Abstract
The recepteur d’origne nantais (RON) is a receptor tyrosine kinase (RTK) in the scatter factor family, which includes the c-Met receptor. RON exhibits increased expression in a significant number of human breast cancer tissues as well as in many established breast cancer cell lines. Recent studies have indicated that in addition to ligand-dependent signaling events, RON also promotes signals in the absence of its only known ligand, the macrophage stimulating protein, when expressed in epithelial cells. In the current study, we found that when expressed in MCF-10A breast epithelial cells, RON exhibits both MSP-dependent and MSP-independent signaling, which lead to distinct biological outcomes. In the absence of MSP, RON signaling promotes cell survival, increased cell spreading and enhanced migration in response to other growth factors. However, both RON-mediated proliferation and migration require the addition of MSP in MCF-10A cells. Both MSP-dependent and MSP-independent signaling by RON is mediated in part by Src-family kinases. These data suggest that RON has two alternative modes of signaling that can contribute to oncogenic behavior in normal breast epithelial cells.
Introduction
The receptor tyrosine kinase (RTK) RON is a member of the c-Met family of scatter-factor receptors. (Ronsin et al., 1993). After binding to its only known ligand, the macrophage stimulating protein (MSP), RON promotes activation of the PI3K/AKT, MAPK and β-catenin pathways, among others (Wang et al., 2003). Increased levels of RON expression have been found in several epithelial human tumors including colon (Chen et al., 2000), pancreatic (Thomas et al., 2007) and breast cancers (Maggiora et al., 1998). Furthermore, clinical studies indicate that increased expression of RON in both human bladder and breast carcinomas correlates with a more aggressive disease and a poor patient prognosis (Hsu et al., 2006; Lee et al., 2005). Recent studies demonstrated that a monoclonal antibody that blocks RON activation by MSP also inhibited the growth of human tumor xenographs in mice, indicating that signaling by RON played a role in tumor growth (O'Toole et al., 2006). Together, these studies provide evidence that RON may play a general role in cancer development.
RON appears to play a significant role in breast cancer. Nearly 47% of primary human breast cancers expressed RON, and increased expression of RON was found in established breast cancer cell lines (Maggiora et al., 1998). Additionally, when mice were engineered to express RON in mammary tissue, 100% of the RON-expressing mice developed tumors, whereas the parental mice did not develop tumors (Zinser et al., 2006) Although increased expression of RON in breast carcinomas is well-documented, less-understood is whether RON can promote cancer progression in the absence of MSP. To date, no naturally occurring mutations of RON have been identified in human breast cancers; therefore, it is likely that interactions with other cell receptors or kinases might be responsible for the ligand-independent activation of RON.
In breast carcinomas, the activity of Src promotes tumor progression at least in part by its ability to synergize with the epidermal growth factor receptor (EGFR) (Biscardi et al., 2000; Wilson et al., 1989). Other RTKs also interact with Src kinases to enhance oncogenic signaling in human cancers, including c-Met (Emaduddin et al., 2008) and platelet-derived growth factor receptor (PDGFR) (Ishizawar & Parsons, 2004). Additionally, Src mediated RON activation downstream of β1 integrins in human keratinocytes (Danilkovitch-Miagkova et al., 2000). The fact that two or more kinases cooperate to increase their oncogenic effects may dramatically impact the clinical treatment for those patients whose tumors are co-expressing RTKs with other kinases (Stommel et al., 2007)
Since Src is highly expressed and deregulated in at least 70% of human breast cancers (Ishizawar & Parsons, 2004), it is likely that RON and Src are co-expressed in a number of breast tumors. Furthermore, Src is recognized as an important contributing factor to breast cancer progression (Ishizawar et al., 2004). In this study, we examined the contributions of RON, and its putative interaction with c-Src, to the progression of breast cancer by expressing RON in the well-characterized MCF-10A human mammary epithelial cell line. MCF-10A cells are a powerful cell system with which to identify the effects of oncogenic signaling by RTKs (Debnath et al., 2003; Muthuswamy et al., 2001). Our current research determined that the RON receptor was activated in the absence MSP when expressed in MCF-10A cells, which gave rise to evasion of cell death, an increase in spreading and an increased migratory potential. MSP-stimulation of RON was required for RON-mediated cell migration and proliferation, which suggests that MSP-independent functions of RON are not solely a consequence of increased RON expression levels. In addition, Src activity is required for RON-mediated, MSP-independent biologic effects. These data imply that RON cooperates with Src in mammary epithelial cells to promote cellular changes that may allow progression toward a fully oncogenic state.
Results
Characterization of RON expression levels in MCF-10A cells
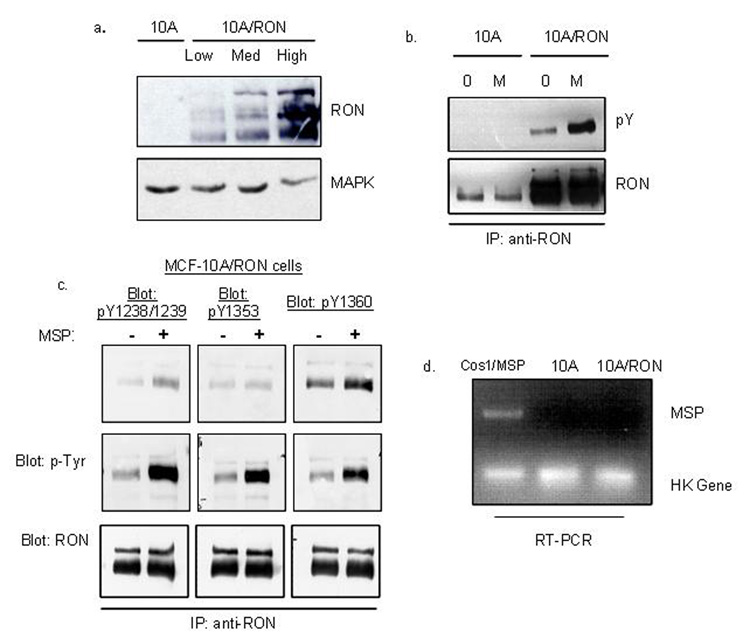
Endogenous levels of RON were low in the relatively normal epithelial cell line, MCF-10A. Consequently, the cells did not respond to MSP in any biological or biochemical assays we have tested to date. Therefore, to examine the contributions of RON to the progression of breast cancer, we transduced MCF-10A cells with a retrovirus expressing wild-type human RON and IRES-promoted GFP. Pools of infected cells were selected for high, medium and low GFP expression levels by FACS, and, as expected, GFP levels were mimicked by expression levels of RON (Figure 1a). We designated these cells as 10A/RON and our vector-only negative control cell line was designated 10A/Vector. The levels of RON expression in 10A/RON cells were well within the range of expression found in human breast carcinomas, some of which had RON levels that were up to 72-fold higher than the level found in benign tissue (Maggiora et al., 1998). High, medium and low 10A/RON cell lines behaved similarly in all of our experiments therefore, we utilized the medium-RON expressing MCF-10A cell line throughout this study.
