SUMMARY
The pervasive influence of secreted Wnt signaling proteins in tissue homeostasis and tumorigenesis has galvanized efforts to identify small molecules that target Wnt-mediated cellular responses. By screening a diverse synthetic chemical library, we have discovered two novel classes of small molecules that disrupt Wnt pathway responses - whereas one class inhibits the activity of Porcupine (Porcn), a membrane-bound acyltransferase that is essential to the production of Wnt proteins, the other abrogates destruction of Axin proteins, suppressors of Wnt/β-catenin pathway activity. With these small molecules we establish a chemical genetic approach for studying Wnt pathway responses and stem cell function in adult tissue. We achieve transient, reversible suppression of Wnt/β-catenin pathway response in vivo, and establish a mechanism-based approach to target cancerous cell growth. The signal transduction mechanisms shown here to be chemically tractable additionally contribute to Wnt-independent signal transduction pathways and thus could be broadly exploited for chemical genetics and therapeutic goals.
INTRODUCTION
The Wnt family of signaling proteins influences most aspects of metazoan embryonic development and post-embryonic tissue homeostasis1. Cellular responses to these proteins are often categorized based on their utilization of β-catenin, a co-activator of the TCF/LEF family of transcriptional effectors. Similar to other signal transduction pathways required for cell fate decision-making, activity of the Wnt/β-catenin (“canonical”) pathway maintains transcriptional programs that enable stem cells to remain multi-potent2,3. Inability to sustain these transcription programs results in compromised ability of stem cells to self-renew2,4-6.
Pathological states that may arise from altered stem cell function, such as degenerative diseases and cancer, are frequently associated with changes in Wnt/β-catenin pathway activity. Indeed, hyperactivation of the Wnt/β-catenin pathway is thought to induce premature senescence of stem cells and age-related loss of stem cell function7,8. In cancer, hyperactivation of the Wnt/β-catenin pathway, often in conjunction with mutations in other cell growth regulatory genes, can lead to aberrant cell growth9. Notably, 90% of colorectal cancers harbor loss-of-function mutations in the adenomatosis polyposis coli (APC) gene, a major suppressor of the Wnt/β-catenin pathway10,11. Less frequently, loss of extracellular inhibitors that normally suppress Wnt protein function may give rise to Wnt ligand-dependent tumors12.
Much of our understanding of Wnt/β-catenin pathway response in adult animal tissues has been limited by the inability to deploy classical genetic approaches without additionally impacting normal embryonic development. Thus, despite a general agreement that targeting the Wnt signal transduction pathway would be potentially useful in a broad range of diseases, the consequences of inhibiting Wnt function in adult tissue homeostasis are unknown. Identification of small molecule inhibitors of the Wnt/β-catenin pathway would enable a chemical genetics-based interrogation of pathway function that bypasses the limitations of typical genetic approaches, and facilitate development of therapeutically useful reagents. So far, efforts to achieve chemical control of the Wnt/β-catenin pathway have been hindered by a lack of knowledge regarding underlying signal transduction mechanisms that are amenable to chemical manipulation. This deficiency is underscored by the fact that there are currently no chemicals targeting this pathway in clinical testing.
In order to identify lead chemical structures that will facilitate the study of Wnt/β-catenin responses in adult tissue homeostasis and disease, we screened a diverse synthetic chemical library and identified two general classes of compounds that target discrete regulatory steps within the pathway. Using these chemicals, we demonstrate the ability to transiently and reversibly suppress regenerative processes in adult tissue and to abolish aberrant Wnt/β-catenin responses in cancerous cells in vitro. Our findings should facilitate the use of chemical genetic approaches to study Wnt protein function in adult animals and the development of therapeutic approaches premised upon attack of Wnt-mediated cellular responses.
RESULTS
Small molecule modulators of the Wnt/β-catenin pathway
We used a high stringency cell-based screening strategy to identify small molecule antagonists of the Wnt/β-catenin pathway from a ~200K compound synthetic chemical library (Supp. Fig. 1). Briefly, mouse L-cells stably harboring a well-characterized Wnt/β-catenin pathway-responsive firefly luciferase (FL) reporter plasmid (SuperTopFlash or STF), a control reporter, and an expression construct encoding the Wnt protein, Wnt3A, were exposed to individual compounds for one day prior to measurement of reporter activities. We selected for further testing chemicals that altered FL but not control reporter activity.
Several secondary tests were employed further select compounds of interest. These tests were designed to identify particularly potent compounds with minimal cellular cytotoxicity (“dose-dependent test”) and specificity for attacking the Wnt/β-catenin pathway (“Hh and Notch pathway tests” and “FL inhibitor/protein exocytosis test”; Supp. Fig. 1). In order to discriminate between compounds that either disrupt ligand production or response, we further tested the ability of our strongest candidates to block transcriptional response in cells treated with exogenously supplied Wnt protein (Supp. Fig. 1, 2a). Among those compounds that failed to block Wnt/β-catenin pathway response in the “exogenous Wnt” test, four inhibited Wnt secretion as determined using a Wntluciferase fusion protein (Supp. 2b). Thus, we identified five and four compounds that act as inhibitors of Wnt response (IWR compounds, 1-5) and inhibitors of Wnt production (IWP compounds, 6-9), respectively (Supp. Fig. 1). These compounds do not appear to be specific for Wnt3A activity, as they were also capable of inhibiting cellular response to Wnt1 and Wnt2 (Supp. Fig. 2c).
