Abstract
Objectives
To identify macrophage-rich atherosclerotic plaque non-invasively by the combined use of systemic administration of superparamagnetic nanoparticles with magnetic resonance imaging (MRI), using a positive contrast off-resonance imaging sequence (Inversion Recovery with ON-resonant water suppression: IRON).
Background
The sudden rupture of macrophage-rich atherosclerotic plaques can trigger the formation of an occlusive thrombus in coronary vessels, resulting in acute myocardial infarction. Therefore, a noninvasive technique that can identify macrophage-rich plaques and thereby assist with risk stratification of patients with atherosclerosis would be of great potential clinical utility.
Methods
Experiments were conducted on a clinical 3T MRI scanner in seven heritable hyperlipidemic and four control rabbits. Monocrystalline iron-oxide nanoparticles (MION)-47 were administrated intravenously (two doses of 250μmol Fe/kg), and animals underwent serial IRON-MRI before injection of the nanoparticles and serially after 1, 3 and 6 days.
Results
After administration of MION-47, a striking signal enhancement was found in areas of plaque only in hyperlipidemic rabbits. The magnitude of enhancement on MR-images had a high correlation with the number of macrophages determined by histology (p<0.001) and allowed for the detection of macrophage-rich plaque with high accuracy (AUC=0.92, SE=0.04, 95% CI=0.84-0.96, p<0.001). No significant signal enhancement was measured in remote areas without plaque by histology and in controls without atherosclerosis.
Conclusion
IRON-MRI in conjunction with superparamagnetic nanoparticles is a promising approach for the noninvasive evaluation of macrophage-rich, vulnerable plaques.
Keywords: atherosclerosis, vulnerable plaque, superparamagnetic nanoparticles, molecular imaging, Inversion Recovery with ON-resonant water suppression (IRON), positive contrast, magnetic resonance imaging
Introduction
Atherosclerosis is the underlying cause of most cardiovascular diseases. Despite major advances in the treatment of coronary artery disease, it remains the leading cause of death in Western societies and the predominant underlying cause of sudden cardiac death(1,2). The role of inflammation in all stages of atherosclerosis is now widely appreciated(3).
Currently, coronary angiography is the clinical gold standard for diagnosing the presence and extent of coronary artery disease. However, this technique is invasive and provides limited information on the presence of inflammation within the vessel wall and future cardiac events(4). Therefore, a noninvasive technique that can identify macrophage-rich plaques and thereby assist with risk stratification of patients with coronary artery disease would be of great potential clinical utility. Several recent molecular imaging strategies have been proposed to detect inflamed atherosclerotic plaque non-invasively, using targeted gadolinium agents(5,6) and superparamagnetic nanoparticles(7-11). The appeal of superparamagnetic nanoparticles lies in the fact that these agents have completed phase-3 clinical trials and thus currently have the greatest potential for clinical translation(12). In previous studies superparamagnetic nanoparticles resulted in local signal loss in areas of plaque. Concerns have been raised, however, that signal loss may also arise from other sources, such as motion, absence of tissue, ‘fibrous cap’ or calcification(8,13,14).
Recently, new MRI methods were reported that create positive signal in areas of superparamagnetic materials(15-18). The purpose of our study was to test whether macrophage-rich plaque can be visualized with positive signal using Inversion Recovery with ON-resonant water suppression (IRON)-MRI(18), and whether the degree of signal enhancement is related to the number of macrophages in atherosclerotic plaque. Experiments were conducted in Watanabe heritable hyperlipidemic and in New Zealand White rabbits as controls on a clinical 3T scanner.
Materials and Methods
Animals
Studies were approved by the Institutional Animal Care and Use Committee. Experiments were conducted in 7 mature male heritable hyperlipidemic Watanabe rabbits (14.9±1.3 months old, 3.0±0.3kg body-weight, Brown Family Enterprises, Odenville, AL). At this age Watanabe rabbits exhibit active plaque formation within their aortic wall(7). Further progression of atherosclerotic lesions was induced by high-cholesterol diet (1% cholesterol, Dyets Inc., Bethlehem, PA) for 6 weeks(19). After 6 weeks of high-cholesterol diet, blood samples were taken from the ear vein to measure total serum cholesterol levels. Four male New Zealand White rabbits (6.6±0.5 months old, 2.9±0.4kg body-weight, Myrtle’s Rabbitry, Inc. Thompson Station, TN) served as negative controls for atherosclerosis.