Figure 1. RON exhibits MSP-independent tyrosine phosphorylation in 10A cells.
(a) GFP-positive cells were selected by FACS analysis to yield a 10A/Vector control cell line and RON-expressing cells. RON-expression levels were detected by Western blot with an anti-RON antibody. This antibody recognizes the three different processed forms of RON: the 170 kDa unprocessed form, the 150 kDa processed and phosphorylated form and the 145 kDa processed and unphosphorylated form. Total MAPK level was used as a loading control. (b) 10A/Vector and 10A/RON cells were immunoprecipitated with an anti-RON antibody and subjected to a Western blot analysis for phospho-tyrosine (4G10 antibody). The blot was reprobed for total RON expression. (c)The same experiment as in (b), blotting with antibodies against catalytically activated RON (pY1238/Y1239), and specific tyrosine phosphorylated docking sites in the C-terminus of RON (pY1353, pY1360). (d) Semi-quantitative RT-PCR detected MSP mRNA levels. COS1 cells were transfected with a vector containing an MSP cassette as a positive control. These experiments were repeated at least three times, and shown here are representative images.
RON exhibits MSP-independent tyrosine phosphorylation in MCF-10A cells
Although MSP is the only known ligand for RON, RON may not require MSP to induce some of its biological effects. For example, RON knockout mice die by E6.5 (Muraoka et al., 1999), whereas mice deficient in MSP exhibit a nearly normal phenotype (Bezerra et al., 1998). We detected MSP-independent tyrosine phosphorylation of RON using an anti-phospho-tyrosine antibody (Figure 1b), antibodies directed specifically against tyrosine phosphorylated residues in the catalytic site (Y1238/1239) and on C-terminal docking sites (Y1353 and Y1360) of RON (Figure 1c). This data suggests that RON may transduce downstream signals in the absence of MSP. RON also exhibited increased phosphorylation on Y1238/1239 and on the Y1360 docking site in the presence of exogenously added MSP (Figure 1b, c). There was no significant increase of phosphorylation on Y1353 with the addition of MSP. RT-PCR analysis indicated that neither the 10A/Vector nor the 10A/RON cells endogenously express MSP (Figure 1d), indicating that the activation of RON is not due to an autocrine loop unique to MCF-10A cells. Similar levels of RON expression in NIH-3T3 cells did not lead to tyrosine phosphorylation of RON in the absence of ligand (Supplementary Figure 1a), indicating that the RON receptor is not constitutively active, but rather that the activation seen in MCF-10A cells is a cell-specific effect. MSP-independent tyrosine phosphorylation of RON is not unique to MCF-10A cells, as we can recapitulate the MSP-independent activation of RON by expressing RON in HeLa cells (Supplementary Figure 1b). Further, we found that a kinase dead mutant of RON, K1114M (Danilkovitch-Miagkova et al., 2000), does not exhibit any tyrosine phosphorylation in HeLa cells (Supplementary Figure 2b), providing evidence that the kinase activity of RON is necessary for phosphorylation.
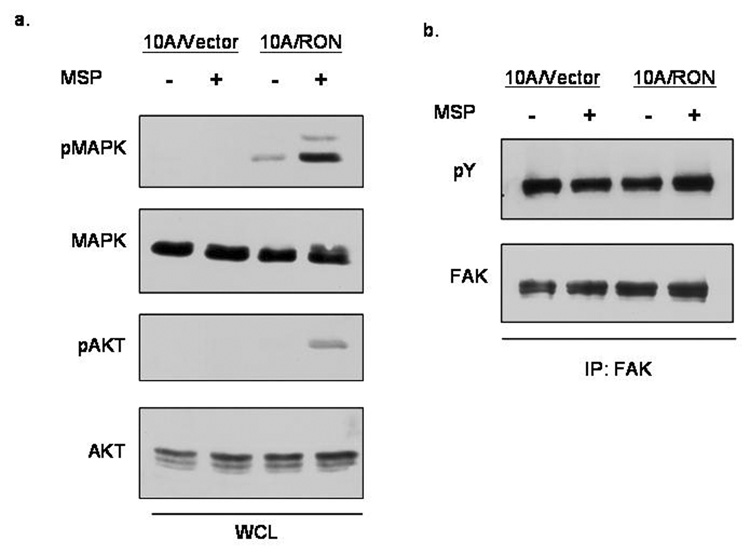
MSP increases stimulation of MAPK and AKT but has no effect on the phosphorylation of FAK
We considered possible molecular differences between MSP-dependent and MSP-independent RON signaling in MCF-10A cells by looking at the activation of MAPK (Erk 1/2) and AKT, which are known to be important for MSP-driven activation of RON in other cell systems. In the absence of MSP, RON promotes a low level of MAPK activation and a barely detectable AKT activation; however, MSP significantly increased the activation of both MAPK and AKT (Figure 2a). We also looked at whether a molecular distinction could be made in focal adhesion kinase (FAK) phosphorylation between MSP-dependent and independent RON signaling because of RON’s known role in cell migration and its previously described interaction with the Src kinase (Danilkovitch-Miagkova et al., 2000), which has a role in FAK signaling. This data demonstrated that in MCF-10A cells, FAK is constitutively phosphorylated, and there was no discernible difference between 10A/Vector and 10A/RON cells in the presence or absence of MSP (figure 2b).
Figure 2. MSP-stimulation of RON leads to MAPK and AKT activation.
(a)Cell lysates were prepared after 3 hours of serum starvation and 30 minute stimulation with 200ng/ml MSP. Whole cell lysates were run on a polyacrylamide gel and subjected to blotting with phospho-MAPK, MAPK, phospho-AKT and AKT antibodies. (b) Focal adhesion kinase was immunoprecipitated from cells and subjected to a Western Blot analysis by 4G10 phospho-tyrosine antibodies. The blot was stripped and reprobed for total FAK expression.