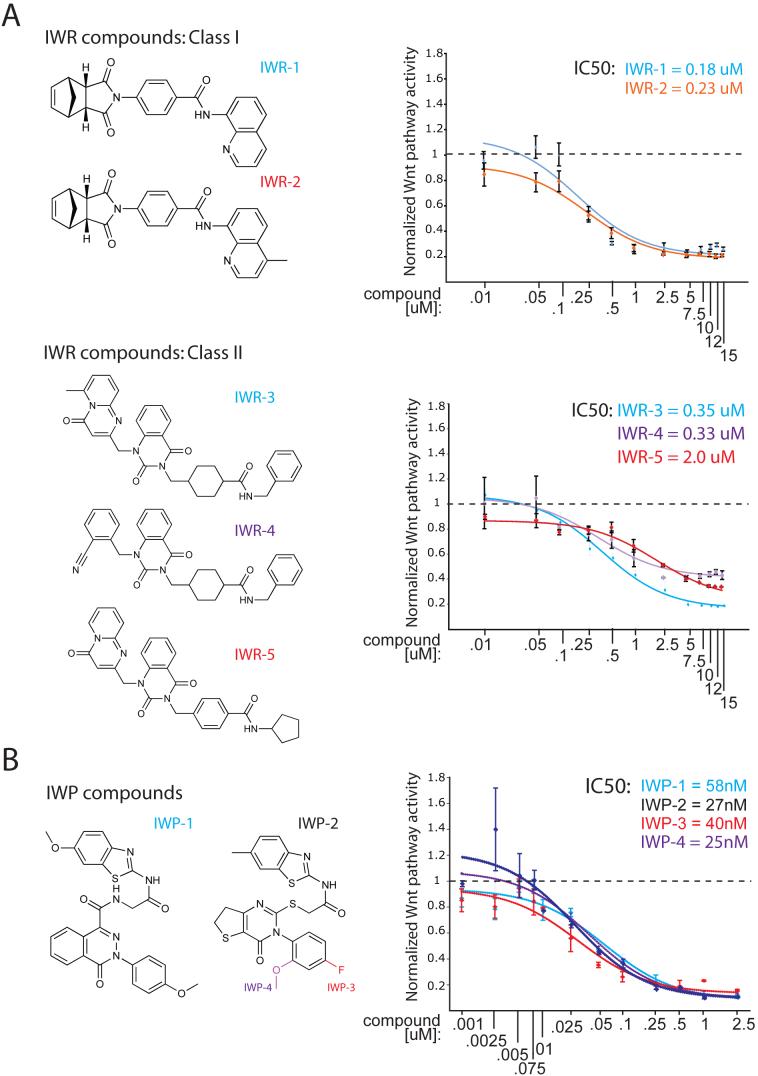
Whereas the IWP compounds all share the same core chemical structure, the IWRs could be grouped into two classes based on structural similarities (Fig. 1a,b; Supp. Fig. 3). In cultured cells, the IWP compounds are more potent pathway antagonists than those in the strongest class of IWRs (~40nM versus ~200nM, respectively). Using biochemical markers of Wnt/β-catenin pathway activation, we localized the site of action for each compound (Fig. 2). Consistent with their predicted effects on Wnt protein production, IWP compounds blocked all Wnt-dependent biochemical changes that were assayed (phosphorylation of the Lrp6 receptor and Dvl2, and β-catenin accumulation). On the other hand, IWR compounds appear to only affect β-catenin levels, suggesting they target regulatory events downstream of Lrp6 and Dvl2.
Figure 1. Chemical structure and potency of IWR and IWP compounds.
(a) Structure and potency of IWR compounds. Two IWR compounds that differ by only a single methyl group and that share similar IC50's (as determined in L-Wnt-STF cells; upper right of graphs) were designated Class | compounds. The remaining three IWRs which share structural similarity (see also Supp. Fig. 3) were designated Class || compounds. (b) Structure and potency of IWP compounds. All IWP compounds share structural similarity and IC50's with IWP compounds 2-4 sharing the same core structure (IWP-2) and differing only by either the presence of an additional fluoro or methoxy adduct (IWP-3 and IWP-4, respectively).
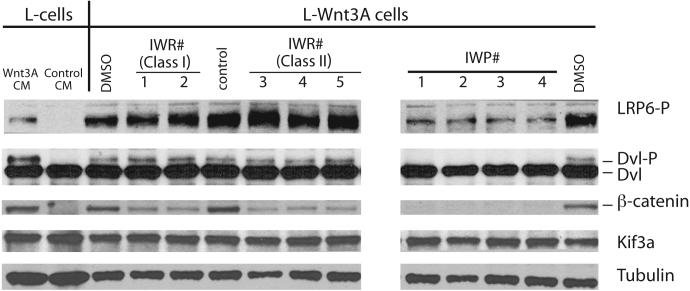
Figure 2. Biochemical evidence for Wnt/β-catenin pathway inhibition by IWR and IWP compounds.
L-Wnt-STF cells that exhibit constitutive Wnt pathway activation were incubated with IWR (10μM) and IWP (5μM) compounds for 24 hrs prior to lysis. Cellular lysates were subjected to Western blot analysis to determine levels of Lrp6 and Dvl2 phosphorylation, and β-catenin accumulation, all biochemical events associated with Wnt/β-catenin pathway activity. Predictably, IWPs blocked all three biochemical events whereas IWR compounds appear to block β-catenin accumulation without affecting Lrp6 and Dvl2 phosphorylation. Kif3A and tubulin serve as loading controls. Wild-type L-cells stimulated with exogenous Wnt3A protein provided in conditioned medium exhibit similar biochemical changes in Wnt pathway components as that observed in the L-Wnt-STF cells.
