Abstract
Obstructive sleep apnea (OSA) is a common disorder characterized by repetitive narrowing or collapse of the pharyngeal airway during sleep. The disorder is associated with major comorbidities including excessive daytime sleepiness and increased risk of cardiovascular disease. The underlying pathophysiology is multifactorial and may vary considerably between individuals. Important risk factors include obesity, male sex, and aging. However, the physiological mechanisms underlying these risk factors are not clearly understood. This brief review summarizes the current understanding of OSA pathophysiology in adults and highlights the potential mechanisms underlying the principal risk factors. In addition, some of the pathophysiological characteristics associated with OSA that may modulate disease severity are illustrated. Finally, the potential for novel treatment strategies, based on an improved understanding of the underlying pathophysiology, is also discussed with the ultimate aim of stimulating research ideas in areas where knowledge is lacking.
Keywords: arousal, genioglossus, lung volume, upper airway, ventilatory control stability
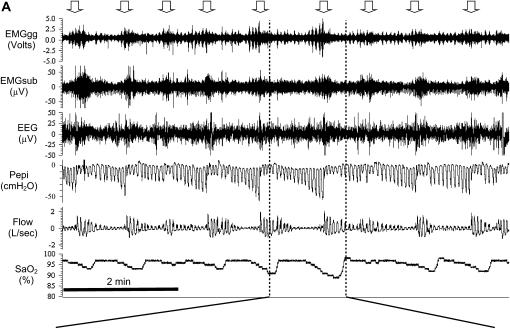
Obstructive sleep apnea (OSA) is characterized by recurrent collapse of the pharyngeal airway during sleep, resulting in substantially reduced (hypopnea) or complete cessation (apnea) of airflow despite ongoing breathing efforts. These disruptions to breathing lead to intermittent blood gas disturbances (hypercapnia and hypoxemia) and surges of sympathetic activation. Loud snoring is a typical feature of OSA and in most cases the culmination of a respiratory event is associated with a brief awakening from sleep (arousal). These events result in a cyclical breathing pattern and fragmented sleep as the patient oscillates between wakefulness and sleep. In severe cases respiratory events can occur more than 100 times per hour and typically each event lasts 20–40 seconds (see Figure 1 for an example).
Figure 1.
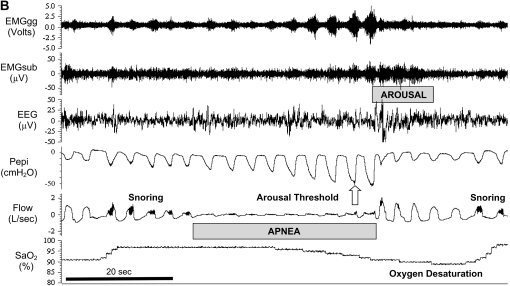
Polysomnographic tracings of obstructive sleep apnea from a detailed experimental study of a patient with severe disease (apnea–hypopnea index = 56 events/h). EMGgg = Electromyogram of the genioglossus muscle (intramuscular); EMGsub = EMG of the submental muscle (surface); EEG = electroencephalogram (C3–A2); Pepi = pressure at the level of the epiglottis; Flow = airflow measured via nasal mask and pneumotachograph; SaO2 = arterial blood oxygen saturation measured via pulse oximetry at the finger. (A) An 8-minute segment during stage 2 sleep, during which the patient is experiencing sleep-disordered breathing. Note the repeated oxygen desaturations as a result of severely impaired (hypopnea) or absent (apnea) airflow despite continual breathing efforts (Pepi) and the cyclical breathing pattern that ensues as the patient oscillates between sleep and arousal (downward pointing arrows). (B) An expanded segment during an obstructive event. Note: Evidence of snoring on the flow tracing, quantification of the arousal threshold, and progressive increases in EMGgg activity throughout the obstructive event, although occurring, were not sufficient to restore flow without arousal in this instance.
OSA is associated with major comorbidities including daytime somnolence, impaired cognition, poor quality of life, and increased risk of motor vehicle accidents. There is emerging evidence to suggest that OSA is an independent risk factor for a variety of adverse cardiovascular outcomes (1, 2). The clinical disorder, defined as more than five abnormal breathing disturbances (hypopneas or apneas) per hour of sleep combined with symptoms of daytime sleepiness, affects at least 2–4% of the adult population (3). Although nonobese individuals may suffer from OSA, obesity is the main epidemiologic risk factor. Indeed, increases in body mass index, central accumulation of adipose tissue, and neck circumference are strong predictors of disease (4). Further, the prevalence of OSA is two to three times greater in men than in women (3, 5, 6) and in older individuals (≥65 yr) compared with middle-aged individuals (30–64 yr) (7).
The pathophysiological causes of OSA likely vary considerably between individuals. Important components likely include upper airway anatomy, the ability of the upper airway dilator muscles to respond to respiratory challenge during sleep, the propensity to wake from increased respiratory drive during sleep (arousal threshold), the stability of the respiratory control system (loop gain), and the potential for state-related changes in lung volume to influence these factors. These various physiological traits and the potential for each to influence sleep apnea pathophysiology have been described in detail in review articles (8–10). The focus of the current article is to (1) briefly review the key physiological factors, discuss how they may interact with one another, and highlight advances in our understanding of OSA pathogenesis; (2) discuss the potential physiological mechanisms underlying the key epidemiologic risk factors for OSA; (3) highlight some of the physiological factors associated with OSA that may modulate disease severity; and (4) briefly discuss potential treatment strategies according to the various underlying physiological causes of OSA.
PATHOPHYSIOLOGY
Upper Airway Anatomy
The human upper airway is a unique multipurpose structure involved in performing functional tasks such as speech, swallowing of food/liquids, and the passage of air for breathing. The anatomy and neural control of the upper airway have evolved to enable these various functions. The airway, therefore, is composed of numerous muscles and soft tissue but lacks rigid or bony support. Most notably, it contains a collapsible portion that extends from the hard palate to the larynx. Although the ability of the upper airway to change shape and momentarily close is essential for speech and swallowing during wakefulness, this feature also provides the opportunity for collapse at inopportune times such as during sleep.
From a purely anatomic perspective, a narrow upper airway is generally more prone to collapse than a larger one. Accordingly, on the whole, the cross-sectional area of the upper airway as measured by computed tomography and magnetic resonance imaging during wakefulness is reduced in patients with OSA compared with subjects without OSA (11–13). Further, the arrangement of the surrounding soft tissues appears to be altered in patients with OSA, which may place the upper airway at risk for collapse (11). Imaging studies during wakefulness, however, are complicated to interpret because ongoing upper airway dilator muscle activity may lead to potential differences between groups, due to factors other than anatomy. In addition to these imaging measures, a methodology to determine the pressure at which the upper airway collapses during sleep (Pcrit) as a gauge of passive upper airway anatomy is also in concordance with reduced upper airway caliber in patients with OSA (14, 15). Perhaps the most definitive data come from Isono and colleagues, who observed increased closing pressure (more collapsible) in OSA as compared with control subjects under conditions of general anesthesia and muscle paralysis (16). Thus, in aggregate, multiple methodologies have shown that patients with OSA have anatomic compromise making these individuals susceptible to pharyngeal collapse during sleep.