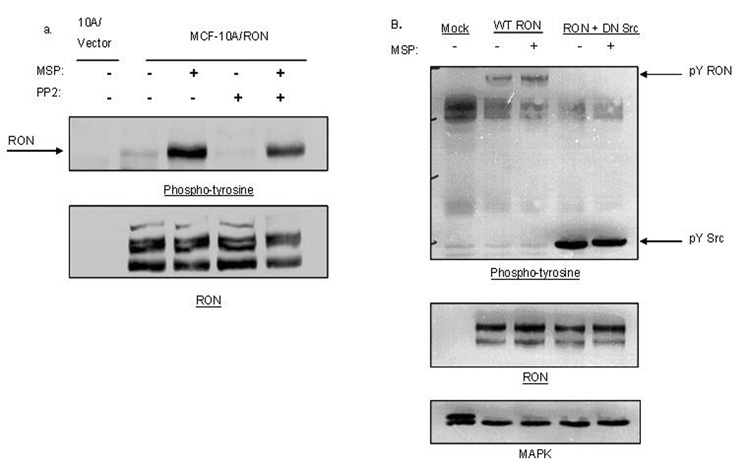
Tyrosine-phosphorylation of RON is Src-dependent
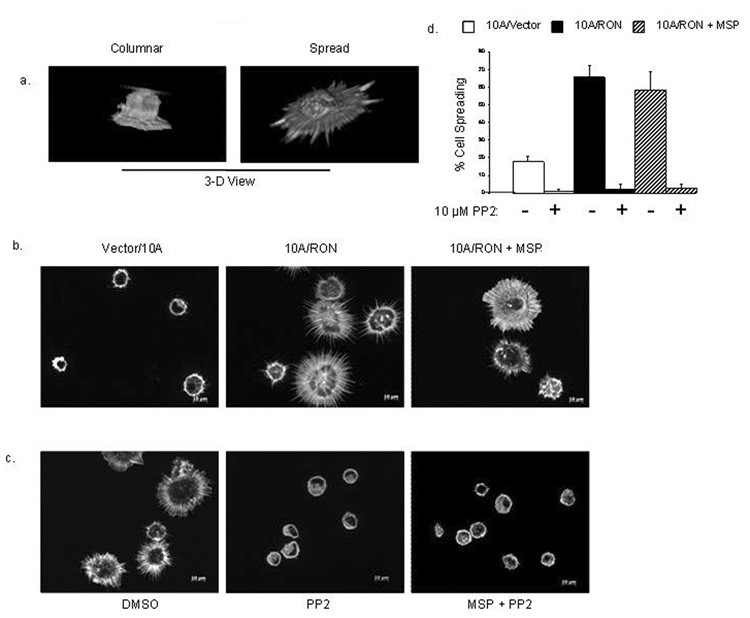
Ligand-independent activation of RTKs can occur as a result of interaction with other cell surface receptors or other intracellular kinases. We considered whether the intracellular Src kinase was required for phosphorylation of RON in 10A/RON cells for three reasons: wild-type Src is frequently upregulated in breast cancers (Ottenhoff-Kalff et al., 1992), it is known to enhance RTK signals in cancer (Tice et al., 1999) and Src was implicated in signaling by RON (Danilkovitch-Miagkova et al., 2000). When Src activity was blocked with the inhibitor PP2, we found that both MSP-dependent and MSP-independent phosphorylation of RON was reduced, although in the presence of MSP, substantially more tyrosine phosphorylation of RON remained after PP2 treatment (Figure 3a). These data suggest that MSP-stimulation of RON may promote the activation of additional Src-independent pathways compared to the pathways activated by MSP-independent signaling of RON. To extend these observations, we tested the effect of a dominant-negative mutant of Src, (in which the Src active site lysine was mutated to arginine), on the tyrosine phosphorylation of RON in HeLa cells. MSP-dependent and MSP-independent phosphorylation of RON were blocked when RON was co-transfected with the dominant-negative Src construct (Figure 3b). These data suggest that Src family kinases play a general role in the tyrosine phosphorylation of RON in epithelial cells.
Figure 3. Tyrosine phosphorylation of RON requires the activity of Src family kinases.
10A/Vector and 10A/RON cells were left untreated, treated with 10 µM PP2 or treated with PP2 and 200 ng/ml MSP. Cell lysates were analyzed by Western Blot with anti-phospho-tyrosine antibodies and anti-RON antibodies (b) HeLa cells were transfected with either RON or RON and DN-Src. Cells were treated or untreated with 200ng/ml MSP for 30 minutes. Lysates were analyzed by Western blot with anti-phospho-tyrosine antibodies, anti-RON antibodies and MAPK antibodies as a loading control.
MSP-dependent and MSP-independent activation of RON give rise to distinct biological consequences
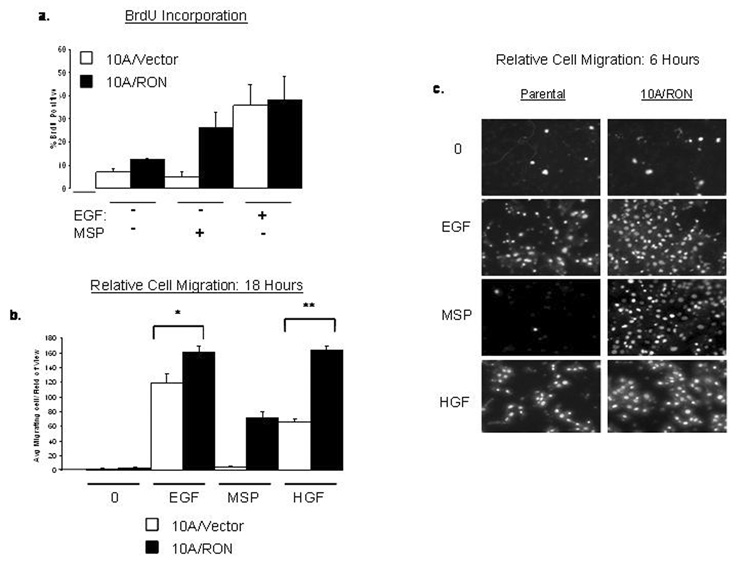
RON-mediated proliferation requires MSP
MCF-10A cells require the addition of EGF to proliferate, therefore, by omitting EGF from the growth media, we determined whether 10A/RON cells could proliferate both in the presence and absence of MSP. Approximately 12% of 10A/RON cells were BrdU positive when cultured without the addition of MSP or EGF compared to approximately 7% BrdU incorporation in 10A/Vector cells (Figure 4a). In contrast, the addition of MSP to the media of 10A/RON cells led to approximately 30% BrdU incorporation whereas the 10A/Vector cells did not proliferate in response to MSP (Figure 4a). With EGF, 35% of both 10A/Vector and 10A/RON cells were BrdU positive. These data indicate that MSP-independent activation of RON is not sufficient for cell proliferation however, due to the increased expression level of RON, MSP can act as a mitogen in 10A/RON cells. Furthermore, the MSP-independent activation of RON does not appear to promote additive proliferative effects with EGFR stimulation.
Figure 4. RON-mediated cell proliferation and migration require MSP.
(a)BrdU incorporation into cells that were cultured for 48 hours in regular MCF-10A media that lacked EGF and MSP or contained either EGF or MSP. The percent BrdU-positive cells were determined by counting at least 500 cells/coverslip. (b) Analysis of the number of migrating cells in the presence or absence of various growth factors. * indicates P≤0.02, n=5 ** indicates P≤0.0001. The error bars for (a) and (b) represent + SEM. (c)Representative images of the migration assay after 6 hours. Each experiment was repeated at least 3 times.