IWP compounds target the acyltransferase Porcupine
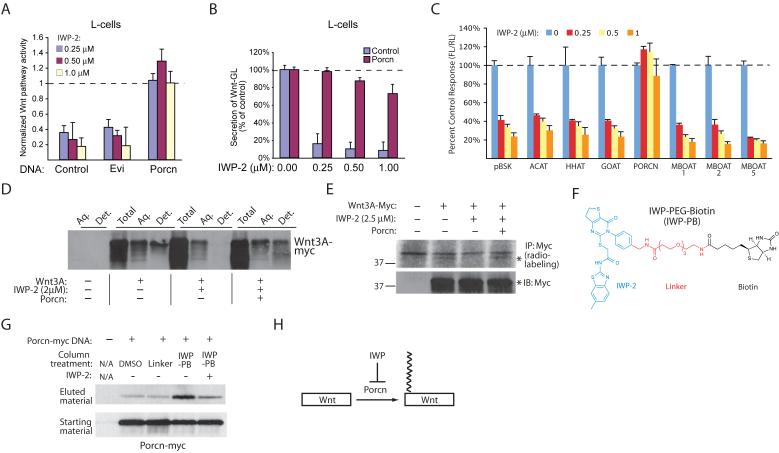
We tested the ability of two genes known to be essential for the production of Wnt ligands, Evenness interrupted (Evi; Wls) and Porcupine (Porcn), to rescue pathway response in cells treated with de-novo synthesized IWP-1 or -2 (Supp. Fig. 4). Expression of Porcn but not Evi alleviated the effects of IWP-2 on pathway activity (Fig. 3a) and Wnt secretion (Fig. 3b), suggesting that in general IWP compounds may act on Porcn. Porcn, a member of the membrane-bound O-acyltransferase (MBOAT) family, adds a palmitoyl group to Wnt proteins that is essential to their signaling ability, and is required for Wnt secretion13. Furthermore, expression of several other MBOAT family members was unable to abrogate the effects of IWP on Wnt pathway response consistent with the specific role of Porcn in Wnt protein maturation (Fig. 3c). Consistent with an attack on Porcn function by IWP compounds, the levels of lipidated Wnt3A as measured using either a detergent solubility fractionation (Fig. 3d) assay or an 3H-palmitate radiolabeling assay (Fig. 3e), are decreased in IWP-2-treated cells but are recovered in those cells over-expressing Porcn. In addition, IWP was able to decrease palmitoylation of Wnt5, a presumed “non-canonical” Wnt14 (Supp. Fig. 5a). In agreement with our previous observations that these compounds have specific effects on the Wnt-mediated cellular responses, IWP compounds failed to abrogate Notch mediated signaling or block general protein secretion (see Supp. Fig. 1), palmitoylation of Sonic hedgehog (Shh), a known substrate of the MBOAT family member Hedgehog acyltransferase (Hhat)15 (Supp. Fig. 5b), or maturation of LRP6, which is dependent upon an intracellular palmitoylation event16 (Supp. Fig. 5c).
Figure 3. IWP compounds target the O-acyltransferase Porcn.
(a) Overexpression Porcn but not the Wnt chaperone Evi counters the effects of IWP compounds on Wnt/β-catenin pathway activity as measured using the STF reporter. (b) Overexpression of Porcn reverses the block in Wnt protein secretion induced by IWP compounds as measured using a Wnt3A-Gaussia luciferase fusion protein (see Fig. 1 and Supp. Fig. 2b). (c) Expression of other MBOAT family members does not abrogate the effects of IWP on Wnt/β-catenin pathway response as measured using the STF reporter. (d) IWP compounds inhibit formation of detergent-soluble Wnt3A in a Porcn-dependent manner. Lipidated Wnt3A protein, found in the detergent fraction of TritonX-114 treated cells, is absent in IWP-treated cells but present in Porcn overexpressing cells. (e) IWP compounds inhibit palmitoylation of Wnt3A. Cells expressing Wnt3A with or without Porcn were treated with H3-palmitate. Palmitoylation of Wnt3A was then determined using autoradiography of immunoprecipitated Wnt3A. (f) Structure of a biotinylated IWP-2 derivative. A linker and biotin group (PEG-biotin; PB) were attached to IWP-2 at the para position in the phenyl group (IWP-PB; see Supp. Fig. 4b for synthetic scheme). (g) Porcn associates with IWP-2. IWP-PB or the control PB molecule (linker) bound to streptavidin-coated sepharose beads were incubated with cellular lysate containing Porcn-myc protein in the presence or absence of soluble IWP-2. IWP-PB-bound Porcn-myc binding was measured using Western blot analysis. (h) IWP compounds inhibit Porcn function thereby blocking palmitoylation of Wnt proteins. For (a-c), data represent mean values ± s.d.
In order to test if Porcn interacts with the IWP compounds, we generated a biochemical reagent that would allow us to isolate IWP-associated proteins [IWP-PEG-Biotin (IWP-PB, 10); Fig. 3f; synthetic scheme shown in Supp. Fig. 6a]. We observed competitive binding of Porcn to IWP-PB with either IWP-2 (Fig. 3g, Supp. Fig. 6b) or IWP-1 (Supp. Fig. 6c), indicating that all IWP compounds share a similar mechanism of action. In addition, IWP-2 increased levels of overexpressed Porcn (Supp. Fig. 7a) without altering its localization to the ER17 (Supp. Fig. 7b), suggesting that IWP compounds target Porcn without inducing Porcn destruction or mislocalization. Using several related compounds (IWP-2-v1, -v2, and -v3; Supp. Fig. 7c), we identified the benzothiazole group as a critical determinant for IWP-mediated inhibition of Porcn function. Considering our biochemical data and that overexpression of Porcn alone is able to abrogate the effects of the IWP compounds, the simplest model for IWP action is that it inactivates Porcn function, either by directly inhibiting the Porcn active site or a Porcn regulator (Fig. 3h).
