DS, Gudas LJ. Reduced lecithin: retinol acyltransferase expression correlates with increased pathologic tumor stage in bladder cancer. Clin Cancer Res. 2004;10:3429–3437. doi: 10.1158/1078-0432.CCR-03-0756. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Park S, Uskokovic MR, Dawson MI, Koeffler HP. Expression of retinoic acid receptor-beta sensitizes prostate cancer cells to growth inhibition mediated by combinations of retinoids and a 19-nor hexafluoride vitamin D3 analog. Endocrinology. 1998;139:1972–1980. doi: 10.1210/endo.139.4.5943. [DOI] [PubMed] [Google Scholar]
- Chen AC, Guo X, Derguini F, Gudas LJ. Human breast cancer cells and normal mammary epithelial cells: retinol metabolism and growth inhibition by the retinol metabolite 4-oxoretinol. Cancer Res. 1997;57:4642–4651. [PubMed] [Google Scholar]
- Chiba H, Clifford J, Metzger D, Chambon P. Distinct retinoid X receptor-retinoic acid receptor heterodimers are differentially involved in the control of expression of retinoid target genes in F9 embryonal carcinoma cells. Mol Cell Biol. 1997;17:3013–3020. doi: 10.1128/mcb.17.6.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya R, Boyle B, Park HD, Kurhanewicz J, Macdonald JM, Narayan P. 13-cis-retinoic acid-mediated growth inhibition of DU-145 human prostate cancer cells. Biochemistry & Molecular Biology International. 1994;32:1–12. [PubMed] [Google Scholar]
- de Vos S, Dawson MI, Holden S, Le T, Wang A, Cho SK, Chen DL, Koeffler HP. Effects of retinoid X receptor-selective ligands on proliferation of prostate cancer cells. Prostate. 1997;32:115–121. doi: 10.1002/(sici)1097-0045(19970701)32:2<115::aid-pros6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- DiPaola RS, Rafi MM, Vyas V, Toppmeyer D, Rubin E, Patel J, Goodin S, Medina M, Medina P, Zamek R, Zhang C, White E, Gupta E, Hait WN. Phase I clinical and pharmacologic study of 13-cis-retinoic acid, interferon alfa, and paclitaxel in patients with prostate cancer and other advanced malignancies. J Clin Oncol. 1999;17:2213–2218. doi: 10.1200/JCO.1999.17.7.2213. [DOI] [PubMed] [Google Scholar]
- Gillespie RF, Gudas LJ. Retinoic acid receptor isotype specificity in F9 teratocarcinoma stem cells results from the differential recruitment of coregulators to retinoic response elements. J Biol Chem. 2007;282:33421–33434. doi: 10.1074/jbc.M704845200. [DOI] [PubMed] [Google Scholar]
- Goodman DS. Plasma retinol-binding protein. Annals of the New York Academy of Sciences. 1980;348:378–390. doi: 10.1111/j.1749-6632.1980.tb21314.x. [DOI] [PubMed] [Google Scholar]
- Gudas LJ, Sporn MB, Roberts AB. Cellular biology and biochemistry of the retinoids. In: MBaR Sporn AB, Goodman DS, editors. The Retinoids: Biology, Chemistry, and Medicine. New York: Raven Press; 1994. pp. 443–520. [Google Scholar]
- Guo X, Knudsen BS, Peehl DM, Ruiz A, Bok D, Rando RR, Rhim JS, Nanus DM, Gudas LJ. Retinol metabolism and lecithin:retinol acyltransferase levels are reduced in cultured human prostate cancer cells and tissue specimens. Cancer Research. 2002;62:1654–1661. [PubMed] [Google Scholar]
- Guo X, Nanus DM, Ruiz A, Rando RR, Bok D, Gudas LJ. Reduced levels of retinyl esters and vitamin A in human renal cancers. Cancer Research. 2001;61:2774–2781. [PubMed] [Google Scholar]