RON-mediated migration requires MSP
MSP-stimulation of RON can increase epithelial cell migration; however, it is not clear whether RON-mediated migration can occur in the absence of MSP. Using Transwell filter migration assays, we confirmed that MSP induced migration in 10A/RON cells, but in the absence of MSP, 10A/RON cells did not significantly migrate (Figure 4b, c). We conclude that MSP-independent RON activation is not sufficient to promote cell migration in the absence of basally-added growth factors.
Both 10A/Vector and 10A/RON cells migrated in response to EGF; however, at 6 hours after EGF addition, approximately 50% more 10A/RON cells had migrated than 10A/Vector cells (Figure 4c). A similar effect was seen 18 hours after adding EGF, when approximately 27% more of 10A/RON cells migrated through the filter than 10A/Vector cells (Figure 4b). To determine whether the increased migratory potential of 10A/RON cells was an EGF-specific phenomenon, we repeated the assay with the addition of the c-Met ligand, hepatocyte growth factor (HGF), since MCF-10A cells express endogenous levels of Met (Montesano et al., 1998). In this case, approximately 30% more 10A/RON cells migrated in response to HGF than 10A/Vector cells within 6 hours and approximately 60% more 10A/RON cells migrated when cells were examined after 18 hours of HGF treatment (Figure 4b, c). These data suggest that although the MSP-independent activation of RON is not sufficient to promote migration on its own, the expression of RON in MCF-10A cells confers an intrinsic migratory advantage of these cells compared to 10A/Vector cells in response to EGF or HGF.
RON-mediated cell spreading is MSP-independent
During the process of cell migration, cells must first adhere to the substratum and then spread before extending lamellipodia to propel them forward (Yamaguchi et al., 2005). We hypothesized that if 10A/RON cells had an ability to adhere and/or spread more efficiently, it would explain the MSP-independent migratory advantage over the 10A/Vector cells. To test this possibility, we allowed the 10A/Vector and 10A/RON cells to briefly attach to poly-d-lysine-coated coverslips. .After 10 minutes the unattached cells were washed away and the remaining cells were fixed and stained with rhodamine-conjugated phalloidin to visualize the actin filaments. Attached cells exhibited two phenotypes: they were either columnar in shape, which indicated a very early point in the cell attachment process, or the cells were flattened and spread, indicating a more advanced stage in the sequence of adhesion events (Figure 5a). When measured, the columnar cells were approximately 15–20 microns in diameter or less, whereas spreading cells ranged from 20–40 microns in diameter (data not shown). We quantified the difference between 10A/Vector and 10A/RON cells, and found that overall; approximately 60% of 10A/RON cells exhibited a flattened appearance within 10 minutes, compared with approximately 20% spreading of the 10A/Vector cells (Figure 5b, d). The addition of MSP to this assay did not increase the percentage of spreading 10A/RON cells. These data demonstrate that 10A/RON cells have acquired an expanded ability to spread on this substratum.
Figure 5. RON promotes cell spreading in the absence of MSP.
(a) Cells were stained with Rhodamine-Phalloidin in a 10 minute attachment assay to poly-d-lysine coated coverslips. The 2 basic phenotypes of attached cells are shown from 3-D rendering of image Z-stacks. (b) Representative 2-D images of comparing the phenotype of 10A/Vector, 10A/RON and 10A/RON cells that were pre-stimulated with MSP in the 10 minute attachment assay. (c) 10 minute attachment of 10A/RON cells that were incubated with MSP for 10 minutes, then PP2 or DMSO for 10 minutes and before attachment to the coverslip for 10 minutes. (d) Quantitation of the percentage of spreading cells. Error bars represent + SEM, n=4, P<0.001.
RON-mediated evasion of cell death is MSP-independent
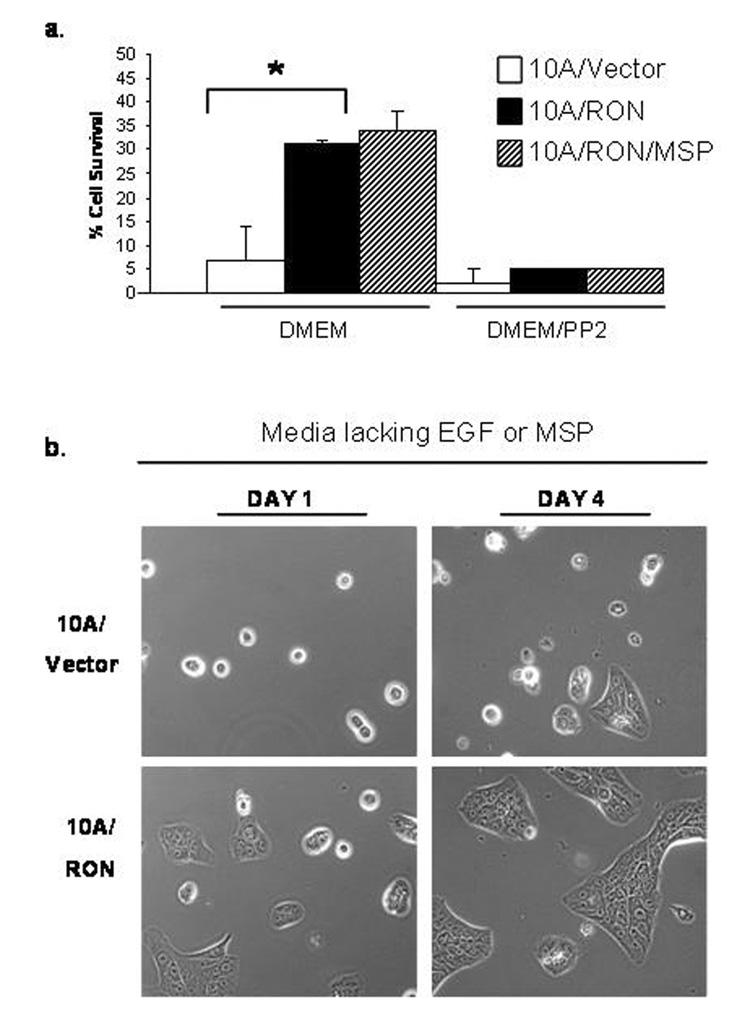
MSP stimulation of RON has been associated with anti-apoptotic activity in epithelial cells (Danilkovitch et al., 2000). To examine whether RON can protect cells from cell death in the absence of MSP, we used a cell death assay in which we incubated cells in DMEM without added growth factors or serum for 72 hours. Floating cells were collected from the media and pooled with the remaining adherent cells after they were removed from the plate by trypsinization. Dead cells were identified by Propidium Iodide labeling and the percentage of cell survival was assessed by FACS analysis. As seen in Figure 6a, only 7% of the 10A/Vector cells survived under these conditions in contrast to 31% of the 10A/RON cells. MSP minimally increased the survival rate to 34%. These data imply that MSP-independent activation of RON improves 10A/RON cell survival in the absence of growth factors, and that MSP does not significantly contribute to this effect. Figure 6b presents the phenotypic difference between 10A/Vector and 10A/RON cells when cultured without EGF. After 4 days, most of the 10A/Vector cells were rounded-up, floating in the media, and stained positive for Propidium Iodide whereas the 10A/RON cells remained strongly adherent and began to proliferate when growth factors were added to the media (data not shown). The MCF-10A cells undergoing cell death in our survival assay appear to be dying by apoptosis, as Poly (ADP-ribose) polymerase (PARP) is cleaved in these cells (Supplementary Figure 2b). It was difficult to collect enough 10A/Vector cells for analysis, which is why in it is not possible to distinguish the cleaved form of PARP in the 10A/Vector cells, but it is clear that PARP is cleaved in dying 10A/RON cells from this data.