IWR compounds abrogate Axin protein turnover
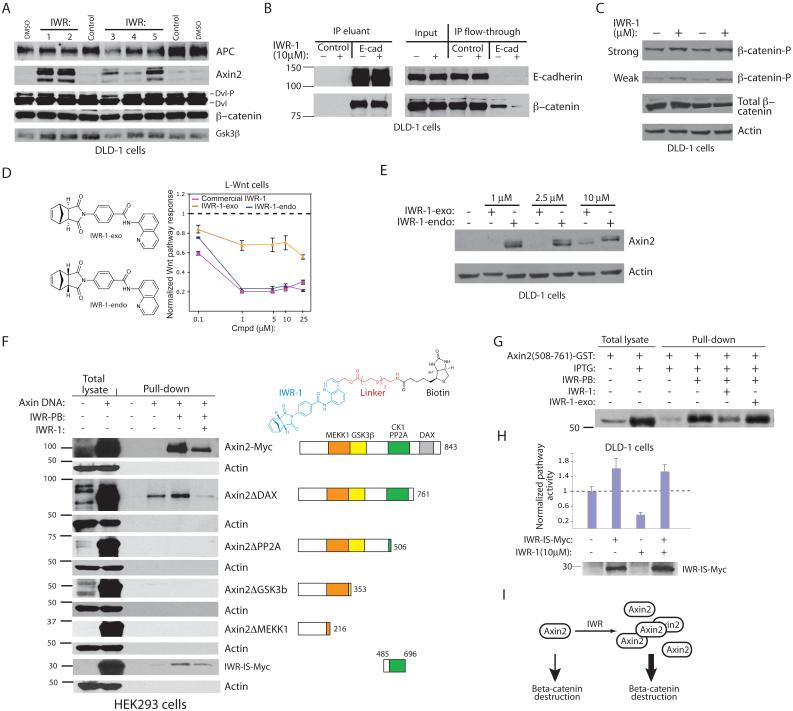
Based on our biochemical evidence, IWR compounds likely inhibit Wnt-induced accumulation of β-catenin by targeting a pathway component that functions downstream of Lrp and Dvl proteins (see Fig. 2). The β-catenin destruction complex, which consists of Apc, Axin, Ck1, and Gsk3β, promotes proteasome-mediated proteolysis of phosphorylated β-catenin18. We examined the biochemical effects of IWR compounds on components of this destruction complex in the DLD-1 colorectal cancer (CRC) cell line. Interestingly, we observed a potent IWR-dependent induction of Axin2 protein with little change in levels of Apc or Gsk3β (Fig. 4a). Despite this increase in Axin protein levels, we did not observe a concomitant decrease in β-catenin levels as would be expected based on our results in L-cells (see Fig. 2). As the majority of β-catenin protein in colonic epithelial cells is sequestered in complexes with the cell-cell adhesion molecule E-cadherin19, we examined the pool of “free” β-catenin that is available for Wnt-mediated response. Indeed, levels of β-catenin not bound to E-cadherin are decreased in DLD-1 cells after addition of IWR-1 (Fig. 4b). The IWR-induced increase in Axin protein levels was accompanied by elevated levels of β-catenin phosphorylation, a prerequisite for proteasome-mediated destruction of β-catenin (Fig. 4c). Thus, IWR compounds promote β-catenin destruction likely by promoting stability of Axin-scaffolded destruction complexes. Consistent with this model, the IWR compounds did not induce de novo synthesis of Axin2 (Supp. Fig. 8a; see also Fig. 7h), a transcriptional target of the Wnt/β-catenin pathway20,21, inhibit the proteasome (Supp. Fig. 8b), alter the affinity of Axin2 for β-catenin or its ability to interact other pathway components (Supp. Fig. 8c-e), or disrupt subcellular localization of Axin (Supp. Fig. 8f).
Figure 4. Stabilization of the Axin2 destruction complex by IWR compounds.
(a) IWR compounds induce accumulation of Axin2 protein as revealed by Western blot analysis of proteins involved in regulating β-catenin levels. (b) Levels of β-catenin not bound to the E-cadherin cell-cell adhesion receptor are decreased in the presence of IWR compounds. (c) IWR compounds induce phosphorylation of β-catenin as measured using an anti-phospho-β-catenin (Ser33/37/Thr41) antibody. Two different exposures of the phospho-β-catenin Western blot are shown. (d) Diastereomeric conformation influences IWR-1 activity. The “exo” form of IWR-1 has decreased Wnt pathway inhibitory activity as measured in L-Wnt cells. (e) The IWR-1-exo compound has reduced ability to stabilize Axin2 as compared to IWR-1-endo. (f) Identification of an Axin2 protein domain necessary for interaction with IWR. Cell lysates with various Axin proteins were incubated with or without sepharose-immobilized IWR-PB (top right) in the presence or absence of competing IWR-1. Bound Axin protein was detected by Western blotting. IWR-IS: IWR-interaction sequence. (g) IWR compounds bind directly to a portion of Axin2 IWR-IS expressed as a glutathione-S-transferase (GST) fusion protein in bacteria. (h) Expression of the IWR-IS protein abrogates the effects of IWR on Wnt/β-catenin pathway response. Inhibition of aberrant Wnt/β-catenin pathway, as measured using the STF reporter assay, by IWR-1 is reversed by expression of IWR-IS protein. Data represent mean values ± s.d. (i) IWR induces stabilization of Axin2 protein with consequential increase in β-catenin destruction.
Figure 7. Chemical inhibition of cancerous Wnt/β-catenin pathway activity.