Upper Airway Dilator Muscle Activity and Reflex Responsiveness
During wakefulness, patients with OSA appear to compensate for an anatomically compromised upper airway through protective reflexes which increase upper airway dilator muscle activity to maintain airway patency (17). Accordingly, the genioglossus, the largest and most extensively studied upper airway dilator muscle in humans, has higher activity in patients with OSA compared with control subjects. One mechanism believed to be important in the pathogenesis of OSA relates to the interaction between pharyngeal anatomy and a diminished ability of the upper airway dilator muscles to maintain a patent airway during sleep (17). In support of this hypothesis, muscle tone measured via multiunit EMG intramuscular electrodes of the genioglossus is reduced at sleep onset in healthy individuals and patients with OSA (18, 19). Thus, whereas healthy individuals experience a loss of upper airway muscle tone at sleep onset and experience some degree of breathing instability (20), an individual reliant on muscle tone due to an anatomic vulnerability will be particularly susceptible to developing OSA. Accordingly, hypopneas and apneas commonly occur at the transition from wakefulness to sleep in OSA. As is discussed below, each event is typically associated with a cortical arousal such that the patient with OSA cycles between wakefulness and sleep, making it difficult to achieve deeper stages of sleep. Unlike the transition to sleep, slow wave sleep is associated with increased, not decreased, upper airway dilator muscle activity (21). Thus, when patients are able to achieve slow wave sleep, increased upper airway dilator muscle activity may be one important factor contributing to the improvement in apnea severity that is commonly observed in this sleep stage (21). Alternatively, patients with apnea may be able to enter slow wave sleep only when muscle activity is increased and breathing is already stabilized.
Mechanistically, in addition to central respiratory drive, the genioglossus is importantly modulated by locally mediated (i.e., in the upper airway) mechanoreceptive reflex mechanisms that respond to negative pharyngeal pressure (22). One such mechanism is the genioglossus negative pressure reflex, whereby the muscle is activated in response to rapid changes in negative intrapharyngeal pressure (i.e., pressures that are subatmospheric or suction pressure) (23). Consistent with the nature of OSA being a state-related disease, the genioglossus negative pressure reflex has been shown to be diminished during non-REM sleep in healthy individuals (24, 25). However, more recent data have demonstrated maintenance of genioglossus reflex activation in non-REM sleep, particularly in the supine posture when gravitational collapsing effects on the upper airway are maximal (26, 27). The identification of a secondary state-dependent suppression component to this reflex arc has raised the possibility that more pronounced reflex inhibition rather than a loss of excitation may mediate diminished pharyngeal reflex responses during sleep (26). Indeed, advances in our understanding of the neuroanatomy of the genioglossus negative pressure reflex and hypoglossal motor nucleus inputs from rat studies have highlighted the extensive presence of inhibitory inputs to the genioglossus muscle (28–30). Nonetheless, although genioglossus muscle responsiveness may be impaired during sleep compared with wakefulness, it is clear that the muscle does respond to sustained negative pressure (Figure 1) and potentially hypercapnia, particularly when combinations of stimuli are provided (31–33). However, there appears to be substantial interindividual variability in the effectiveness of these compensatory responses to restore airflow during respiratory loading in sleep (32).
To further advance our understanding of pharyngeal muscle control and its role in OSA pathogenesis, single motor unit–recording techniques have been employed to assess genioglossus muscle activity in humans. This technique is based on high-frequency sampling and allows sorting of individual motor units to gain insight into their unique characteristics and regulation. Although these studies are in their infancy, they have highlighted the heterogeneity of the genioglossus muscle and provide a powerful tool for studying the neural control of muscle activity (34, 35). It is hoped that, by combining neuroanatomic knowledge from animal models with sensitive neurophysiological techniques in humans, novel therapeutic targets to increase muscle activity may ultimately be identified for some patients with OSA. Although such approaches may lead to reduced severity of OSA for some patients, as is discussed below, given the heterogeneity of OSA pathogenesis such an approach will likely not resolve sleep-disordered breathing for all patients. Nonetheless, there is evidence to suggest that novel training exercises of upper airway muscles may lead to some improvement in sleep-disordered breathing (36, 37). However, on the basis of the state dependence of OSA, muscle training during wakefulness is unlikely to have major effects on airway patency during sleep unless the increased muscle activity/efficiency is maintained during sleep.
Arousal from Sleep
Arousal from sleep at the cessation of a hypopnea or an apnea has long been believed to be an important protective mechanism for airway reopening (38, 39). In fact, most respiratory events are associated with cortical arousal and more severe events result in longer arousals (40). However, work by Younes has provided insight into the functional role of arousal from sleep in OSA and challenged the notion that it is essential for airway reopening (41). In studying the response to experimentally induced transient continuous positive airway pressure (CPAP) reductions in patients with OSA, Younes noted that inspiratory flow increased in 22% of instances before arousal and was restored in 17% of trials in the absence of arousal (41). More recently, Jordan and colleagues conducted a study to examine the mechanisms underlying these arousal-free restorations of airflow (32). Transient pressure reductions for up to 5 minutes resulted in increases in genioglossus muscle activity and changes in duty cycle. These compensatory responses were similar between patients with OSA and healthy individuals. However, patients with OSA were less able to restore ventilation without cortical arousal than were healthy individuals given stimuli of similar magnitude (32).
The findings that patients with OSA are able to restore ventilation in the face of respiratory loading without cortical arousal at least some of the time, albeit to a lesser extent than healthy individuals, raises the possibility that some patients may be able to maintain a patent airway during sleep if they are able to remain asleep for a sufficient duration to recruit compensatory mechanisms. For example, because combinations of stimuli such as carbon dioxide and negative pressure can activate upper airway dilator muscles during sleep, delaying of arousals may be beneficial if it allows sufficient accumulation of respiratory stimuli to restore pharyngeal patency (31, 42). Should this be the case, strategies to prevent arousal from sleep (increase the arousal threshold) are likely to be most beneficial in patients who awaken easily (low arousal threshold) to respiratory loads during sleep (10, 41). However, increasing the arousal threshold in patients with a preexisting high arousal threshold, for example, in patients with severe sleep-disordered breathing, may be deleterious because of worsening of blood gas abnormalities (43).
Most of the available evidence suggests that the level of pleural pressure, generated by respiratory effort regardless of the stimulus (e.g., hypoxia, hypercapnia, and respiratory loading), is likely to be the key trigger for inducing arousal from non-REM sleep (44, 45). Experimentally, the arousal threshold is measured as the minimal esophageal pressure (or pressure at the level of the epiglottis, which is likely to be similar during airway occlusion) generated on the breath preceding arousal during a respiratory load or occlusion. Figure 1B depicts quantification of the arousal threshold, in this instance during airway occlusion during a naturally occurring apnea. Although there is wide interindividual variability, patients with OSA tend to have an impaired arousal response to airway occlusion (more negative pressure required or a higher arousal threshold) than control subjects (46). Treating OSA with CPAP tends to lower the arousal threshold (47). These findings suggest that OSA (e.g., sleep fragmentation, hypoxia, and repeated airway obstruction) rather than an inherent abnormality in the arousal threshold is responsible for the impaired arousal responses in patients with OSA.