Figure 6. MSP-independent activation of RON promotes cell survival.
(a)After 72 hours in serum-free media, floating and attached cells were pooled together, stained for Propidium Iodide (PI) and the percentage of PI-positive cells was determined by FACS. * indicates a P<0.002. Error bars represent + SEM, n=4. (b)Cells were cultured in regular MCF-10A media that lacked EGF. Phase contrast images were taken on Day 1 and Day 4 to demonstrate cell morphology
Effects of kinase inhibitors on RON-mediated biological effects
Because tyrosine phosphorylation of RON depends on the activity of Src family kinases (Figure 3), we examined whether Src signaling is required for RON-mediated biological effects. We found that PP2 nearly abolished the increased survival of 10A/RON cells under serum-starved conditions regardless of whether MSP was added (Figure 6a). PP2 treatment had no effect on 10A/Vector and 10A/RON cells cultured in regular growth media for 72 hours (Supplementary Figure 2a). RON-mediated cell spreading was also completely abrogated by PP2 in both the absence and presence of MSP (Figure 6c,d). Src activity was not necessary for cell proliferation in response to EGF or MSP, as PP2 treatment did not influence BrdU incorporation (data not shown). However, PP2 abolished cell migration in response to either MSP or EGF (data not shown), which could potentially be due to the effect of PP2 on cell attachment. We also determined the influence of PI3K/AKT (LY294002) and MEK/ERK (U0126) inhibitors on RON-mediated biological effects. In contrast to PP2 these inhibitors had no effects on cell spreading either with or without MSP. These two inhibitors did, however, completely abrogate the cell survival properties of RON and reduced MSP-induced Transwell migration by 60–80% (data not shown). These data imply the activity of Src family kinases are required for RON-mediated MSP-independent events, but it remains unclear if all MSP-mediated events also depend on Src.
Discussion
Our study describes a novel and significant role for MSP-independent RON signaling in cell spreading and evasion of cell death. We found that increased expression of RON in breast epithelial cells leads to both MSP-dependent and MSP-independent biological events. In the absence of MSP, RON promoted an increase in cell spreading, cell survival and enhanced growth-factor mediated migration in MCF-10A cells. MSP was required, however, to induce RON-mediated migration and proliferation, implying that RON possesses two distinct modes of signaling. Furthermore, we found that Src family kinases contribute to the activity of RON in both the presence and absence of MSP.
Ligand-independent activation of non-mutated RTKs is commonly due to increased expression that allows unregulated receptor dimerization and activation, promoting tumor development (Weiss et al., 1997). In epithelial cells that express high amounts of c-Met, cell attachment mediates ligand-independent tyrosine phosphorylation of c-Met (Wang et al., 2001; Wang et al., 1996). In our study, we did not find that MSP-independent tyrosine phosphorylation of RON was a direct result of cell attachment (data not shown). However, we found that 10A/RON cells exhibited a significant increase in cell attachment and spreading compared to 10A/Vector cells, suggesting that cell adhesion may generally regulate the scatter factor receptors.
We hypothesize that the increased adhesive and spreading property of 10A/RON cells explains their enhanced migratory response toward EGF and HGF, as well as their ability to avoid cell death under serum starved conditions. During migration, 10A/RON cells may attach to the filter more quickly than 10A/Vector cells, allowing for more efficient movement through the pores. Cell adhesion itself activates survival pathways via integrins or other cell surface receptors (Hofmann et al., 2007; Kang et al., 2007; Muller et al., 2008). The ability of cells to evade intrinsic cell death pathways is a major contributor to tumor development (Mehlen & Puisieux, 2006). Even at the lowest level of RON expression, at least 30% of 10A/RON cells survived serum-free conditions compared to almost no survival of the 10A/Vector cells. It is not known whether the adhesion effects are mediated by interactions of RON with receptors, such as integrins. MCF-10A cells have been reported to secrete laminin 5 ((Stahl et al., 1997) and our own unpublished results). RON is known to interact with β1 integrins (Danilkovitch-Miagkova et al., 2000; Santoro et al., 2003), one of the receptors for laminin 5, however we could not demonstrate that 10A/RON cells bound more strongly to laminin 5 than did 10A/Vector cells (data not shown).
Src mediates a diverse range of biological processes downstream of RTKs, such as cell survival (Yamamoto et al., 2006), proliferation (Riggins et al., 2006), adhesion and migration (Van Slambrouck et al., 2007). Furthermore, RON signals via Src downstream of integrins (Danilkovitch-Miagkova et al., 2000) and Src was responsible for RON-mediated constitutive activation of MAPK in the absence of MSP (Wei et al., 2005). Our data confirms that Src family kinases play a significant role in both MSP-dependent and MSP-independent RON signaling. We found that inhibition of Src family kinases reduced tyrosine phosphorylation of RON and abrogated RON-mediated biological events, with the exception of cell proliferation, implying that RON utilizes a Src-independent pathway to mediate proliferation in MCF-10A cells. In the case of EGFR, Src mediates EGF-independent transactivation, which promotes differential biological effects than EGF-stimulation of EGFR (Moro et al., 2002; Wu et al., 2002) This is similar to the observations reported here on the MSP-independent activation of RON.
In the presence of MSP, RON activates the MAPK and PI3K pathways ((Danilkovitch-Miagkova, 2003).and Figure 2). We found that MSP-independent signaling by RON also activates MAPK (Figure 2), however, the reduced level of stimulation was not sufficient for RON-mediated proliferation or migration. To examine the role of these pathways further we used the inhibitors LY294002 to block PI3K/AKT or U0126 to block MEK/ERK. These pathways were clearly not involved in cell-spreading but did play a role in MSP-dependent and independent cell survival and also in MSP-induced migration. In addition to MAPK and AKT, there is also the potential that MSP regulates additional or alternative signaling pathways to MSP-independent signaling.