- Guo X, Ruiz A, Rando RR, Bok D, Gudas LJ. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis. 2000;21:1925–1933. doi: 10.1093/carcin/21.11.1925. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Yokozaki H, Goodison S, Oue N, Suzuki T, Lotan R, Yasui W, Tahara E. Inactivation of retinoic acid receptor beta by promoter CpG hypermethylation in gastric cancer. Differentiation. 2001;68:13–21. doi: 10.1046/j.1432-0436.2001.068001013.x. [DOI] [PubMed] [Google Scholar]
- Herr FM, MacDonald PN, Ong DE. Solubilization and partial characterization of lecithin-retinol acyltransferase from rat liver. J Nutr Biochem. 1991:503–511. [Google Scholar]
- Hu L, Crowe DL, Rheinwald JG, Chambon P, Gudas LJ. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51:3972–3981. [PubMed] [Google Scholar]
- Huang D, Chen SW, Langston AW, Gudas LJ. A conserved retinoic acid responsive element in the murine Hoxb-1 gene is required for expression in the developing gut. Development. 1998;125:3235–3246. doi: 10.1242/dev.125.16.3235. [DOI] [PubMed] [Google Scholar]
- Jones HE, Eaton CL, Barrow D, Dutkowski C, Griffiths K. Response of cell growth and retinoic acid receptor expression to retinoic acid in neoplastic and non-neoplastic prostate cell lines. Prostate. 1997;30:174–182. doi: 10.1002/(sici)1097-0045(19970215)30:3<174::aid-pros5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kim H, Lapointe J, Kaygusuz G, Ong DE, Li C, van de Rijn M, Brooks JD, Pollack JR. The retinoic acid synthesis gene ALDH1a2 is a candidate tumor suppressor in prostate cancer. Cancer Res. 2005;65:8118–8124. doi: 10.1158/0008-5472.CAN-04-4562. [DOI] [PubMed] [Google Scholar]
- Kim YK, Wassef L, Hamberger L, Piantedosi R, Palczewski K, Blaner WS, Quadro L. Retinyl ester formation by lecithin: Retinol acyltransferase (LRAT) is a key regulator of retinoid homeostasis in mouse embryogenesis. J Biol Chem. 2007;283:5611–5621. doi: 10.1074/jbc.M708885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Xu J, Wilen EW, Sylvester R, Derguini F, Gudas LJ. LIF removal increases CRABPI and CRABPII transcripts in embryonic stem cells cultured in retinol or 4-oxoretinol. Mol Cell Endocrinol. 2008;280:63–74. doi: 10.1016/j.mce.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston AW, Thompson JR, Gudas LJ. Retinoic acid-responsive enhancers located 3′ of the Hox A and Hox B homeobox gene clusters. Functional analysis. J Biol Chem. 1997;272:2167–2175. doi: 10.1074/jbc.272.4.2167. [DOI] [PubMed] [Google Scholar]
- Leonard M, Brice M, Engel JD, Papayannopoulou T. Dynamics of GATA transcription factor expression during erythroid differentiation. Blood. 1993;82:1071–1079. [PubMed] [Google Scholar]
- Liu C, Glasser SW, Wan H, Whitsett JA. GATA-6 and thyroid transcription factor-1 directly interact and regulate surfactant protein-C gene expression. J Biol Chem. 2002;277:4519–4525. doi: 10.1074/jbc.M107585200. [DOI] [PubMed] [Google Scholar]
- Liu L, Gudas LJ. Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J Biol Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- Liu L, Tang XH, Gudas LJ. Homeostasis of retinol in lecithin: retinol acyltransferase gene knockout mice fed a high retinol diet. Biochem Pharmacol. 2008;75:2316–2324. doi: 10.1016/j.bcp.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R. Effect of vitamin A and its analogs (retinoids) on normal and neplastic cells. Biochim Biophys Acta. 1981;605:33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Lotan Y, Xu XC, Shalev M, Lotan R, Williams R, Wheeler TM, Thompson TC, Kadmon D. Differential expression of nuclear retinoid receptors in normal and malignant prostates. J Clin Oncol. 2000;18:116–121. doi: 10.1200/JCO.2000.18.1.116. [DOI] [PubMed] [Google Scholar]