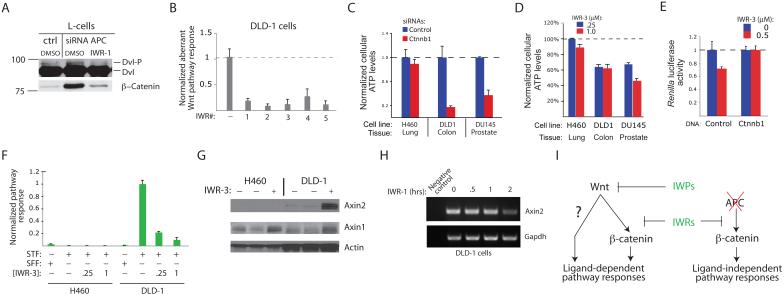
(a) IWR-1 blocks β-catenin accumulation induced by loss of APC tumor suppressor. (b) IWRs block aberrant Wnt/βbcatenin pathway activity in the colorectal cancer (CRC) cells. Constitutive Wnt/β-catenin pathway activity in DLD-1 cells consequential to APC loss-of-function, is abrogated by IWR compounds as measured using the STF reporter. (c) β-catenin-dependent growth of several cancer cell lines. Cells from lung, colon, and prostate cancers transiently transfected with a β-catenin siRNA pool were seeded at clonal density and cell viability measured using Cell-Titer Glo assay 10 days later. (d) Growth-inhibitory effects of IWR compounds on cancerous cells. The same assay in (c) was performed except cells were treated with IWR-3 for 6 days. (e) Overexpression of β-catenin can rescue the growth-inhibitory effects of an IWR compound in DLD-1 cells as indicated by levels of Renilla luciferase (RL) activity in cells transfected with or without a β-catenin expression construct and the RL reporter DNA. (f) H460 cells lack aberrant Wnt/β-catenin pathway activity as measured by STF reporter assay. (g) IWR-3 induces Axin1 protein stabilization in H460 and DLD-1 cells. (h) IWR-1 stabilization of Axin2 results in decreased transcription of Axin2, a Wnt/β-catenin target gene as measured using RT-PCR. (i) The predicted utility of IWP and IWR compounds for inhibiting Wnt ligand-dependent and -independent pathway responses. For (b-f), data represent mean values ± s.d.
As part of our effort to synthesize IWR-1, we produced two diastereomeric forms of IWR-1 (Fig. 4d, left; Supp. Fig. 9a), with the “exo” form (IWR-1-exo; 11) exhibiting decreased activity against the Wnt/β-catenin pathway as compared to the “endo” form (Fig. 4d, right; Fig. 4e). The sensitivity of IWR action to diastereomeric conformation is consistent with a high degree of specificity of IWR for its target protein. We also demonstrate that a fragment of IWR (IWR-frag, 12) lacking the quinoline group is incapable of inhibiting Wnt/β-catenin pathway activity (Supp. Fig. 9b). We use IWR-1-exo and IWR-frag hereafter as specificity controls for IWR-1 action.
We observed in vitro interaction of Axin2 with a biotinylated IWR compound (IWR-PB, 13) that can be competed with soluble IWR suggesting that IWR compounds either directly target Axin2 or an Axin2-associated protein (Fig. 4f; Supp. Fig. 9c-e). Under the same binding conditions, we are unable to detect proteins that bound to IWR-PB using silver staining or known pathway components by Western blot analysis, consistent with a specific but rather low affinity in vitro interaction between Axin2 and IWR-PB (Supp. Fig. 9f,g). We mapped the IWR interaction sequence (IWR-IS) in Axin2 to a region that also mediates association with other known Wnt pathway components (Fig. 4f). A bacterially expressed fusion protein containing a portion of the IWR-IS is capable of binding IWR-PB (Fig. 4g), supporting a direct interaction between Axin2 and IWR compounds. Expression of the IWR-IS protein is sufficient to abrogate the Wnt/β-catenin pathway inhibitory effects of IWR in DLD-1 cells (Fig. 4h) as well as IWR-induced stabilization of Axin proteins (Supp. Fig. 9h).
The simplest model that accounts for our observations is that IWR compounds induce stabilization of Axin proteins via a direct interaction. Indeed, the sensitivity of IWR activity to diastereomeric conformation and the ability of IWR compounds to directly bind to Axin2, suggests that the IWR-IS contains a binding pocket that may accommodate an endogenous regulatory small molecule. Although the evidence provided here is preliminary, small molecules that occupy this pocket may induce Axin proteins to adopt a stabilizing conformation (Supp. Fig. 10) perhaps similar to that reported following Wnt pathway activation22. Taken together, the IWR compounds have revealed a chemically tractable regulatory mechanism within this pathway, stabilization of Axin proteins, that could be exploited to control levels of Wnt/β-catenin pathway response (Fig. 4i).
Reversible chemical disruption of tissue regeneration
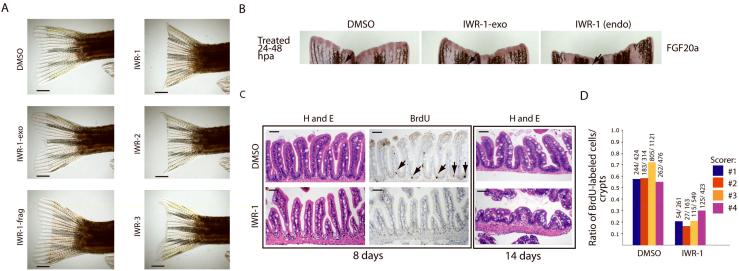
In order to test the in vivo activity of our IWR and IWP compounds, we first turned to a simple and rapid assay of Wnt/β-catenin pathway activity - regeneration of the zebrafish caudal fin following resection23. The addition of IWR-1, -2, and -3, but not IWR-1-exo and IWR-frag to the aquarium water of zebrafish suppressed fin regeneration after mechanical resection (Fig. 5a). However, the addition of IWP compounds failed to do the same suggesting that IWP compounds either have poor bioavailability, or that the determinants in the gene product that they target are not conserved in zebrafish. Accordingly, IWR-1 but not IWR-1-exo inhibited expression of FGF20a, a gene induced by Wnt/β-catenin pathway activity following tailfin resection23 and that is required for fin regeneration24 (Fig. 5b).
Figure 5. Chemical inhibition of the Wnt/β-catenin pathway in regeneration of zebrafish tissue.