When initiated, arousal from sleep is associated with heightened upper airway dilator muscle activity for an equivalent level of negative pharyngeal pressure during sleep and a brisk ventilatory response (48) (Figure 1B). Although these changes are beneficial in rapidly restoring airflow and reversing oxygen desaturation/hypercapnia, as is discussed in the following section, they can also destabilize breathing and perpetuate apnea severity (49).
Ventilatory Control Stability
As stated, a typical feature of OSA is the cyclical breathing pattern that develops, whereby the patient oscillates between obstructive breathing events (sleep) and arousal (wakefulness) (Figure 1A). Further, obstructive events tend to occur during periods of low respiratory drive. Thus, ventilatory control stability is believed to be an important contributor to OSA pathogenesis.
Ventilatory control stability can be described using the engineering concept loop gain (50). Essentially, loop gain is a term used to describe the stability of a system controlled by feedback loops. In the context of ventilatory control, loop gain refers to the stability of the respiratory system and how responsive the system is to a perturbation to breathing (e.g., arousal). In other words, loop gain can be considered as the propensity for the ventilatory control system to develop cyclical fluctuations in ventilatory output (as seen in periodic breathing). There are two principal components to loop gain: controller gain and plant gain. As it relates to respiratory control, controller gain refers to the chemoresponsiveness of the system (i.e., hypoxic and hypercapnic ventilatory responses). Plant gain reflects primarily the efficiency of CO2 excretion (i.e., the ability of a given level of ventilation to excrete CO2). A third factor, known as mixing gain, appears to be less crucial, but is a function of circulatory delay as well as hemoglobin binding of O2 and CO2. Mixing gain tends to be fairly constant, although circulatory delays may make mixing gain more clinically relevant in patients with congestive heart failure (51). The physical separation of the sensors and effectors makes the ventilatory feedback control system vulnerable to instability. An inherently high loop gain system is unstable (i.e., robust ventilatory response to a respiratory stimulus) compared with a low loop gain system (i.e., dampened ventilatory response to an equivalent respiratory stimulus). A commonly used analogy is the regulation of room temperature, whereby temperature will be prone to oscillation in a situation where there is a particularly sensitive thermostat and an overly powerful heater (i.e., high loop gain) (52). Techniques have been developed to measure loop gain of the respiratory system, such as the proportional assist ventilation (PAV) technique (53, 54). Studies using PAV have demonstrated that patients with OSA do in fact have an elevated loop gain and suggest that ventilatory instability is an important mechanism contributing to sleep-disordered breathing (55, 56). However, the PAV technique relies on a stable upper airway and stable state (no arousals). Thus, information about transient responses that are also likely to be important in perpetuating cyclical breathing is currently beyond the scope of this technique.
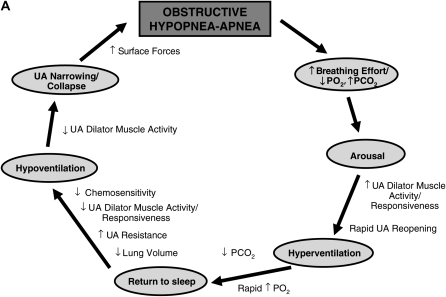
Debate exists regarding how elevated loop gain may affect the propensity for apnea. There are two key potential mechanisms that are likely to be important, although neither is definitively proven. First, elevated loop gain would be expected to increase oscillations from the brainstem central pattern generator. One would predict that pharyngeal obstruction occurs when ventilatory motor output is at its nadir (i.e., when neural output to the upper airway muscles is low). Second, elevated loop gain may also increase the ventilatory response to arousal, which may drive PaCO2 below the apnea threshold during subsequent sleep. Obstructive or central apnea could then occur depending on the prevailing upper airway mechanics. Thus, further work is required in this area. A schematic representation of the typical physiological characteristics associated with airway narrowing/closure during sleep and the potential role of a high loop gain in contributing to OSA pathogenesis are illustrated in Figure 2.
Figure 2.
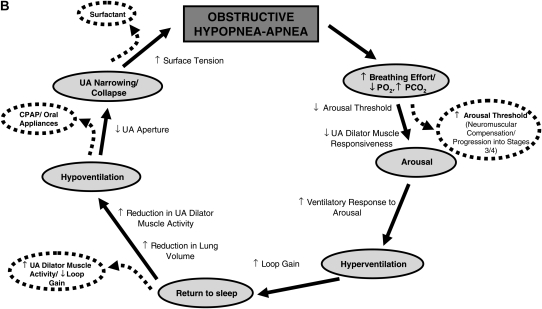
(A) Schematic representation of the typical pathophysiological sequence that occurs in obstructive sleep apnea (OSA) (shown in gray) and the associated physiological processes that occur throughout the cycle that are either protective/restorative (outside the circle) or perpetuating (inside the circle). UA = upper airway. (B) Schematic representation of the possible sites where each of the various pathophysiological traits would either predispose or tend to worsen OSA (inside the main circle). Note that some of the effects of these traits may be interrelated (i.e., a high loop gain may increase the ventilatory response to arousal and the propensity for cyclical breathing, and also lead to periods of decreased upper airway dilator muscle activity). Refer to the text for further detail. Some of the potential factors that may alleviate OSA at various points throughout the typical physiological cycle (dashed arrows and ovals) are located outside the main circle. Note that some factors are theoretical and largely untested whereas others are more proven therapies (i.e., continuous positive airway pressure [CPAP]).
Lung Volume
The interaction between pharyngeal patency and lung volume is believed to be an important contributor to OSA pathogenesis. Indeed, upper airway mechanics can be modulated by changes in lung volume during wakefulness and sleep in healthy individuals (57–61). Further, Hoffstein and colleagues demonstrated that across the range from residual volume to total lung capacity there is a lung volume dependence on upper airway cross-sectional area measured during wakefulness (62). In addition, the lung volume dependence appears to be more pronounced in patients with OSA compared with control subjects (62, 63). However, studies during wakefulness are confounded by behavioral influences because a maximal inhalation to total lung capacity is likely to activate upper airway muscles behaviorally as well. During sleep, upper airway resistance increases as lung volume is reduced (64, 65). Increasing end-expiratory lung volume decreases airway collapsibility in healthy control subjects and improves sleep-disordered breathing in patients with OSA (60, 66, 67).