Superparamagnetic nanoparticles
Monocrystalline iron-oxide nanoparticle (MION)-47 (Center for Molecular Imaging Research, Harvard Medical School, Charlestown, MA) is a stable colloid, which shortens the longitudinal (T1) and transverse (T2)-relaxation times of tissue(20,21). MION-47 consists of a central monocrystalline magnetite-like single crystal core coated by multiple 10kD dextran molecules. The mean size of the nanoparticles is 27.5±6.8nm, and the R1-and R2-relaxivities are 25.5mM-1s-1 and 53.7mM-1s-1 respectively, in an aqueous solution at 37° and 0.47T. The plasma half-life time of MION-47 is 11.4±0.6h in mice(21). MION-47 is a laboratory preparation developed by Drs. Josephson and Weissleder, which has similar size, relaxivity and biological properties as ferumoxtran-10, which is an FDA compliant clinical preparation(12).
Experimental Design
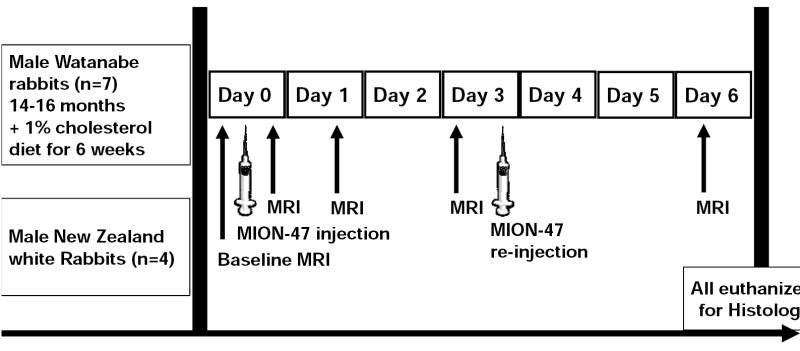
The experiments included MRI before and after the administration of MION-47. Animals were imaged before and after the injection of 250μmol Fe/kg on ‘Day 0’. Imaging was repeated on ‘Day 1’, on ‘Day 3’ and on ‘Day 6’. Based on quantitative T1-measurements of blood performed in preliminary experiments (T1 of arterial blood of 37±6ms within 2 hours after injection, 125±32ms on ‘Day 1’, 1629±75 on ‘Day 3’ and 1652±58ms at baseline), we observed that the T1-value of blood on ‘Day 3’ approached the T1-value of blood at baseline, which indicated complete clearance of the agent from blood pool at this time point. To maximize the time of exposure of the macrophages to the iron particles, a re-injection of 250μmol Fe/kg was performed on Day 3 and animals were imaged again on ‘Day 6’, and were subsequently sacrificed with an overdose of thiopental (figure 1). All rabbits that were followed on this protocol survived until ‘Day 6’, and showed no clinical signs of respiratory or cardiac failure during the studies.
Figure 1. Experimental protocol.
Experimental protocol illustrating the serial MR-imaging and the contrast agent injections performed in Watanabe and controls.
Magnetic Resonance Imaging
Animals were imaged under general anesthesia in a Philips 3T Achieva system (Philips Medical Systems, Best, The Netherlands), and using a 4-element human carotid receiver coil (Pathway MRI Inc, Seattle, WA). A standardized protocol was followed, aiming at the visualization of the aortic wall with high spatial resolution:
T1-weighted conventional magnetic resonance angiography (MRA). Coronal 3D-gradient-echo images were obtained using the following sequence parameters: repetition-time/echo-time (TR/TE)=25/2.7ms, 20° flip-angle (FA), 200×100mm2 field-of-view (FOV) and a 0.5×0.5×1mm3 voxel size. Fifty sagittal (thoracic aorta) and fifty coronal (abdominal aorta) slices were acquired.
Black-Blood Turbo Field-Echo-Imaging (BB-TFE). For BB-TFE, a dual-inversion pre-pulse with an inversion delay of 370ms was used for blood-signal nulling. Typical imaging parameters were: TR/TE=21/10ms, 30° FA, 100×100mm2 FOV and a 0.35×0.35×2mm3 voxel size.