(a) Specific inhibition of tailfin regeneration with IWR compounds. IWR-1, -2, and -3 but not the inactive IWR-1-exo and IWR-frag inhibit tailfin regeneration. Four animals in each group were analyzed. Scale bar: 2.5mm. (b) IWR-1 inhibits FGF20A expression in resected tailfins. Fish with resected tailfins were allowed to recover for 24hrs and then treated with small molecules for an additional 24hrs post amputation (hpa). Fins were probed for FGF20A expression by in situ hybridization. Arrows highlight a region that more readily reveals differences in FGF20a expression. (c) IWR-1 blocks normal homeostatic renewal of the GI tract. Representative histological sections of mid-intestinal tissue from fish treated with carrier or IWR-1 (10μM) for 8 or 14 days stained either with hematoxylin and eosin (H&E) or for BrdU incorporation. Loss of BrdU-labeled cells in the base of intestinal folds in IWR-1-treated fish (8 days; arrows) is followed by gross changes in intestinal tissue architecture after prolonged chemical exposure (14 days). Eight animals in each group were analyzed. Scale bar: 50μM. (d) Quantification of BrdU-labeled cells in the intestinal tract of control or IWR-1-treated fish. Histological sections as seen in b (middle column) were scored for the percentage of intestinal folds that contain BrdU-labeled cells. Four independent scorers analyzed sections from eight fish either from control or IWR-1 treated groups. Ratio: BrdU-labeled cells in the numerator and the number of intestinal folds scored in the denominator.
We also examined the effects of IWR-1 treatment on maintenance of dividing cells in the zebrafish gastrointestinal (GI) tract, another Wnt-dependent process6. Several lines of evidence suggests that the GI tissue in metazoans is particularly sensitive to perturbations in Wnt pathway activity1. Consistent with the specific activity of IWR-1 against the Wnt/β-catenin pathway in zebrafish, we observed a decreased number of the bromodeoxyuridine (BrdU)-labeled cells typically found at the base of the intestinal folds in IWR-1-treated fish, signifying a loss of stem/progenitor cell function (Fig. 5c,d). We also noted that fish treated for periods longer than 5 days exhibit lethargy and decreased appetite (data not shown), correlating with gross histological changes in the architecture of GI tissue. The effectiveness of IWR compounds to inhibit two regenerative processes genetically shown to be dependent upon Wnt/β-catenin signaling in zebrafish, tailfin regeneration and epithelial stem cell self-renewal in the intestines, is consistent with their ability to target the Wnt/β-catenin pathway in vitro. However, further studies will be required to ascertain whether or not stabilization of Axin proteins by IWR compounds will alter other signal transduction pathways important to tissue homeostasis, or if other targets of IWR compounds exist in vivo.
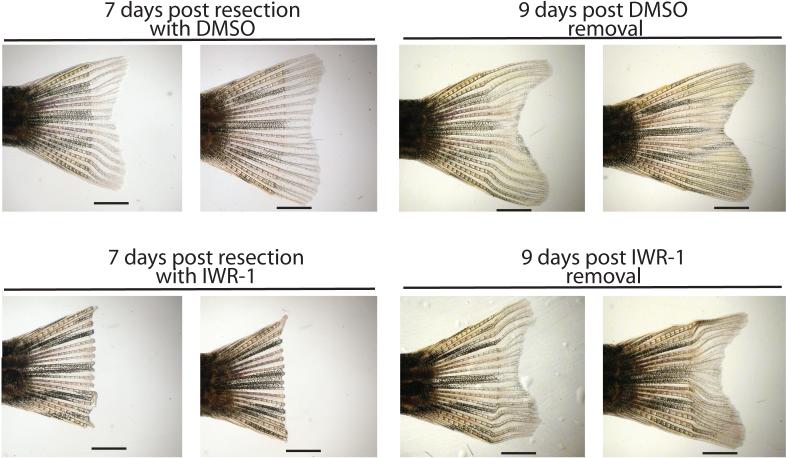
Achieving transient repression of pathological Wnt response without incurring permanent damage to normal stem cell function is a key anti-cancer therapeutic goal. We tested for the ability of zebrafish to resume regenerative processes following a chemically induced blockade of fin re-growth. Fish with resected caudal fins that were breed in water containing IWR-1 for 7 days were able to regenerate tissue to nearly normal levels subsequent to chemical removal, suggesting that transient inhibition of Wnt/β-catenin response did not permanently alter the ability of stem cells to self-renew (Fig. 6).
Figure 6. The effects of IWR-1 on caudal fin regeneration are reversible.
Adult zebrafish with resected caudal fins were placed in water containing DMSO carrier or IWR-1 (10μM) for 7 days with replenishment of breeding water and compounds every day. Consistent with inhibition of Wnt/β-catenin pathway response by IWR-1, fish treated with IWR-1 but not DMSO failed to regenerate fin tissue. Nine days post-removal of chemicals, fish that were treated with IWR-1 display tissue regrowth suggesting the pluripotent cells required for regeneration are able to resume normal function. Numbers designate specific animals. Four fish were analyzed in each group. Scale bar: 2.5mm.
Chemical inhibition of Wnt/β-catenin response in cancer
Aberrant Wnt-mediated pathway responses, sustained either by genetic changes that result in altered Wnt ligand activity or the function of pathway regulators, has been associated with a broad range of cancers1,12. Pointedly, more than 90% of colorectal cancer (CRC) tumors harbor a loss-of-function mutation in APC, a suppressor of the Wnt/β-catenin pathway11. The ability of IWR compounds to stabilize Axin proteins and induce β-catenin destruction even in the absence of normal APC protein function (see Fig. 4a), suggests that they may block aberrant cell growth supported by hyperactivation of Wnt/β-catenin response. Indeed, IWR compounds are able to inhibit aberrant Wnt/β-catenin activity as a consequence of Apc loss in either mouse L-cells (using Apc siRNAs; Fig. 7a) or DLD-1 colorectal cancer cells (that harbor a loss-of-function mutation in APC; Fig. 7b). Next, we tested the ability of IWR-3 to mimic the cell growth effects of β-catenin siRNAs in several cancer cell lines that exhibit differences in growth-dependency on Wnt/β-catenin pathway activity (Fig. 7c, d). Notably, IWR-3 mimicked the effects of β-catenin siRNAs on the growth of cells derived from cancers of the colon (DLD-1) and prostate (DU145) but not lung (H460), suggesting that IWR-3 successfully targeted the Wnt/β-catenin pathway in these cells. Indeed, overexpression of β-catenin can overcome the effects of IWR-3 on DLD-1 cell growth (Fig. 7e). The difference in growth sensitivity of DLD-1 and H460 cells to IWR-3 is more likely due to differences in pre-existing Wnt/β-catenin pathway activity in these cells (Fig. 7f) rather than pharmacokinetic considerations as IWR-3 was nevertheless able to induce Axin protein stabilization in H460 cells (Fig. 7g).