While the aforementioned studies demonstrate that changes in lung volume are capable of modulating upper airway patency in OSA, the underlying mechanisms have not been well defined in humans. One likely mechanism, which has been clearly demonstrated in animal models (68–70), is the concept of a loss of caudal traction on upper airway structures during decreased lung volume. Briefly, when lung volume is reduced there is a displacement of the diaphragm and thorax toward the head. This movement results in a loss of caudal traction on the upper airway, yielding a more collapsible airway. Data obtained by examining the interaction between passive pharyngeal airway and lung volume independent of neuromuscular factors in patients with sleep-disordered breathing suggest that similar mechanisms may contribute to OSA pathogenesis (71).
GENETICS
Studies have clearly shown a common familial basis to the development of OSA (72, 73). This finding is true for both obese and nonobese patients with OSA (73–75). Studies using linkage analysis have provided initial insight into the potential link between specific areas of the genome and OSA pathogenesis (76–78). Future studies using genome-wide association analyses and other novel technologies may also provide important information in this regard (79, 80). Anatomy (obesity, craniofacial structure) clearly have genetic underpinnings (81–83). Furthermore, traits such as the size of the upper airway soft tissue structures (84), ventilatory control abnormalities (85), and respiratory responses to resistive loading during sleep may also have a genetic basis (86). Nonetheless, further work is required to determine by which physiological mechanisms genes influence the risk of sleep apnea. Moreover, which of these genetic factors will be modifiable in therapeutic studies remains unclear.
PHYSIOLOGICAL MECHANISMS UNDERLYING EPIDEMIOLOGIC RISK FACTORS
The major risk factors for OSA include aging, male sex, and obesity although the underlying mechanisms remain unclear. Given the pathophysiological mechanisms that have been discussed, these risk factors are likely to be explained by increased anatomic compromise, increased pharyngeal dilator muscle dysfunction, lowered arousal threshold, increased ventilatory control instability, and/or reduced lung volume tethering.
Obesity
Although it is clear that obesity is a key risk factor for the development of OSA and modest reductions in weight lead to improvement in OSA severity (87), the physiological mechanisms remain less than certain. Deposition of fat around the pharyngeal airway is likely to increase the collapsibility of the pharyngeal airway (88, 89). Weight loss leads to important improvements in Pcrit (90). Fat deposition around the abdomen leads to reductions in functional residual capacity (91), which would be predicted to reduce lung volume tethering effects on the upper airway. Low lung volumes are also associated with diminished oxygen stores, which would contribute to ventilatory control instability (high loop gain). Finally, obesity has been associated with functional impairment in upper airway muscles (92).
Male Sex
Similar to obesity, it is not entirely clear why OSA is more common in males than females. Imaging studies have revealed that men have increased fat deposition around the pharyngeal airway as compared with women (93) and an increased length of the pharyngeal airway as compared with women (94). Modeling studies have revealed an important role for pharyngeal length in contributing to airway collapsibility (94–97). A cross-sectional study of children at the age of puberty has suggested that boys experience lengthening of their airway during puberty compared with girls, independent of systemic growth (98). In addition, the pharyngeal airway is longer in postmenopausal as compared with premenopausal women (99). Thus, changes in pharyngeal airway length may explain some of the sex-related observations in OSA. Despite these anatomic and modeling studies, there has been no consistency in studies regarding sex differences in pharyngeal collapsibility (100–102). In addition, although initial studies suggested important differences in pharyngeal dilator muscle activation between men and women (103), several subsequent studies have failed to reproduce these results (94, 102, 104). Similarly, loop gain does not appear to be systematically different between men and women in either healthy subjects (105) or among those with OSA (100). However, other techniques for assessing ventilatory instability (such as the PCO2 apnea threshold) have been shown to differ between the sexes (106, 107). This effect may, importantly, be mediated via hormonal mechanisms (106, 108). Hormonal differences between men and women have long been proposed to contribute to the increased male prevalence in OSA and to the propensity for women to develop OSA after menopause (109, 110). Indeed, testosterone administration in hypogonadal men has been shown to induce sleep-disordered breathing in some patients (111–113). However, thus far, attempts to manipulate OSA severity on the basis of hormone-targeted approaches have revealed little benefit (114, 115). In addition to sex-related changes in the apnea threshold, the ventilatory response to arousal from sleep is greater in men than women, suggesting that men may be more prone to cyclical breathing (48, 116). Sex differences in arousal threshold and lung volume effects during sleep have not been systematically evaluated to our knowledge. However, it is conceivable that men who tend to store adipose tissue more centrally may have a greater susceptibility to lung volume–related changes in upper airway collapsibility via a loss of caudal traction compared with women. Nonetheless, the majority of the available data suggest that the male sex predisposition to OSA appears to be primarily anatomically based although other factors such as ventilatory control may also be important.
Aging
Substantial literature suggests that the frequency of apnea increases with aging, with a number of studies reporting a remarkable prevalence of sleep-disordered breathing in older individuals (3, 4). However, the increase in prevalence appears to plateau after 65 years (4) and when body mass index is controlled for, the severity appears to decrease with age (7), potentially because of survivor effects. Historically, it has been proposed that the increased prevalence of sleep apnea in the elderly may be attributed to impaired respiratory control with aging. However, data based on the PAV loop gain technique have challenged this notion (117). Although several small studies have attempted to address the cause of the age-related impact on apnea frequency, no definitive conclusions have been reached. Anatomic susceptibility to OSA appears to worsen with aging and there appears to be a preferential deposition of fat around the pharynx with aging, independent of systemic fat (99, 118). Similar to many upper airway reflexes (119), the genioglossus negative pressure reflex appears to deteriorate with aging (99, 120). Indeed, these anatomic and physiological factors both likely contribute to increased upper airway collapsibility with aging (121). Other pathophysiological factors such as arousal threshold appear to be less important in mediating the aging predisposition to OSA (121). Finally, although lung compliance is known to increase with aging, we are not aware of systematic studies that have assessed how loss of lung elastic recoil with aging affects upper airway mechanics.
OTHER FACTORS
In addition to the major epidemiologic risk factors described above that contribute to OSA pathogenesis, there are multiple other physiological variables that may impact OSA. Some of these components are described below and are illustrated schematically in Figure 2B.
REM Sleep
Hypopneas and apneas increase in duration and are associated with more pronounced hypoxemia during REM compared with non-REM sleep in OSA (122). Some patients have OSA only during REM sleep (123). Although human physiological data are challenging to obtain and the available literature is relatively scarce, REM sleep is associated with decreased upper airway muscle tone (124, 125), impaired genioglossus reflex responsiveness to negative pressure (26, 126, 127), and reduced chemosensitivity (128, 129). These factors may worsen apnea during REM sleep. However, the arousal threshold appears higher during REM sleep, which would tend to reduce, not prolong, event duration (130, 131). Further, Pcrit is similar during REM compared with non-REM sleep in patients with OSA, suggesting that upper airway anatomy is not further impaired in this sleep state (14). Thus, the precise underlying causes of increased REM-related apnea severity remain largely unknown. Further studies are required to address this important issue.