- Loudig O, Babichuk C, White J, Abu-Abed S, Mueller C, Petkovich M. Cytochrome P450RAI(CYP26) promoter: a distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol Endocrinol. 2000;14:1483–1497. doi: 10.1210/mend.14.9.0518. [DOI] [PubMed] [Google Scholar]
- Loudig O, Maclean GA, Dore NL, Luu L, Petkovich M. Transcriptional co-operativity between distant retinoic acid response elements in regulation of Cyp26A1 inducibility. Biochem J. 2005;392:241–248. doi: 10.1042/BJ20050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader S, Leroy P, Chen JY, Chambon P. Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J Biol Chem. 1993;268:591–600. [PubMed] [Google Scholar]
- Mangelsdorf DJ. Vitamin A receptors. Nutrition Reviews. 1994;52:S32–44. doi: 10.1111/j.1753-4887.1994.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Martin DI, Zon LI, Mutter G, Orkin SH. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990;344:444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Mongan NP, Gudas LJ. Valproic acid, in combination with all-trans retinoic acid and 5-aza-2′-deoxycytidine, restores expression of silenced RARbeta2 in breast cancer cells. Mol Cancer Ther. 2005;4:477–486. doi: 10.1158/1535-7163.MCT-04-0079. [DOI] [PubMed] [Google Scholar]
- Mongan NP, Gudas LJ. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75:853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Mouthon MA, Bernard O, Mitjavila MT, Romeo PH, Vainchenker W, Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993;81:647–655. [PubMed] [Google Scholar]
- Nakayama T, Watanabe M, Yamanaka M, Hirokawa Y, Suzuki H, Ito H, Yatani R, Shiraishi T. The role of epigenetic modifications in retinoic acid receptor beta2 gene expression in human prostate cancers. Lab Invest. 2001;81:1049–1057. doi: 10.1038/labinvest.3780316. [DOI] [PubMed] [Google Scholar]
- Nanus DM, Gudas LJ. Retinoids and prostate cancer. Prostate J. 2000;2:68–73. [Google Scholar]
- Niles RM. Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutation Research. 2004;555:81–96. doi: 10.1016/j.mrfmmm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT) J Biol Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong DE, MacDonald PN, Gubitosi AM. Esterification of retinol in rat liver. Possible participation by cellular retinol-binding protein and cellular retinol-binding protein II. Journal of Biological Chemistry. 1988;263:5789–5796. [PubMed] [Google Scholar]
- Peehl DM, Sellers RG, Arnstein P, Kung HF, Rhim JS. Altered growth regulation and loss of response to retinoic acid accompany tumorigenic transformation of prostatic cells. Anticancer Res. 1999;19:3857–3864. [PubMed] [Google Scholar]
- Peehl DM, Wong ST, Stamey TA. Vitamin A regulates proliferation and differentiation of human prostatic epithelial cells. Prostate. 1993;23:69–78. doi: 10.1002/pros.2990230107. [DOI] [PubMed] [Google Scholar]
- Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph RK, Ross AC. Vitamin A status regulates hepatic lecithin: retinol acyltransferase activity in rats. J Biol Chem. 1991;266:16453–16457. [PubMed] [Google Scholar]
- Sambrook JFEF, Maniatis T. Molecular cloning: a laboratory manual. New York: Cold Cold Spring Harber Laboratory Press; 1989. [Google Scholar]
- Sheren-Manoff M, Shin SJ, Su D, Bok D, Rando RR, Gudas LJ. Reduced lecithin:retinol acyltransferase expression in human breast cancer. Int J Oncol. 2006;29:1193–1199. [PubMed] [Google Scholar]
- Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- Su D, Gudas LJ. Gene expression profiling elucidates a specific role for RARgamma in the retinoic acid-induced differentiation of F9 teratocarcinoma stem cells. Biochem Pharmacol. 2008a;75:1129–1160. doi: 10.1016/j.bcp.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su D, Gudas LJ. Retinoic acid receptor gamma activates receptor tyrosine kinase Tie1 gene transcription through transcription factor GATA4 in F9 stem cells. Exp Hematol. 2008b;36:624–641. doi: 10.1016/j.exphem.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Suh YA, Lee HY, Virmani A, Wong J, Mann KK, Miller WH, Jr, Gazdar A, Kurie JM. Loss of retinoic acid receptor beta gene expression is linked to aberrant histone H3 acetylation in lung cancer cell lines. Cancer Res. 2002;62:3945–3949. [PubMed] [Google Scholar]
- Sumi K, Tanaka T, Uchida A, Magoori K, Urashima Y, Ohashi R, Ohguchi H, Okamura M, Kudo H, Daigo K, Maejima T, Kojima N, Sakakibara I, Jiang S, Hasegawa G, Kim I, Osborne TF, Naito M, Gonzalez FJ, Hamakubo T, Kodama T, Sakai J. Cooperative interaction between hepatocyte nuclear factor 4 alpha and GATA transcription factors regulates ATP-binding cassette sterol transporters ABCG5 and ABCG8. Mol Cell Biol. 2007;27:4248–4260. doi: 10.1128/MCB.01894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Lotan R. Retinoids and their receptors in cancer development and chemoprevention. Crit Rev Oncol Hematol. 2002;41:41–55. doi: 10.1016/s1040-8428(01)00144-5. [DOI] [PubMed] [Google Scholar]
- Swisshelm K, Ryan K, Lee X, Tsou HC, Peacocke M, Sager R. Down-regulation of retinoic acid receptor beta in mammary carcinoma cell lines and its up-regulation in senescing normal mammary epithelial cells. Cell Growth Differ. 1994;5:133–141. [PubMed] [Google Scholar]
- Szeto W, Jiang W, Tice DA, Rubinfeld B, Hollingshead PG, Fong SE, Dugger DL, Pham T, Yansura DG, Wong TA, Grimaldi JC, Corpuz RT, Singh JS, Frantz GD, Devaux B, Crowley CW, Schwall RH, Eberhard DA, Rastelli L, Polakis P, Pennica D. Overexpression of the retinoic acid-responsive gene Stra6 in human cancers and its synergistic induction by Wnt-1 and retinoic acid. Cancer Res. 2001;61:4197–4205. [PubMed] [Google Scholar]
- Taneja R, Bouillet P, Boylan JF, Gaub MP, Roy B, Gudas LJ, Chambon P. Reexpression of retinoic acid receptor (RAR) gamma or overexpression of RAR alpha or RAR beta in RAR gamma-null F9 cells reveals a partial functional redundancy between the three RAR types. Proc Natl Acad Sci U S A. 1995;92:7854–7858. doi: 10.1073/pnas.92.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalasila A, Poplin E, Shih J, Dvorzhinski D, Capanna T, Doyle-Lindrud S, Beers S, Goodin S, Rubin E, DiPaola RS. A phase I trial of weekly paclitaxel, 13- cis-retinoic acid, and interferon alpha in patients with prostate cancer and other advanced malignancies. Cancer Chemother Pharmacol. 2003;52:119–124. doi: 10.1007/s00280-003-0644-6. [DOI] [PubMed] [Google Scholar]
- Touma SE, Goldberg JS, Moench P, Guo X, Tickoo SK, Gudas LJ, Nanus DM. Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model. Clin Cancer Res. 2005;11:3558–3566. doi: 10.1158/1078-0432.CCR-04-1155. [DOI] [PubMed] [Google Scholar]