Aberrant transcriptional induction of Wnt/β-catenin target genes is considered nearly universal in CRC cells that harbor loss-of-function mutations in the APC tumor suppressor25. Consistent with the ability of IWR compounds to inhibit cancerous Wnt/β-catenin pathway response, we observed a decrease in the expression of Axin2 in DLD-1 cells after exposure to IWR-1 for 2 hrs (Fig. 7h). Thus, Axin protein stability can be chemically controlled in order to suppress cancerous Wnt/β-catenin activity as demonstrated by the IWR compounds.
DISCUSSION
Achieving chemical control of cell fate determination in post-embryonic tissues is a key goal of both regenerative medicine and anti-cancer efforts. We have focused our attention on Wnt-mediated cellular responses given their central role to stem cell self-renewal and cancer. The ability of IWP compounds to selectively target a member of the MBOAT family of acyltransferases suggests that chemical inhibition of regulatory events underlying production of Wnt proteins may be as therapeutically viable as blocking mechanisms of cellular response (Figure 7i). Indeed, the chemical inhibition of Wnt protein production by the IWP compounds represents a departure from current small molecule-based strategies that are primarily focused on targeting transducers of Wnt-mediated cellular responses26. As other important signaling molecules frequently associated with disease such as the Hedgehog proteins and the appetite-regulating hormone Ghrelin are also substrates of MBOAT family members15,27, our findings could be broadly exploited for therapeutic purposes.
The effectiveness of IWR compounds for inhibiting Wnt/β-catenin pathway response can in part be explained by the rate-limiting role that Axin proteins occupies within the pathway as predicted by mathematical modeling28. Importantly, elevated Axin protein levels induced by IWR compounds can compensate for the loss of APC tumor suppressor function by re-establishing regulation of β-catenin protein levels and countering aberrant transcriptional activation of growth-promoting genes (Fig. 7i). As cancerous Wnt/β-catenin pathway activity can also arise from misregulated Wnt ligand function, the distinct druggable targets established using the IWR and IWP compounds could form the basis for therapeutic strategies to be selectively deployed depending upon the underlying genetics of disease.
METHODS
Primary screen and secondary reporter-based assays
For the “primary screen” and “dose-dependent test”, ~5K L-Wnt-STF cells were seeded into each well of a white opaque 384 well plate and individual compounds from the UTSouthwestern chemical library (see http://www.utsouthwestern.edu/utsw/cda/dept24734/files/342125.html for more information regarding source of chemicals) added 24hrs later to each well. Luciferase activities were measured 24 hrs later. To identify FL inhibitors and compounds that blocked protein secretion, L-cells were transiently transfected with CMV-FL and CMV-GL constructs, immediately incubated with compounds, and analyzed for GL and FL activities, respectively, 24 hrs later. For the “exogenous Wnt test”, Wnt3A-containing conditioned medium, prepared following the protocol provided ATCC, was applied to HEK293 cells transiently transfected with STF and control reporters. For Hh and Notch tests, NIH-3T3 cells or L-cells, respectively, were transiently transfected with indicated reporter constructs, immediately incubated with compounds, and luciferase activities measured 24 hrs later. The “Wnt secretion test” was performed in L-cells transiently transfected with the Wnt-GL expression construct, immediately incubated with compounds, and analyzed 48 hrs later for GL activity. Assays used to calculate IC50's for compounds were performed in L-Wnt-STF cells.
Biochemical studies
Biochemical studies involving L-Wnt-STF or DLD-1 cells were performed in either 48- or 6-well format with IWR (10μM) or IWP (5μM) compounds and/or cycloheximide (100μM) in a 48 hr assay period. E-cadherin depletion studies were performed at 4°C using DLD-1 cells lysed in PBS/1% NP-40/protease inhibitors. For Wnt3A and ShhN phase separation assays, expression constructs encoding mPorcn, hWnt3A-myc, or mShhN were transfected into HEK293 cells using Effectene (Qiagen). After 48 hrs, cells were lysed for 15 min, RT with distilled water, 10 mM Tris-HCl, 150 mM NaCl /1% TritonX-114 (PL buffer). Lysate was briefly chilled on ice, pelleted for 10 min 4°C, and the supernatant combined with an equal volume of PL buffer/3.5% TX-114. Solutions were rotated for 15 min at 4°C, placed at 37°C for 5 min followed by an additional centrifugation for 5 min at 2000g, RT. Distinct phases were collected and combined with PL buffer to a total volume of 1 ml. Samples were chilled on ice, ConA sepharose (for Wnt protein) or 5E1 mAb/protein A sepharose (for ShhN protein) added, and samples rotated for 2 hrs at 4°C. Beads were washed 2x with PL buffer, and a Western blot performed with eluted proteins using an anti-c-myc antibody. For IWP-PB and IWR-PB binding studies, cell lysate (10mM Na2HPO4 pH7.4, 0.15mM NaCl, 1%NP-40) derived from HEK293 cells transfected with the Porcn-myc or various Axin2 constructs, or bacterially expressed Axin2 (508-761)-GST protein were incubated with either DMSO, linker (0.17 mM), IWP-3 (0.6 mM), or IWR-1 (0.2mM) for 1 hr at 4°C prior to addition of NeutrAvidin agarose resin (Pierce) and either DMSO, IWP-PB (0.17 mM) or IWR-PB (.05mM). Samples were rotated overnight at 4°C, washed with 3×10min with lysis buffer, and bound material eluted with sample loading buffer. For in vitro trypsinization studies, lysate derived from HEK293 cells expressing Axin2-myc protein and treated with or without IWR-1 was incubated at RT with 0.25 mg/ml of trypsin and 0.10 mM EDTA for indicated time periods.