Surface Tension
Surface tension of the liquid lining of the upper airway influences pharyngeal patency and changes in surface forces may perpetuate disease severity. Indeed, lining the upper airway with surfactant before sleep reduces apnea severity and improves the Pcrit in OSA (132, 133). Patients with OSA appear to have increased surface forces acting on the upper airway despite similar salivary flow compared with healthy individuals (134). Further, the route of breathing during sleep appears to influence salivary flow and perhaps as a result may affect surface tension. Nasal breathing may reduce, and oral breathing may increase, surface tension forces (135). Thus, changes in breathing route in OSA may be one of many factors contributing to the elevated levels of surface tension observed in OSA. Although these studies highlight a role for surface tension in OSA pathogenesis, the clinical relevance of surface tension in OSA remains less certain.
Upper Airway Sensory Neuropathy/Impaired Sensory Processing
It has been proposed that the repeated mechanical trauma and/or hypoxemia associated with OSA may lead to sensory impairment of upper airway structures (136). Should this be the case, prolonged untreated OSA may perpetuate disease severity because of an impaired ability of the upper airway to respond to negative pharyngeal pressure. However, although epidemiologic data support the progression of disease severity over time, the effect appears to be modest and may be largely explained by progressive increases in body weight (137). Nonetheless, upper airway sensory function has been shown to be impaired in OSA during wakefulness (138–141). However, given the state-dependent nature of the disorder, how sensory impairment during wakefulness affects upper airway function during sleep is unclear (142). Consistent with a state-dependent phenomenon, sensory processing of respiratory afferent information as measured by cortical evoked potentials has been shown to be impaired in OSA during sleep but not during wakefulness (143, 144). Thus, adaptive compensatory mechanisms may ensure a patent airway during wakefulness in OSA despite impaired upper airway sensory function, but are less able to do so during sleep. Thus, further studies are required to elucidate the role of upper airway sensory impairment in modulating disease severity.
SUMMARY AND POSSIBLE FUTURE TREATMENTS
Some of the treatment strategies that are either known to, or theoretically may, alleviate the cycle of OSA are illustrated schematically in Figure 2B. OSA has long been recognized as a heterogeneous disorder with potentially multiple contributing pathophysiological causes, the relative contributions of which may vary considerably between patients. Major progress has been made in our understanding of the key physiological components underlying the disorder. However, despite substantial research effort, the goal of determining which patients will respond most favorably to certain treatment options ahead of time (i.e., CPAP vs. oral appliances vs. surgery) and the development of alternative treatments remains largely elusive. To achieve this goal, a clear understanding of the physiological causes of OSA on an individual patient basis may be required. As such, approaches that enable determination of the relative importance of the various physiological causes of OSA may lead to novel therapeutic treatment options (as highlighted in Figure 2B) for certain patients.
Thus, measures to stabilize ventilation (e.g., oxygen) may be particularly effective in those with unstable ventilatory control or high loop gain. Similarly, those with a low arousal threshold may benefit from sedatives, whereas some sedatives may have a deleterious effect on patients with a high arousal threshold. Finally, although agents that lead to major increases in pharyngeal dilator muscle activity are not currently available, such an agent would best be provided to those with impaired muscle responsiveness. Thus, defining various OSA phenotypes may be critical to the success of novel therapeutic approaches.
Acknowledgments
The authors thank Dr. Amy Jordan and Dr. Susie Yeh for helpful feedback on the manuscript. Dr. Sanjay Patel provided valuable advice on the section concerning the role of genetics in OSA pathogenesis.
Supported by the Thoracic Society of Australia and by a New Zealand/Allen and Hanbury's respiratory research fellowship (D.J.E.) and by NIH grants P50 HL060292-09, AG024837-01, and RO1-HL73146 (A.M.).
Conflict of Interest Statement: D.J.E. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.M. has received consulting income of less than $20,000 from Respironics, NMT Medical, Inspiration Medical Restore Medical, Cephalon, and Pfizer. He has received research funding from Respironics and Restore Medical.
References
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 2.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J 2007;29:156–178. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–1235. [DOI] [PubMed] [Google Scholar]
- 4.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004;291:2013–2016. [DOI] [PubMed] [Google Scholar]
- 5.Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med 1996;154:279–289. [DOI] [PubMed] [Google Scholar]
- 6.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 2001;163:608–613. [DOI] [PubMed] [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men. I. Prevalence and severity. Am J Respir Crit Care Med 1998;157:144–148. [DOI] [PubMed] [Google Scholar]
- 8.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med 2005;172:1363–1370. [DOI] [PubMed] [Google Scholar]
- 9.White DP. The pathogenesis of obstructive sleep apnea: advances in the past 100 years. Am J Respir Cell Mol Biol 2006;34:1–6. [DOI] [PubMed] [Google Scholar]
- 10.White DP. Sleep apnea. Proc Am Thorac Soc 2006;3:124–128. [DOI] [PubMed] [Google Scholar]
- 11.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing: significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995;152:1673–1689. [DOI] [PubMed] [Google Scholar]
- 12.Haponik EF, Smith PL, Bohlman ME, Allen RP, Goldman SM, Bleecker ER. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis 1983;127:221–226. [DOI] [PubMed] [Google Scholar]
- 13.Burger CD, Stanson AW, Sheedy PF II, Daniels BK, Shepard JW Jr. Fast-computed tomography evaluation of age-related changes in upper airway structure and function in normal men. Am Rev Respir Dis 1992;145:846–852. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 1998;157:1051–1057. [DOI] [PubMed] [Google Scholar]
- 15.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest 2007;132:325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol 1997;82:1319–1326. [DOI] [PubMed] [Google Scholar]
- 17.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 1992;89:1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med 1996;153:1880–1887. [DOI] [PubMed] [Google Scholar]
- 19.Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol 1998;85:908–920. [DOI] [PubMed] [Google Scholar]
- 20.Trinder J, Whitworth F, Kay A, Wilkin P. Respiratory instability during sleep onset. J Appl Physiol 1992;73:2462–2469. [DOI] [PubMed] [Google Scholar]