- Tsuzuki S, Kitajima K, Nakano T, Glasow A, Zelent A, Enver T. Cross talk between retinoic acid signaling and transcription factor GATA-2. Mol Cell Biol. 2004;24:6824–6836. doi: 10.1128/MCB.24.15.6824-6836.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wering HM, Huibregtse IL, van der Zwan SM, de Bie MS, Dowling LN, Boudreau F, Rings EH, Grand RJ, Krasinski SD. Physical interaction between GATA-5 and hepatocyte nuclear factor-1alpha results in synergistic activation of the human lactase-phlorizin hydrolase promoter. J Biol Chem. 2002;277:27659–27667. doi: 10.1074/jbc.M203645200. [DOI] [PubMed] [Google Scholar]
- Vasios GW, Gold JD, Petkovich M, Chambon P, Gudas LJ. A retinoic acid-responsive element is present in the 5′ flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989;86:9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongsiriroj N, Piantedosi R, Palczewski K, Goldberg IJ, Johnston TP, Li E, Blaner WS. The molecular basis of retinoid absorption: a genetic dissection. J Biol Chem. 2008;283:13510–13519. doi: 10.1074/jbc.M800777200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Li Y, Liu R, Agadir A, Lee MO, Liu Y, Zhang X. Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. Embo J. 1997;16:1656–1669. doi: 10.1093/emboj/16.7.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Zhang D, Zhang ZP, Soprano DR, Soprano KJ. Critical role of both retinoid nuclear receptors and retinoid-X-receptors in mediating growth inhibition of ovarian cancer cells by all-trans retinoic acid. Oncogene. 1998;17:2839–2849. doi: 10.1038/sj.onc.1202208. [DOI] [PubMed] [Google Scholar]
- Yost RW, Harrison EH, Ross AC. Esterification by rat liver microsomes of retinol bound to cellular retinol-binding protein. Journal of Biological Chemistry. 1988;263:18693–18701. [PubMed] [Google Scholar]
- Zhan HC, Gudas LJ, Bok D, Rando R, Nanus DM, Tickoo SK. Differential expression of the enzyme that esterifies retinol, lecithin:retinol acyltransferase, in subtypes of human renal cancer and normal kidney. Clin Cancer Res. 2003;9:4897–4905. [PubMed] [Google Scholar]
- Zheng X, Chang RL, Cui XX, Avila GE, Lee S, Lu YP, Lou YR, Shih WJ, Lin Y, Reuhl K, Newmark H, Rabson A, Conney AH. Inhibitory effect of 12-O-tetradecanoylphorbol-13-acetate alone or in combination with all-trans-retinoic acid on the growth of LNCaP prostate tumors in immunodeficient mice. Cancer Res. 2004;64:1811–1820. doi: 10.1158/0008-5472.can-03-2848. [DOI] [PubMed] [Google Scholar]
- Zochbauer-Muller S, Fong KM, Virmani AK, Geradts J, Gazdar AF, Minna JD. Aberrant promoter methylation of multiple genes in non-small cell lung cancers. Cancer Res. 2001;61:249–255. [PubMed] [Google Scholar]
- Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase from mouse and rat liver. CDNA cloning and liver-specific regulation by dietary vitamin a and retinoic acid. J Lipid Res. 2000;41:2024–2034. [PubMed] [Google Scholar]
- Zolfaghari R, Ross AC. Lecithin:retinol acyltransferase expression is regulated by dietary vitamin A and exogenous retinoic acid in the lung of adult rats. J Nutr. 2002;132:1160–1164. doi: 10.1093/jn/132.6.1160. [DOI] [PubMed] [Google Scholar]
- Zolfaghari R, Ross AC. Cloning, gene organization and identification of an alternative splicing process in lecithin:retinol acyltransferase cDNA from human liver. Gene. 2004;341:181–188. doi: 10.1016/j.gene.2004.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI, Mather C, Burgess S, Bolce ME, Harland RM, Orkin SH. Expression of GATA-binding proteins during embryonic development in Xenopus laevis. Proc Natl Acad Sci U S A. 1991;88:10642–10646. doi: 10.1073/pnas.88.23.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]