Chemical synthesis
Synthesis of IWP-1, IWP-2, IWP-PB, IWR-1, and IWR-PB are described in Supp. Fig. 4, 6a, and 9a,c. Synthesis of IWR-1 along with IWP-1 and IWR-3 are further described in Supplementary Methods.
Cancer cell growth studies
Cancer cells were seeded into a 24 well format (2.5K cells/well) in the presence of carrier alone or IWR-4 (0.5% DMSO final). Media with compound was changed every 24 hrs for 5 days. On day 6, ATP levels were quantified via Cell Titer Glo assay (Promega). In experiments involving siRNA transfections, cells were transfected with 50nM control or β-catenin siRNAs using Effectene and seeded into a 96 well plate, 7.5K cells/well, in triplicate. After 48 hrs, 2.5K cells were transferred from each well into a six well format. ATP levels were measured 120 hrs later via Cell Titer Glo assay.
Zebrafish studies
Six month-old zebrafish were incubated 8 or 14 days at 28.5°C in aquarium water supplemented with 10 μM IWR-1 or in 0.1% DMSO as a control. Fish were fed standard diet, and solutions were changed daily. For BrdU-labeling studies, zebrafish were incubated in 1 mM BrdU in aquarium water for 2 hrs at room temperature (RT) at the end of an 8-day chemical exposure period, then washed several times in aquarium water, anesthetized with 0.1% Tricaine, and fixed in 4% paraformaldehyde for 48 hrs at 4°C. The intestine was dissected out, dehydrated, paraffin embedded and sectioned at 5mm intervals. Sections were stained with Hematoxylin and Eosin or processed for BrdU immunohistochemistry as described29. The total number of crypts and total number of BrdU-positive nuclei in the mid and distal sections of the intestine were counted from each section. For caudal fin regeneration assays, zebrafish, 3-6 months of age, were anaesthetized in 0.02% Tricaine and half of the fin was resected using a razor blade to remove. Amputees were reared at 28°C in tanks containing either DMSO or IWR (10μM). Water and compounds were replenished daily for the total indicated assay period. For recovery experiments, fish were then breed in a chemical-free environment at 28°C for an additional 9 days. All zebrafish experiments were performed in accordance to regulatory standards as accepted by the Institutional Animal Care and Use Committee (IACUC) at UTSouthwestern. A 494□bp fragment of the Fgf20a coding sequence was amplified with primers AGATGGGACGTCCAGAGATG (forward) and GTCCATGCCAGTGTTTTGTG (reverse) and subcloned into pGE□T Easy (Promega). The resultant plasmid was linearized and an antisense riboprobe was synthesized using Digoxigenin□UTP labeling mix and T7 RNA polymerase (both from Roche) according to the manufacturer's instructions. In situ hybridization of fixed tails was carried out according as described in30 except that Proteinase K digestion was carried out for 20 min at RT in 20 mcg/ml Proteinase K.
Other methods and chemical compound information
See Supplementary Methods online for remaining methods, including description of reagents and radiolabeling experiments. Chemical compound information can be found on the Nature Chemical Biology website.
Supplementary Material
ACKNOWLEDGEMENTS
We thank M. Bienz (Medical Research Council Laboratory of Molecular Biology), R.T. Moon (University of Washington), P.T. Chuang (University of California, San Francisco), J. Laborda (University of Castilla-La Mancha), G. Johnson (University of Alabama at Birmingham), P. Beachy (Stanford University), M. Brown, and J. Goldstein (UTSouthwestern Medical Center) for reagents; D. Frantz, K. Lillard, the UTSW Pathology Core, and S. McKnight and HTS Core for support with the chemical screen; this work was supported by NIH/NCI (PO1 CA095471; Z. Ma, J. Kilgore, and N. S. Williams), the NIH/NIGMS (1R01GM076398-01), the American Cancer Society (RSGGMC□112251), the Welch Foundation (I-1665), a High Risk/High Impact award from UTSouthwestern, and an endowment from Virginia Murchison Linthicum.
Footnotes
COMPETING FINANCIAL INTEREST STATEMENT The authors declare competing financial interests in the form of a pending patent application.
REFERENCES
- 1.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008;22:746–55. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Flier LG, et al. The Intestinal Wnt/TCF Signature. Gastroenterology. 2007;132:628–32. doi: 10.1053/j.gastro.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551–9. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 6.Muncan V, et al. T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 2007;8:966–73. doi: 10.1038/sj.embor.7401071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–10. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 9.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 10.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 11.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 12.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Takada R, et al. Monounsaturated fatty acid modification of Wnt protein: its role in Wnt secretion. Dev Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Kurayoshi M, Yamamoto H, Izumi S, Kikuchi A. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402:515–23. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamoun Z, et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–4. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 16.Abrami L, Kunz B, Iacovache I, van der Goot FG. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0710389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka K, Okabayashi K, Asashima M, Perrimon N, Kadowaki T. The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur J Biochem. 2000;267:4300–11. doi: 10.1046/j.1432-1033.2000.01478.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008 doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112(Pt 8):1237–45. doi: 10.1242/jcs.112.8.1237. [DOI] [PubMed] [Google Scholar]
- 20.Jho EH, et al. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lustig B, et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cselenyi CS, et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc Natl Acad Sci U S A. 2008;105:8032–7. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoick-Cooper CL, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–89. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead GG, Makino S, Lien CL, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310:1957–60. doi: 10.1126/science.1117637. [DOI] [PubMed] [Google Scholar]
- 25.Sabates-Bellver J, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–75. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- 26.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–96. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepard JL, et al. A zebrafish bmyb mutation causes genome instability and increased cancer susceptibility. Proc Natl Acad Sci U S A. 2005;102:13194–9. doi: 10.1073/pnas.0506583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poss KD, et al. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222:347–58. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.