Black-Blood Turbo Spin-Echo-Imaging (BB-TSE). For BB-TSE, blood-signal nulling was obtained as mentioned above and typical imaging parameters were: TR/TE=857/8.2ms, 90° FA, 100×100mm2 FOV and a 0.35×0.35×2mm3 voxel size. Seven coronal and twenty axial slices were acquired to include the abdominal aorta and seven sagittal and twenty axial slices were acquired for the thoracic aorta.
Inversion Recovery with ON-resonant water suppression (IRON). The concept of IRON-imaging comprises the use of a spectrally selective saturation pre-pulse on-resonance with the bandwidth of BWIRON to suppress the signal originating from on-resonant protons(18). This saturation pulse does not affect off-resonant protons in close proximity to the superparamagnetic nanoparticles. Therefore, signal enhancement adjacent to these particles can be generated, while the on-resonant background appears signal attenuated. For IRON-imaging, an on-resonant IRON-pre-pulse with a BWIRON of 100Hz and a flip angle of 100° was used. This IRON-pre-pulse was followed by a frequency selective pre-pulse for fat saturation and was combined with TFE and TSE imaging sequences.
Image analysis
Aortic wall thickness. The thickness of the aortic wall was measured on pre-contrast BB-TFE images using the Deriche edge detection, as previously shown(22).
- Enhancement of the aortic lumen on conventional and on IRON-MRA. For quantification of signal-to-noise-ratio (SNR) and contrast-to-noise-ratio (CNR), regions-of-interest (ROI) were placed manually in the aortic lumen to measure the mean blood signal (SBlood). Signal intensity of adjacent muscle (SMuscle) was measured by choosing a ROI of similar size in muscle adjacent to the aorta. ROI were also placed in the air outside of the rabbit to measure the standard deviation of the background signal (σBackground), and CNR was calculated as follows:
[1] - Enhancement of the aortic wall on IRON-images. To quantify signal enhancement in the aortic wall, signal intensity measurements were performed as follows. ROI were manually positioned in the vessel wall and the vessel lumen in 5mm intervals extending from the aortic root to the iliac bifurcation to measure the vessel wall signal SWall and mean blood signal SBlood, respectively. To allow for exact matching between the 4 imaging sessions for each rabbit, anatomical landmarks (the position of the aortic arch, renal arteries and iliac bifurcation) were used to guide ROI positioning. For each matched slice, the normalized enhancement ratio (NER) of the aortic wall (5) was calculated by dividing the post-contrast SNR of the vessel wall by baseline SNR as follows:
[2]
With this approach, the baseline measurements of each animal serve as their own internal reference. Vessel wall enhancement will be indicated by a ratio of NER>1. To investigate the contribution of partial volume effects on positive signal detected by IRON in areas of plaque, the mean NER on ‘Day 6’ was compared between areas with plaque (confirmed by histology) in Watanabe rabbits and in areas without plaque in controls, which were matched for wall thickness (range 0.4-0.6mm). To achieve matched wall thicknesses between Watanabe and control rabbits, different parts of the aortic wall were compared (abdominal wall with plaque in Watanabe versus thoracic aortic wall without plaque in control rabbits).
Post-mortem analysis
Euthanasia was performed after the final imaging session on ‘Day 6’. Subsequently, in vivo perfusion fixation was performed and the entire aorta from the aortic root to below the iliac bifurcation was harvested. To account for in vitro tissue shrinkage of the aorta, which may confound the matching of histologic slides and MR-images, the aorta was aligned to a hard copy of a 3D-multi-planar reformatted MRA, and was then cut at 5mm intervals. Co-registration was performed carefully, considering anatomical landmarks. The specimens were then frozen, and serial prepared cryosections (10μm thick) were obtained. Sections of the aorta were stained with: 1) oil Red O and counterstained with hematoxylin for specific lipid staining, 2) fast nuclear red for determination of morphology and for measurement of wall thickness, 3) acid phosphatase (Sigma-Aldrich Corp. St. Louis, MO) to detect phagocytic cells and counterstained with Prussian blue to detect superparamagnetic nanoparticles(7,13), and 4) RAM-11 (Dako Corp., Carpinteria, CA), a marker of the rabbit macrophage cytoplasm. These sections were counterstained with 4′,6-diamidino-2-phenylindole-dihydrochloride reagent (DAPI) and were processed for immunofluorescence.