- 21.Basner RC, Ringler J, Schwartzstein RM, Weinberger SE, Weiss JW. Phasic electromyographic activity of the genioglossus increases in normals during slow-wave sleep. Respir Physiol 1991;83:189–200. [DOI] [PubMed] [Google Scholar]
- 22.Pillar G, Fogel RB, Malhotra A, Beauregard J, Edwards JK, Shea SA, White DP. Genioglossal inspiratory activation: central respiratory vs mechanoreceptive influences. Respir Physiol 2001;127:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol 1991;436:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horner RL, Innes JA, Morrell MJ, Shea SA, Guz A. The effect of sleep on reflex genioglossus muscle activation by stimuli of negative airway pressure in humans. J Physiol 1994;476:141–151. [PMC free article] [PubMed] [Google Scholar]
- 25.Wheatley JR, Mezzanotte WS, Tangel DJ, White DP. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis 1993;148:597–605. [DOI] [PubMed] [Google Scholar]
- 26.Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol 2007;581:1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra A, Trinder J, Fogel R, Stanchina M, Patel SR, Schory K, Kleverlaan D, White DP. Postural effects on pharyngeal protective reflex mechanisms. Sleep 2004;27:1105–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chamberlin NL, Eikermann M, Fassbender P, White DP, Malhotra A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol 2007;579:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horner RL. Respiratory motor activity: influence of neuromodulators and implications for sleep disordered breathing. Can J Physiol Pharmacol 2007;85:155–165. [DOI] [PubMed] [Google Scholar]
- 30.Rukhadze I, Kubin L. Mesopontine cholinergic projections to the hypoglossal motor nucleus. Neurosci Lett 2007;413:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stanchina ML, Malhotra A, Fogel RB, Ayas N, Edwards JK, Schory K, White DP. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med 2002;165:945–949. [DOI] [PubMed] [Google Scholar]
- 32.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 2007;62:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RC, Schory K, Dover L, Fogel RB, White DP. Genioglossal muscle response to CO2 stimulation during NREM sleep. Sleep 2006;29:470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol 2007;97:933–936. [DOI] [PubMed] [Google Scholar]
- 35.Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 2006;95:2213–2221. [DOI] [PubMed] [Google Scholar]
- 36.Puhan MA, Suarez A, Lo Cascio C, Zahn A, Heitz M, Braendli O. Didgeridoo playing as alternative treatment for obstructive sleep apnoea syndrome: randomised controlled trial. BMJ 2006;332:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randerath WJ, Galetke W, Domanski U, Weitkunat R, Ruhle KH. Tongue-muscle training by intraoral electrical neurostimulation in patients with obstructive sleep apnea. Sleep 2004;27:254–259. [DOI] [PubMed] [Google Scholar]
- 38.Phillipson EA, Sullivan CE. Arousal: the forgotten response to respiratory stimuli. Am Rev Respir Dis 1978;118:807–809. [DOI] [PubMed] [Google Scholar]
- 39.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978;44:931–938. [DOI] [PubMed] [Google Scholar]
- 40.Nigro CA, Rhodius EE. Variation in the duration of arousal in obstructive sleep apnea. Med Sci Monit 2005;11:CR188–CR192. [PubMed] [Google Scholar]
- 41.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004;169:623–633. [DOI] [PubMed] [Google Scholar]
- 42.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep 2007;30:478–488. [DOI] [PubMed] [Google Scholar]
- 43.Dyken ME, Yamada T, Glenn CL, Berger HA. Obstructive sleep apnea associated with cerebral hypoxemia and death. Neurology 2004;62:491–493. [DOI] [PubMed] [Google Scholar]
- 44.Vincken W, Guilleminault C, Silvestri L, Cosio M, Grassino A. Inspiratory muscle activity as a trigger causing the airways to open in obstructive sleep apnea. Am Rev Respir Dis 1987;135:372–377. [DOI] [PubMed] [Google Scholar]
- 45.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis 1990;142:295–300. [DOI] [PubMed] [Google Scholar]
- 46.Berry RB, Kouchi KG, Der DE, Dickel MJ, Light RW. Sleep apnea impairs the arousal response to airway occlusion. Chest 1996;109:1490–1496. [DOI] [PubMed] [Google Scholar]
- 47.Haba-Rubio J, Sforza E, Weiss T, Schroder C, Krieger J. Effect of CPAP treatment on inspiratory arousal threshold during NREM sleep in OSAS. Sleep Breath 2005;9:12–19. [DOI] [PubMed] [Google Scholar]
- 48.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med 2003;168:1512–1519. [DOI] [PubMed] [Google Scholar]
- 49.Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: pathophysiology and treatment. Chest 2007;131:595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol 1982;53:644–659. [DOI] [PubMed] [Google Scholar]
- 51.Stanchina ML, Ellison K, Malhotra A, Anderson M, Kirk M, Benser ME, Tosi C, Carlisle C, Millman RP, Buxton A. The impact of cardiac resynchronization therapy on obstructive sleep apnea in heart failure patients: a pilot study. Chest 2007;132:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malhotra A, Jordan AS. Did fat boy Joe need hormone replacement? Sleep 2006;29:16–18. [PubMed] [Google Scholar]
- 53.Younes M. Proportional assist ventilation, a new approach to ventilatory support: theory. Am Rev Respir Dis 1992;145:114–120. [DOI] [PubMed] [Google Scholar]
- 54.Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol 1998;85:1929–1940. [DOI] [PubMed] [Google Scholar]
- 55.Wellman A, Jordan AS, Malhotra A, Fogel RB, Katz ES, Schory K, Edwards JK, White DP. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:1225–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med 2001;163:1181–1190. [DOI] [PubMed] [Google Scholar]
- 57.Series F, Cormier Y, Desmeules M. Influence of passive changes of lung volume on upper airways. J Appl Physiol 1990;68:2159–2164. [DOI] [PubMed] [Google Scholar]
- 58.Series F, Marc I. Influence of lung volume dependence of upper airway resistance during continuous negative airway pressure. J Appl Physiol 1994;77:840–844. [DOI] [PubMed] [Google Scholar]
- 59.Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis 1990;141:854–860. [DOI] [PubMed] [Google Scholar]
- 60.Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep 2003;26:851–856. [DOI] [PubMed] [Google Scholar]
- 61.Burger CD, Stanson AW, Daniels BK, Sheedy PF II, Shepard JW Jr. Fast-CT evaluation of the effect of lung volume on upper airway size and function in normal men. Am Rev Respir Dis 1992;146:335–339. [DOI] [PubMed] [Google Scholar]
- 62.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis 1984;130:175–178. [DOI] [PubMed] [Google Scholar]
- 63.Rubinstein I, Hoffstein V, Bradley TD. Lung volume–related changes in the pharyngeal area of obese females with and without obstructive sleep apnoea. Eur Respir J 1989;2:344–351. [PubMed] [Google Scholar]
- 64.Hudgel DW, Devadatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol 1984;57:1319–1322. [DOI] [PubMed] [Google Scholar]