MSP-stimulation of RON induces conformational changes (Yokoyama et al., 2005) that could potentially expose alternative residues to activate distinct pathways, or alter the signal intensity and duration. MSP-activation of RON increased phosphorylation of the docking site Y1360 as well as Y1238/1239 in the catalytic domain (Figure 1c), implying that some structural changes do occur in the presence of MSP. In the mouse RON receptor, docking site tyrosines of RON were required for MSP-independent activation of MAPK, but were dispensable for MSP-induced MAPK activation (Wei et al., 2005). A future comprehensive analysis of human RON tyrosine phosphorylation sites would appear merited.
RON activation in cancer is thought to be a late stage event, due to its roles in migration, invasion and development of EMT in tissue culture (Cote et al., 2007; Wang et al., 2004). We propose that in early stage breast cancers, co-expression of Src and RON is sufficient to initiate oncogenic behavior in the absence of MSP. In support of this, one recent study concluded that co-expression of RON, MSP and the MSP-activating enzyme (MT-SP1) was a strong prognostic marker for a poor patient survival; however, less than 20% of the samples actually exhibited expression of all three mRNAs (Welm et al., 2007). Nonetheless, clinical studies have shown that RON expression alone is predictive for a poor patient prognosis in breast cancers (Lee et al., 2005). Taken together, these data offer two major implications. First is that in the majority of RON-expressing breast cancers, RON contributes to oncogenicity in the absence of activated MSP. The second implication is that RON expression and its ligand-independent signaling in breast cancer are early events, and that the later stage disease is characterized by further activation of MSP and MT-SP1.
Nearly all cancer deaths stem from metastatic growths rather than the primary tumors (Mehlen & Puisieux, 2006), and therefore, it is critical to design cancer therapies that target events occurring in early stages of cancer to prevent secondary growths. Several therapeutic strategies have recently been developed to target RTKs in human cancers. These agents are either small molecule inhibitors that block RTK kinase activity or monoclonal antibodies that bind to the RTK and inhibit its signaling (Tagliaferri et al., 2005). Both of these molecular approaches have proven to be clinically successful in many cases, however, strategies targeting the extracellular ligand-binding domain of RTKs may not be successful in treating patients whose tumors exhibit signaling in the absence of ligand. We provide evidence that in the case of RON-expressing mammary tumors, targeting Src in addition to RON could potentially reduce oncogenic behaviors.
Materials and Methods
Materials and antibodies
MSP and the anti-MSP Receptor antibody were from R&D Systems (Minneapolis, MN, USA). EGF was from Peprotech (Rocky Hill, NJ, USA). HGF was a gift from Amgen (Thousand Oaks, CA, USA). Poly-d-Lysine (P6407), Propidium Iodide (P4864) and anti-BrdU antibody (Clone BU-33) were from Sigma-Aldrich (St. Louis, MO, USA). AlexFluor 546 (A11030), rhodamine-phalloidin (R415) and Hoechst 33342 were from Molecular Probes/Invitrogen (Eugene, OR, USA). Antibodies for Western Blots were: 4G10 and anti-MAPK from Upstate Biotechnology, Inc (Lake Placid, NY, USA), anti-phospho-MAPK and anti-FAK from BD Transduction Laboratories (San Diego, CA, USA), anti-RON (sc-22) and all anti-phospho-RON rabbit polyclonal antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and anti-phospho-AKT was from Cell Signaling (Danvers, MA, USA), PP2 was from BioMol (Plymouth Meeting, PA, USA).
Cell culture
Cell culture materials were purchased from Gibco/Invitrogen (Carlsbad, CA, USA). NIH-3T3 and HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and antibiotics. 10A/Vector and 10A/RON cells were cultured as described (Debnath et al., 2003). pUSE-DN-Src, was provided by Dr. WT Miller (SUNY StonyBrook). HeLa cells were transfected with Fugene (Roche Applied Science, Indianapolis, IN, USA). The K1114M Ron mutant was generated using a GeneEditor mutagenesis kit (Promega, Madison, WI, USA) with the previously reported oligonucleotide (Danilkovitch-Miagkova et al., 2000).
Generation of MCF-10A/RON cell lines
Retroviruses expressing wild-type human RON were generated by transfection of Phoenix E cells with REBNA-RON-IRES-GFP retroviral vector (Petrenko et al., 1999). Virus-containing supernatant from the Phoenix E cells was added to MCF-10A/ecoR cells for 6 hours. See the Nolan Laboratory website for further details on generating retroviruses with the Phoenix E cell system (http://www.stanford.edu/group/nolan/index.html).
Proliferation Assays
Cells were cultured on coverslips for 48 hours in specified media, pulsed with 5-bromo-2′-deoxyuridine (BrdU) for 2 hour, washed with phosphate-buffered saline, fixed with methanol, and treated with 4N HCl for 30 minutes. BrdU was labeled with an anti-BrdU antibody and detected using AlexaFluor 546 secondary antibody. The percent of BrdU-positive cells was determined by counting at least 500 cells per coverslip.
Transwell migration assay
Cells were starved overnight in DMEM/F-12 media containing 1% horse serum. 100,000 cells were added to the top well of a 24-well, 0.8 micron pore Transwell filter insert (Costar, Corning, NY, USA). Media added to the bottom well contained no growth factors, 10ng/ml EGF, 100 ng/ml MSP or 2 ng/ml HGF. After 6 or 18 hours, cells that remained in the top chamber were removed with a cotton swab, the migrated cells were fixed, and DNA was labeled with Hoechst. The number of migrating cells per field of view was counted on a fluorescent microscope under a 20X magnification.
Immunoblotting
All immunoprecipitations and immunoblots were performed as described (Yokayama et al 2005) using a 9% polyacrylamide gel.
Apoptosis assay
Cells were stained with Propidium Iodide and the percentage of PI-positive cells was determined using a BD FACSCalibur™ system (BD, Franklin Lakes, NJ, USA). PP2 was added to the cultures after the cells were allowed to settle on the dish overnight.
Adhesion and attachment assays
Cells were incubated on poly-d-lysine coated coverslips in DMEM for 10 minutes. Unbound cells were washed away with PBS. Attached cells were fixed with 3.7% paraformaldehyde for 15 minutes at 4°C, incubated with 0.1% Triton-X 100 for 2 minutes and rhodamine-conjugated phalloidin was added as per commercial protocol. Cells were visualized with an Axiovert 200M (Zeiss, Thornwood, NY, USA) using a 63X oil DIC lens and the images were analyzed using the Axiovision software (Zeiss).
Reverse-Transcriptase PCR
Cellular RNA was isolated with RNeasy kit (Qiagen, Valencia, CA, USA), and reverse transcribed to cDNA using the Superscript II reverse transcriptase system (Invitrogen). MSP was detected using primers previously described (Zalcenstein et al., 2006).