For quantitative analysis, the area of acid phosphatase positive red cells was determined by manual contouring and was related to the total vessel wall area on the same histology slide. By this approach a measure for macrophage ‘density’ was assessed on histology slides, which was then related to the magnitude of signal enhancement in corresponding slices on IRON-images. A cut-off value of macrophage density >5% was selected in order to differentiate between macrophage-rich plaque and areas with plaque but with a low density of macrophages. Quantitative analysis of histological specimen was performed using Image J (NIH) software. For the determination of intramacrophage iron-oxide uptake, a quantitative Ferrozine-based spectrophotometric assay was performed(23).
Statistical analysis
Statistical analysis was performed using Stata 9.2 (StataCorp, Collage Station, TX). Data are presented as mean±one standard deviation. Differences in aortic wall thickness and differences in iron-oxide uptake between Watanabe and control rabbits were compared using repeated measures regression analysis and unpaired, heteroscedastic, t-tests. In order to correct for the lack of independence of our data, due to repeated observations on rabbits, a clustered regression approach was used to compare differences in NER between different time-points and between different groups of rabbits, taking into account the repeated measures performed in rabbits. Furthermore, receiver operating characteristics (ROC) were used and a cut-off value was selected for NER, to provide an optimal trade-off between sensitivity and specificity for the detection of macrophage-rich plaque in matched slices on histology. Concordance analysis using the Lin’s concordance correlation coefficient(24) was used for the comparison of vessel wall diameter between MRI and histology. Because this approach does not accommodate clustering considerations we refined a bootstrapping approach for this aspect of our analysis. Differences were considered statistically significant at p<0.05.
Results
Cholesterol levels and characterization of the atherosclerotic lesions
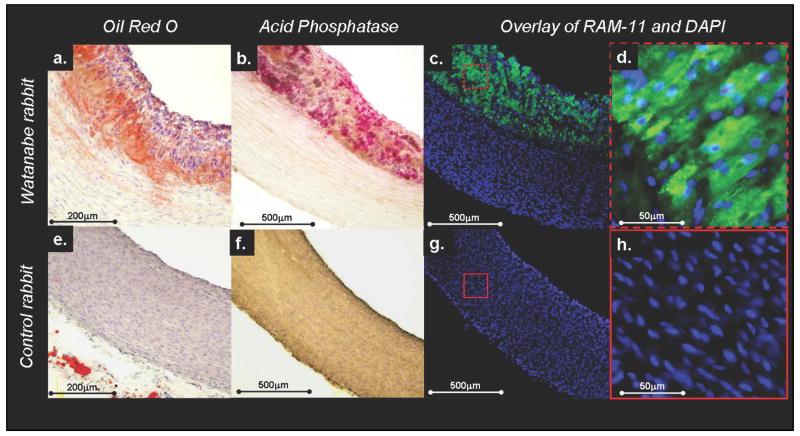
For the Watanabe rabbits, the serum cholesterol was 2119±243 mg/dl after 6 weeks of high cholesterol diet. The animals exhibited lipid-rich (figure 2a) and macrophage-rich (figure 2b) atherosclerotic plaque formation. The plethora of macrophages in the atherosclerotic plaques was confirmed by RAM-11 immunostraining (figure 2c-d). As expected, no plaque and no macrophages were found in controls (figure 2e-h).
Figure 2. Histology of hyperlipidemic versus control rabbits.
Watanabe rabbits exhibited lipid-rich plaque formation (a) with high density of macrophages (acid phosphatase staining in b). The high density of macrophages in the atherosclerotic plaque could be confirmed by RAM-11 immunostraining (macrophages stained green by RAM-11 and cell nuclei stained blue by DAPI, c-d). No plaque formation was observed in controls (e-h).