- 65.Kay A, Trinder J, Kim Y. Progressive changes in airway resistance during sleep. J Appl Physiol 1996;81:282–292. [DOI] [PubMed] [Google Scholar]
- 66.Heinzer RC, Stanchina ML, Malhotra A, Fogel RB, Patel SR, Jordan AS, Schory K, White DP. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med 2005;172:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo YL, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax 2006;61:435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol 1988;65:2124–2131. [DOI] [PubMed] [Google Scholar]
- 69.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol 1991;70:1328–1336. [DOI] [PubMed] [Google Scholar]
- 70.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep 2007;30:179–186. [DOI] [PubMed] [Google Scholar]
- 71.Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol 2007;103:1379–1385. [DOI] [PubMed] [Google Scholar]
- 72.Strohl KP, Saunders NA, Feldman NT, Hallett M. Obstructive sleep apnea in family members. N Engl J Med 1978;299:969–973. [DOI] [PubMed] [Google Scholar]
- 73.Mathur R, Douglas NJ. Family studies in patients with the sleep apnea–hypopnea syndrome. Ann Intern Med 1995;122:174–178. [DOI] [PubMed] [Google Scholar]
- 74.Pillar G, Lavie P. Assessment of the role of inheritance in sleep apnea syndrome. Am J Respir Crit Care Med 1995;151:688–691. [DOI] [PubMed] [Google Scholar]
- 75.Redline S, Tishler PV, Tosteson TD, Williamson J, Kump K, Browner I, Ferrette V, Krejci P. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med 1995;151:682–687. [DOI] [PubMed] [Google Scholar]
- 76.Larkin EK, Patel SR, Redline S, Mignot E, Elston RC, Hallmayer J. Apolipoprotein E and obstructive sleep apnea: evaluating whether a candidate gene explains a linkage peak. Genet Epidemiol 2006;30:101–110. [DOI] [PubMed] [Google Scholar]
- 77.Palmer LJ, Buxbaum SG, Larkin E, Patel SR, Elston RC, Tishler PV, Redline S. A whole-genome scan for obstructive sleep apnea and obesity. Am J Hum Genet 2003;72:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palmer LJ, Buxbaum SG, Larkin EK, Patel SR, Elston RC, Tishler PV, Redline S. Whole genome scan for obstructive sleep apnea and obesity in African-American families. Am J Respir Crit Care Med 2004;169:1314–1321. [DOI] [PubMed] [Google Scholar]
- 79.Polotsky VY, O'Donnell CP. Genomics of sleep-disordered breathing. Proc Am Thorac Soc 2007;4:121–126. [DOI] [PubMed] [Google Scholar]
- 80.Drazen JM, Phimister EG. Publishing genomewide association studies. N Engl J Med 2007;357:496. [Google Scholar]
- 81.Patel SR. Shared genetic risk factors for obstructive sleep apnea and obesity. J Appl Physiol 2005;99:1600–1606. [DOI] [PubMed] [Google Scholar]
- 82.Riha RL, Brander P, Vennelle M, Douglas NJ. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep 2005;28:315–320. [PubMed] [Google Scholar]
- 83.Guilleminault C, Partinen M, Hollman K, Powell N, Stoohs R. Familial aggregates in obstructive sleep apnea syndrome. Chest 1995;107:1545–1551. [DOI] [PubMed] [Google Scholar]
- 84.Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med 2006;173:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Redline S, Leitner J, Arnold J, Tishler PV, Altose MD. Ventilatory-control abnormalities in familial sleep apnea. Am J Respir Crit Care Med 1997;156:155–160. [DOI] [PubMed] [Google Scholar]
- 86.Pillar G, Schnall RP, Peled N, Oliven A, Lavie P. Impaired respiratory response to resistive loading during sleep in healthy offspring of patients with obstructive sleep apnea. Am J Respir Crit Care Med 1997;155:1602–1608. [DOI] [PubMed] [Google Scholar]
- 87.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015–3021. [DOI] [PubMed] [Google Scholar]
- 88.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis 1993;148:462–466. [DOI] [PubMed] [Google Scholar]
- 89.Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burman ED, Longmore DB, Guz A. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J 1989;2:613–622. [PubMed] [Google Scholar]
- 90.Schwartz AR, Gold AR, Schubert N, Stryzak A, Wise RA, Permutt S, Smith PL. Effect of weight loss on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis 1991;144:494–498. [DOI] [PubMed] [Google Scholar]
- 91.Ray CS, Sue DY, Bray G, Hansen JE, Wasserman K. Effects of obesity on respiratory function. Am Rev Respir Dis 1983;128:501–506. [DOI] [PubMed] [Google Scholar]
- 92.Carrera M, Barbe F, Sauleda J, Tomas M, Gomez C, Santos C, Agusti AG. Effects of obesity upon genioglossus structure and function in obstructive sleep apnoea. Eur Respir J 2004;23:425–429. [DOI] [PubMed] [Google Scholar]
- 93.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax 1999;54:323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med 2002;166:1388–1395. [DOI] [PubMed] [Google Scholar]
- 95.Huang Y, Malhotra A, White DP. Computational simulation of human upper airway collapse using a pressure-/state-dependent model of genioglossal muscle contraction under laminar flow conditions. J Appl Physiol 2005;99:1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang Y, White DP, Malhotra A. The impact of anatomic manipulations on pharyngeal collapse: results from a computational model of the normal human upper airway. Chest 2005;128:1324–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang Y, White DP, Malhotra A. Use of computational modeling to predict responses to upper airway surgery in obstructive sleep apnea. Laryngoscope 2007;117:648–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ronen O, Malhotra A, Pillar G. The influence of gender and age on upper airway length during development. Pediatrics 2007;120:e1028–e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malhotra A, Huang Y, Fogel R, Lazic S, Pillar G, Jakab M, Kikinis R, White DP. Aging influences on pharyngeal anatomy and physiology: the predisposition to pharyngeal collapse. Am J Med 2006;119:72.e9–72.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jordan AS, Wellman A, Edwards JK, Schory K, Dover L, MacDonald M, Patel SR, Fogel RB, Malhotra A, White DP. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol 2005;99:2020–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rowley JA, Zhou X, Vergine I, Shkoukani MA, Badr MS. Influence of gender on upper airway mechanics: upper airway resistance and Pcrit. J Appl Physiol 2001;91:2248–2254. [DOI] [PubMed] [Google Scholar]
- 102.Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med 2000;162:1627–1632. [DOI] [PubMed] [Google Scholar]
- 103.Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med 1995;152:725–731. [DOI] [PubMed] [Google Scholar]
- 104.Jordan AS, Catcheside PG, O'Donoghue FJ, Saunders NA, McEvoy RD. Selected contribution: genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol 2002;92:410–417. [DOI] [PubMed] [Google Scholar]
- 105.Wellman A, Malhotra A, Fogel RB, Edwards JK, Schory K, White DP. Respiratory system loop gain in normal men and women measured with proportional-assist ventilation. J Appl Physiol 2003;94:205–212. [DOI] [PubMed] [Google Scholar]
- 106.Rowley JA, Zhou XS, Diamond MP, Badr MS. The determinants of the apnea threshold during NREM sleep in normal subjects. Sleep 2006;29:95–103. [DOI] [PubMed] [Google Scholar]