Supplementary Material
(a)Lysates of NIH-3T3 cells stably expressing wild-type RON were analyzed by an anti-phospho-tyrosine Western blot, with an without stimulation by MSP. The bottom panel shows total RON expression, including the 170 kDa unprocessed form, the 150 kDa processed/phosphorylated form and the 145 kDa processed/unphosphorylated form of RON. . (b)HeLa cells were transiently transfected with an empty vector (Mock), wild-type RON or K1114M kinase dead RON mutant. Lysates were analyzed by Western blot with 4G10 phospho-tyrosine antibodies, anti-RON antibody and MAPK antibody as a loading control.
(a) Cells were cultured for 72 hours in regular media with and without the addition of PP2, trypsinized, stained for PI and the % of PI-positive cells was determined by FACS analysis. (b) After 72 hours in DMEM, floating and attached cells were collected and lysed. Shown is a Western Blot analysis of PARP using whole cell lysates.
Acknowledgements
The authors would like to thank Roger Daly and Joan S. Brugge for providing the MCF-10A/ecoR cell line, James Keller for RT-PCR assistance, Dr. W. Todd Miller for the DN-Src construct, Kyeisha Hodge for generating the K114M mutant and Dr. Edward Chan for various reagents used throughout this study. We would also like to thank members of our laboratory plus Dr Mina Bissell for helpful discussions. The studies were funded by NCI grant CA28146, the Walk for Beauty Foundation and Long Island League to Abolish Cancer (LILAC) to MJH. KJF was supported by NIH T32-CA009176.
Abbreviations
- EGF
Epidermal Growth Factor
- EGFR
Epidermal Growth Factor Receptor
- HGF
Hepatocyte Growth Factor
- MAPK
Mitogen-Activated Protein Kinase
- MSP
Macrophage Stimulating Protein
References
- Bezerra JA, Carrick TL, Degen JL, Witte D, Degen SJ. Biological effects of targeted inactivation of hepatocyte growth factor-like protein in mice. J Clin Invest. 1998;101:1175–1183. doi: 10.1172/JCI1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Zhou YQ, Angeloni D, Kurtz AL, Qiang XZ, Wang MH. Overexpression and activation of the RON receptor tyrosine kinase in a panel of human colorectal carcinoma cell lines. Exp Cell Res. 2000;261:229–238. doi: 10.1006/excr.2000.5012. [DOI] [PubMed] [Google Scholar]
- Cote M, Miller AD, Liu SL. Human RON receptor tyrosine kinase induces complete epithelial-to-mesenchymal transition but causes cellular senescence. Biochem Biophys Res Commun. 2007;360:219–225. doi: 10.1016/j.bbrc.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilkovitch-Miagkova A. Oncogenic signaling pathways activated by RON receptor tyrosine kinase. Curr Cancer Drug Targets. 2003;3:31–40. doi: 10.2174/1568009033333745. [DOI] [PubMed] [Google Scholar]
- Danilkovitch-Miagkova A, Angeloni D, Skeel A, Donley S, Lerman M, Leonard EJ. Integrin-mediated RON growth factor receptor phosphorylation requires tyrosine kinase activity of both the receptor and c-Src. J Biol Chem. 2000;275:14783–14786. doi: 10.1074/jbc.C000028200. [DOI] [PubMed] [Google Scholar]
- Danilkovitch A, Donley S, Skeel A, Leonard EJ. Two independent signaling pathways mediate the antiapoptotic action of macrophage-stimulating protein on epithelial cells. Mol Cell Biol. 2000;20:2218–2227. doi: 10.1128/mcb.20.6.2218-2227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Emaduddin M, Bicknell DC, Bodmer WF, Feller SM. Cell growth, global phosphotyrosine elevation, and c-Met phosphorylation through Src family kinases in colorectal cancer cells. Proc Natl Acad Sci U S A. 2008;105:2358–2362. doi: 10.1073/pnas.0712176105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann C, Obermeier F, Artinger M, Hausmann M, Falk W, Schoelmerich J, et al. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology. 2007;132:587–600. doi: 10.1053/j.gastro.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Hsu PY, Liu HS, Cheng HL, Tzai TS, Guo HR, Ho CL, et al. Collaboration of RON and epidermal growth factor receptor in human bladder carcinogenesis. J Urol. 2006;176:2262–2267. doi: 10.1016/j.juro.2006.07.048. [DOI] [PubMed] [Google Scholar]
- Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Ishizawar RC, Tice DA, Karaoli T, Parsons SJ. The C terminus of c-Src inhibits breast tumor cell growth by a kinase-independent mechanism. J Biol Chem. 2004;279:23773–23781. doi: 10.1074/jbc.M312368200. [DOI] [PubMed] [Google Scholar]
- Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, et al. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res. 2007;67:3094–3105. doi: 10.1158/0008-5472.CAN-06-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Chen HH, Chow NH, Su WC, Lin PW, Guo HR. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin Cancer Res. 2005;11:2222–2228. doi: 10.1158/1078-0432.CCR-04-1761. [DOI] [PubMed] [Google Scholar]
- Maggiora P, Marchio S, Stella MC, Giai M, Belfiore A, De Bortoli M, et al. Overexpression of the RON gene in human breast carcinoma. Oncogene. 1998;16(22):2927–2933. doi: 10.1038/sj.onc.1201812. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Montesano R, Soriano JV, Malinda KM, Ponce ML, Bafico A, Kleinman HK, et al. Differential effects of hepatocyte growth factor isoforms on epithelial and endothelial tubulogenesis. Cell Growth Differ. 1998;9:355–365. [PubMed] [Google Scholar]
- Moro L, Dolce L, Cabodi S, Bergatto E, Boeri Erba E, Smeriglio M, et al. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J Biol Chem. 2002;277:9405–9414. doi: 10.1074/jbc.M109101200. [DOI] [PubMed] [Google Scholar]
- Muller EJ, Williamson L, Kolly C, Suter MM. Outside-in signaling through integrins and cadherins: a central mechanism to control epidermal growth and differentiation? J Invest Dermatol. 2008;128:501–516. doi: 10.1038/sj.jid.5701248. [DOI] [PubMed] [Google Scholar]
- Muraoka RS, Waltz SE, Degen SJ. Expression of hepatocyte growth factor-like protein is repressed by retinoic acid and enhanced by cyclic adenosine 3′,5′-monophosphate response element-binding protein (CREB)-binding protein (CBP) Endocrinology. 1999;140:187–196. doi: 10.1210/endo.140.1.6441. [DOI] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole JM, Rabenau KE, Burns K, Lu D, Mangalampalli V, Balderes P, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res. 2006;66:9162–9170. doi: 10.1158/0008-5472.CAN-06-0283. [DOI] [PubMed] [Google Scholar]
- Ottenhoff-Kalff AE, Rijksen G, van Beurden EA, Hennipman A, Michels AA, Staal GE. Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product. Cancer Res. 1992;52:4773–4778. [PubMed] [Google Scholar]