Aortic wall thickness
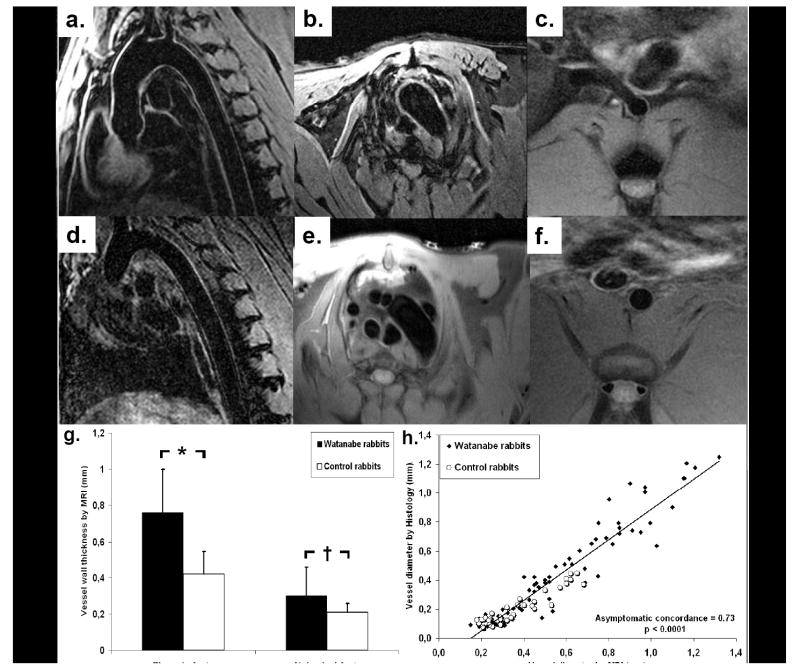
Figure 3 illustrates representative baseline images of the thoracic and the abdominal aortic wall of Watanabe (figure 3a-c) and of controls (figure 3d-f). Watanabe rabbits exhibited increased thickness both in the thoracic (0.76±0.24mm versus 0.42±0.13mm, p<0.001) and in the abdominal aortic wall (0.30±0.16 versus 0.21±0.05, p<0.01, figure 3g) compared to controls. Wall thickness, measured on MR-images, correlated closely with that measured by histology (asymptotic concordance of 0.73, p<0.0001, figure 3h).
Figure 3. Increased wall thickness in hyperlipidemic rabbits.
Representative BB-TFE (a, b and d) and BB-TSE baseline images (c, e and f) of the aortic wall in Watanabe (a-c) and control rabbits (d-f). Watanabe rabbits exhibited increased wall thickness in the thoracic and abdominal aorta (g) compared to controls. Wall thickness measured on MRI correlated closely with histology, on matched slices (h), (asymptotic concordance of 0.73, p<0.0001).
Detection of superparamagnetic nanoparticles in the aortic lumen
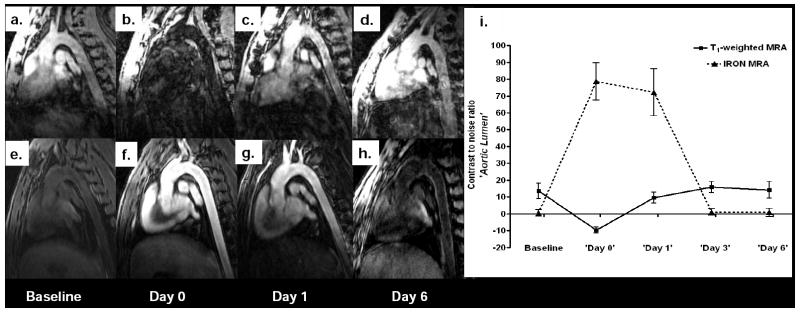
Immediately after MION-47 injection (‘Day 0’), intravascular signal decreased on conventional T1-weighted MRA, reflecting T2*-shortening of the blood-pool. Signal intensity returned on ‘Day 1’ and approached baseline on ‘Day 3’ and on ‘Day 6’ (figure 4a-d and i). Using IRON, superparamagnetic nanoparticles contributed to strong intravascular off-resonance enhancement in the lumen of the aorta in both control and Watanabe rabbits. The intra-luminal signal remained high on ‘Day 1’, and approached baseline on ‘Day 3’ and on ‘Day 6’, allowing for better definition of the aortic wall (figure 4e-i).
Figure 4. Intravascular off-resonance enhancement after MION-47 administration.