- 107.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol 2000;89:192–199. [DOI] [PubMed] [Google Scholar]
- 108.Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol 2003;94:101–107. [DOI] [PubMed] [Google Scholar]
- 109.Block AJ, Wynne JW, Boysen PG. Sleep-disordered breathing and nocturnal oxygen desaturation in postmenopausal women. Am J Med 1980;69:75–79. [DOI] [PubMed] [Google Scholar]
- 110.Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab 2001;86:517–520. [DOI] [PubMed] [Google Scholar]
- 111.Schneider BK, Pickett CK, Zwillich CW, Weil JV, McDermott MT, Santen RJ, Varano LA, White DP. Influence of testosterone on breathing during sleep. J Appl Physiol 1986;61:618–623. [DOI] [PubMed] [Google Scholar]
- 112.White DP, Schneider BK, Santen RJ, McDermott M, Pickett CK, Zwillich CW, Weil JV. Influence of testosterone on ventilation and chemosensitivity in male subjects. J Appl Physiol 1985;59:1452–1457. [DOI] [PubMed] [Google Scholar]
- 113.Matsumoto AM, Sandblom RE, Schoene RB, Lee KA, Giblin EC, Pierson DJ, Bremner WJ. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clinic Endocrinol 1985;22:713–721. [DOI] [PubMed] [Google Scholar]
- 114.Cistulli PA, Barnes DJ, Grunstein RR, Sullivan CE. Effect of short-term hormone replacement in the treatment of obstructive sleep apnoea in postmenopausal women. Thorax 1994;49:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stewart DA, Grunstein RR, Berthon-Jones M, Handelsman DJ, Sullivan CE. Androgen blockade does not affect sleep-disordered breathing or chemosensitivity in men with obstructive sleep apnea. Am Rev Respir Dis 1992;146:1389–1393. [DOI] [PubMed] [Google Scholar]
- 116.Jordan AS, McEvoy RD, Edwards JK, Schory K, Yang CK, Catcheside PG, Fogel RB, Malhotra A, White DP. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol 2004;558:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wellman A, Malhotra A, Jordan AS, Schory K, Gautam S, White DP. Chemical control stability in the elderly. J Physiol 2007;581:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J 1997;10:2087–2090. [DOI] [PubMed] [Google Scholar]
- 119.Erskine RJ, Murphy PJ, Langton JA, Smith G. Effect of age on the sensitivity of upper airway reflexes. Br J Anaesth 1993;70:574–575. [DOI] [PubMed] [Google Scholar]
- 120.Marcus CL, Fernandes Do Prado LB, Lutz J, Katz ES, Black CA, Galster P, Carson KA. Developmental changes in upper airway dynamics. J Appl Physiol 2004;97:98–108. [DOI] [PubMed] [Google Scholar]
- 121.Eikermann M, Jordan AS, Chamberlin NL, Gautam S, Wellman A, Lo YL, White DP, Malhotra A. The influence of aging on pharyngeal collapsibility during sleep. Chest 2007;131:1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Findley LJ, Wilhoit SC, Suratt PM. Apnea duration and hypoxemia during rem sleep in patients with obstructive sleep apnea. Chest 1985;87:432–436. [DOI] [PubMed] [Google Scholar]
- 123.Kass JE, Akers SM, Bartter TC, Pratter MR. Rapid-eye-movement-specific sleep-disordered breathing: a possible cause of excessive daytime sleepiness. Am J Respir Crit Care Med 1996;154:167–169. [DOI] [PubMed] [Google Scholar]
- 124.Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol 1976;51:160–170. [DOI] [PubMed] [Google Scholar]
- 125.Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol 1991;71:488–497. [DOI] [PubMed] [Google Scholar]
- 126.Shea SA, Edwards JK, White DP. Effect of wake–sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol 1999;520:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Issa FG, Edwards P, Szeto E, Lauff D, Sullivan C. Genioglossus and breathing responses to airway occlusion: effect of sleep and route of occlusion. J Appl Physiol 1988;64:543–549. [DOI] [PubMed] [Google Scholar]
- 128.Douglas NJ, White DP, Weil JV, Pickett CK, Martin RJ, Hudgel DW, Zwillich CW. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis 1982;125:286–289. [DOI] [PubMed] [Google Scholar]
- 129.White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis 1982;126:530–533. [DOI] [PubMed] [Google Scholar]
- 130.Gugger M, Bogershausen S, Schaffler L. Arousal responses to added inspiratory resistance during REM and non-REM sleep in normal subjects. Thorax 1993;48:125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Issa FG, Sullivan CE. Arousal and breathing responses to airway occlusion in healthy sleeping adults. J Appl Physiol 1983;55:1113–1119. [DOI] [PubMed] [Google Scholar]
- 132.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Relationship between surface tension of upper airway lining liquid and upper airway collapsibility during sleep in obstructive sleep apnea hypopnea syndrome. J Appl Physiol 2003;95:1761–1766. [DOI] [PubMed] [Google Scholar]
- 133.Jokic R, Klimaszewski A, Mink J, Fitzpatrick MF. Surface tension forces in sleep apnea: the role of a soft tissue lubricant: a randomized double-blind, placebo-controlled trial. Am J Respir Crit Care Med 1998;157:1522–1525. [DOI] [PubMed] [Google Scholar]
- 134.Kirkness JP, Madronio M, Stavrinou R, Wheatley JR, Amis TC. Surface tension of upper airway mucosal lining liquid in obstructive sleep apnea/hypopnea syndrome. Sleep 2005;28:457–463. [DOI] [PubMed] [Google Scholar]
- 135.Verma M, Seto-Poon M, Wheatley JR, Amis TC, Kirkness JP. Influence of breathing route on upper airway lining liquid surface tension in humans. J Physiol 2006;574:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petrof BJ, Hendricks JC, Pack AI. Does upper airway muscle injury trigger a vicious cycle in obstructive sleep apnea? A hypothesis. Sleep 1996;19:465–471. [DOI] [PubMed] [Google Scholar]
- 137.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 2002;165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 138.Kimoff RJ, Sforza E, Champagne V, Ofiara L, Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med 2001;164:250–255. [DOI] [PubMed] [Google Scholar]
- 139.Nguyen AT, Jobin V, Payne R, Beauregard J, Naor N, Kimoff RJ. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep 2005;28:585–593. [DOI] [PubMed] [Google Scholar]
- 140.Boyd JH, Petrof BJ, Hamid Q, Fraser R, Kimoff RJ. Upper airway muscle inflammation and denervation changes in obstructive sleep apnea. Am J Respir Crit Care Med 2004;170:541–546. [DOI] [PubMed] [Google Scholar]
- 141.Akay M, Leiter JC, Daubenspeck JA. Reduced respiratory-related evoked activity in subjects with obstructive sleep apnea syndrome. J Appl Physiol 2003;94:429–438. [DOI] [PubMed] [Google Scholar]
- 142.Berry RB, White DP, Roper J, Pillar G, Fogel RB, Stanchina M, Malhotra A. Awake negative pressure reflex response of the genioglossus in OSA patients and normal subjects. J Appl Physiol 2003;94:1875–1882. [DOI] [PubMed] [Google Scholar]
- 143.Afifi L, Guilleminault C, Colrain IM. Sleep and respiratory stimulus specific dampening of cortical responsiveness in OSAS. Respir Physiolo Neurobiol 2003;136:221–234. [DOI] [PubMed] [Google Scholar]
- 144.Gora J, Trinder J, Pierce R, Colrain IM. Evidence of a sleep-specific blunted cortical response to inspiratory occlusions in mild obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2002;166:1225–1234. [DOI] [PubMed] [Google Scholar]