- Petrenko O, Beavis A, Klaine M, Kittappa R, Godin I, Lemischka IR. The molecular characterization of the fetal stem cell marker AA4. Immunity. 1999;10:691–700. doi: 10.1016/s1074-7613(00)80068-0. [DOI] [PubMed] [Google Scholar]
- Riggins RB, Thomas KS, Ta HQ, Wen J, Davis RJ, Schuh NR, et al. Physical and functional interactions between Cas and c-Src induce tamoxifen resistance of breast cancer cells through pathways involving epidermal growth factor receptor and signal transducer and activator of transcription 5b. Cancer Res. 2006;66:7007–7015. doi: 10.1158/0008-5472.CAN-05-3952. [DOI] [PubMed] [Google Scholar]
- Ronsin C, Muscatelli F, Mattei MG, Breathnach R. A novel putative receptor protein tyrosine kinase of the met family. Oncogene. 1993;8:1195–1202. [PubMed] [Google Scholar]
- Santoro MM, Gaudino G, Marchisio PC. The MSP receptor regulates alpha6beta4 and alpha3beta1 integrins via 14-3-3 proteins in keratinocyte migration. Dev Cell. 2003;5:257–271. doi: 10.1016/s1534-5807(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Stahl S, Weitzman S, Jones JC. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J Cell Sci. 1997;110(Pt 1):55–63. doi: 10.1242/jcs.110.1.55. [DOI] [PubMed] [Google Scholar]
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- Tagliaferri P, Tassone P, Blotta S, Viscomi C, Grillone F, Budillon A, et al. Antitumor therapeutic strategies based on the targeting of epidermal growth factor-induced survival pathways. Curr Drug Targets. 2005;6:289–300. doi: 10.2174/1389450053765897. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Toney K, Fenoglio-Preiser C, Revelo-Penafiel MP, Hingorani SR, Tuveson DA, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res. 2007;67:6075–6082. doi: 10.1158/0008-5472.CAN-06-4128. [DOI] [PubMed] [Google Scholar]
- Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Slambrouck S, Grijelmo C, De Wever O, Bruyneel E, Emami S, Gespach C, et al. Activation of the FAK-src molecular scaffolds and p130Cas-JNK signaling cascades by alpha1-integrins during colon cancer cell invasion. Int J Oncol. 2007;31:1501–1508. [PubMed] [Google Scholar]
- Wang D, Shen Q, Chen YQ, Wang MH. Collaborative activities of macrophage-stimulating protein and transforming growth factor-beta1 in induction of epithelial to mesenchymal transition: roles of the RON receptor tyrosine kinase. Oncogene. 2004;23:1668–1680. doi: 10.1038/sj.onc.1207282. [DOI] [PubMed] [Google Scholar]
- Wang MH, Wang D, Chen YQ. Oncogenic and invasive potentials of human macrophage-stimulating protein receptor, the RON receptor tyrosine kinase. Carcinogenesis. 2003;24:1291–1300. doi: 10.1093/carcin/bgg089. [DOI] [PubMed] [Google Scholar]
- Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Kobayashi R, Bishop JM. Cellular adherence elicits ligand-independent activation of the Met cell-surface receptor. Proc Natl Acad Sci U S A. 1996;93:8425–8430. doi: 10.1073/pnas.93.16.8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Ni S, Correll PH. Uncoupling ligand-dependent and -independent mechanisms for mitogen-activated protein kinase activation by the murine Ron receptor tyrosine kinase. J Biol Chem. 2005;280:35098–35107. doi: 10.1074/jbc.M505737200. [DOI] [PubMed] [Google Scholar]
- Weiss FU, Daub H, Ullrich A. Novel mechanisms of RTK signal generation. Curr Opin Genet Dev. 1997;7:80–86. doi: 10.1016/s0959-437x(97)80113-x. [DOI] [PubMed] [Google Scholar]
- Welm AL, Sneddon JB, Taylor C, Nuyten DS, van de Vijver MJ, Hasegawa BH, et al. The macrophage-stimulating protein pathway promotes metastasis in a mouse model for breast cancer and predicts poor prognosis in humans. Proc Natl Acad Sci U S A. 2007;104:7570–7575. doi: 10.1073/pnas.0702095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LK, Luttrell DK, Parsons JT, Parsons SJ. pp60c-src tyrosine kinase, myristylation, and modulatory domains are required for enhanced mitogenic responsiveness to epidermal growth factor seen in cells overexpressing c-src. Mol Cell Biol. 1989;9:1536–1544. doi: 10.1128/mcb.9.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Graves LM, Gill GN, Parsons SJ, Samet JM. Src-dependent phosphorylation of the epidermal growth factor receptor on tyrosine 845 is required for zinc-induced Ras activation. J Biol Chem. 2002;277:24252–24257. doi: 10.1074/jbc.M200437200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Mammadova G, Song RX, Fukami Y, Sato K. Tyrosine phosphorylation of p145met mediated by EGFR and Src is required for serum-independent survival of human bladder carcinoma cells. J Cell Sci. 2006;119:4623–4633. doi: 10.1242/jcs.03236. [DOI] [PubMed] [Google Scholar]
- Yokoyama N, Ischenko I, Hayman MJ, Miller WT. The C terminus of RON tyrosine kinase plays an autoinhibitory role. J Biol Chem. 2005;280:8893–8900. doi: 10.1074/jbc.M412623200. [DOI] [PubMed] [Google Scholar]
- Zalcenstein A, Weisz L, Stambolsky P, Bar J, Rotter V, Oren M. Repression of the MSP/MST-1 gene contributes to the antiapoptotic gain of function of mutant p53. Oncogene. 2006;25:359–369. doi: 10.1038/sj.onc.1209061. [DOI] [PubMed] [Google Scholar]
- Zinser GM, Leonis MA, Toney K, Pathrose P, Thobe M, Kader SA, et al. Mammary-specific Ron receptor overexpression induces highly metastatic mammary tumors associated with beta-catenin activation. Cancer Res. 2006;66:11967–11974. doi: 10.1158/0008-5472.CAN-06-2473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a)Lysates of NIH-3T3 cells stably expressing wild-type RON were analyzed by an anti-phospho-tyrosine Western blot, with an without stimulation by MSP. The bottom panel shows total RON expression, including the 170 kDa unprocessed form, the 150 kDa processed/phosphorylated form and the 145 kDa processed/unphosphorylated form of RON. . (b)HeLa cells were transiently transfected with an empty vector (Mock), wild-type RON or K1114M kinase dead RON mutant. Lysates were analyzed by Western blot with 4G10 phospho-tyrosine antibodies, anti-RON antibody and MAPK antibody as a loading control.
(a) Cells were cultured for 72 hours in regular media with and without the addition of PP2, trypsinized, stained for PI and the % of PI-positive cells was determined by FACS analysis. (b) After 72 hours in DMEM, floating and attached cells were collected and lysed. Shown is a Western Blot analysis of PARP using whole cell lysates.