Conventional T1-weighted MRA in a Watanabe rabbit at baseline (a) and after the injection of superparamagnetic nanoparticles (b). Injection of the contrast agent resulted in decreased intravascular signal, due to T2*-shortening of the blood. Using IRON, blood, fat and muscle were homogenously suppressed at baseline (e). MION-47 injection contributed to strong intravascular off-resonance enhancement on ‘Day 0’ (f) and ‘Day 1’ (g), which approached baseline at later time points (h-i) allowing for better judgment of the aortic wall.
Deposition of superparamagnetic nanoparticles in the aortic wall
Using conventional images, on ‘Day 6’, susceptibility artifacts could be detected in Watanabe rabbits in the wall of the thoracic (figure 5b) and abdominal aorta (figure 5d), which were not present at baseline (figure 5a and 5c). However, these signal voids were subtle and could not exclusively be attributed to contrast agent deposition, due to respiratory motion artifacts or absence of tissue in the same areas. Using IRON, a striking contrast enhancement was observed on ‘Day 6’ in aortic wall of Watanabe rabbits (figures 5f and 5h) compared to baseline (figures 5e and 5g). This positive signal on MR-images corresponded to the deposition of superparamagnetic nanoparticles in macrophage-rich atherosclerotic plaques on histology (figures 5i-l). Enhancement was not observed in the aortic wall of controls after MION-47 injection (figures 5m and 5o), which as expected, showed no evidence of atherosclerosis (figures 5n and 5p).
Figure 5. Striking positive signal enhancement corresponding to macrophage-rich plaque on IRON-images.
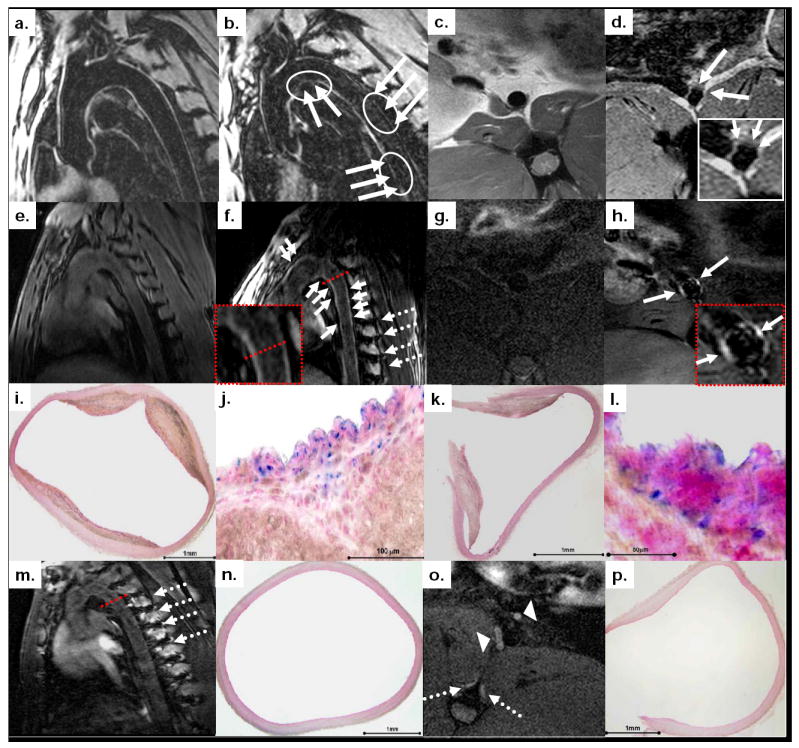
On conventional MR-images subtle susceptibility artifacts were detected in the aorta (solid arrows and overlaid regions of interest in b and d) of hyperlipidemic rabbits after MION-47 injection. Positive signal was seen on IRON-images after MION-47 injection (solid arrows in f and h), which corresponds to iron deposition in matched slices on histology (fast nuclear red staining in i and k and combined acid phosphatase and Prussian blue staining in j and l). The dotted red lines on the MR-images correspond to the cross section of the vessel. In both Watanabe and control rabbits, positive signal was present in para-spinal ribs (dotted arrows in f, m and o) and in lymph nodes (arrowheads in o), (see also text for details).
Quantitative analysis of IRON-images
Quantification of the aortic wall enhancement showed that NER significantly increased in areas of plaque (confirmed by histology on matched slices) in Watanabe rabbits on ‘Day 3’ (1.48±0.25, p<0.001 versus baseline) and even more by ‘Day 6’ (1.72±0.46, p<0.05 versus ‘Day 3’). NER of the aortic wall did not significantly change over time in remote areas without plaque in both Watanabe and control rabbits (figure 6a). A comparison of areas with plaque in Watanabe rabbits to areas without plaque in controls (n=20), which were matched for wall thickness (0.51±0.07mm in Watanabe versus 0.51±0.06mm in controls), showed that the mean NER on ‘Day 6’ was significantly higher in areas with plaque (1.64±0.43 versus 1.15±0.19, p<0.001).
Figure 6. Correlation of positive signal observed on IRON-images with macrophage density in atherosclerotic plaques on matched slices.
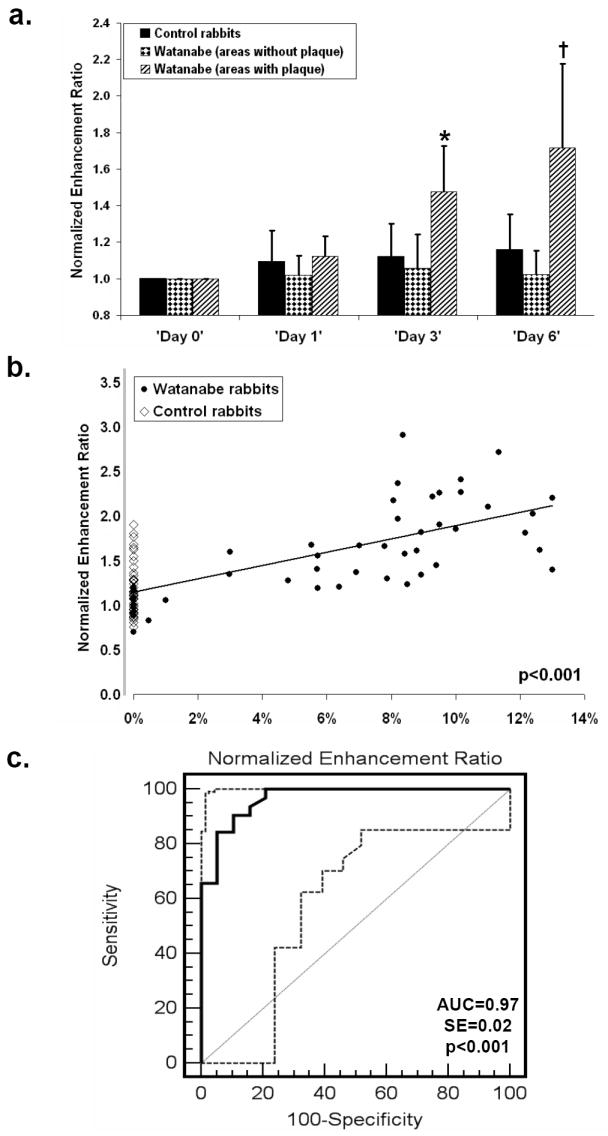
(a) NER increased in areas of plaque in Watanabe rabbits on ‘Day 3’ (*, p<0.001 versus baseline) and further increased on ‘Day 6’ (†, p<0.05 versus ‘Day 3’) only in the hyperlipidemic group. (b) NER of the aortic wall on ‘Day 6’ correlated with macrophage density in atherosclerotic plaques on matched slices (p<0.001 by clustered regression analysis, taking into account the repeated measures performed). (c) A cut-off value of NER=1.27 provided sensitivity of 91% and specificity of 89% (AUC=0.97, SE=0.02, 95% CI: 0.87-0.99, p<0.001) for the detection of macrophage-rich atherosclerotic plaque. The dashed curves represent the 95% confidence bounds of the ROC curve.
Furthermore, NER of the aortic wall on ‘Day 6’ significantly correlated with the macrophage density within atherosclerotic plaques, and for each unit increase of NER, the odds of observing a macrophage increased by 10.8% (CI of 5 to 17%), p<0.001 (figure 6b). Selecting a cut-off value of NER=1.27 provided high sensitivity (91%) and acceptable specificity (89%) (AUC=0.97, SE=0.02, 95% CI: 0.87-0.99, p<0.001) for the detection of macrophage-rich atherosclerotic plaque (macrophage density>5%), (figure 6c).
Ferrozine assays
Ferrozine-based spectrophotometric assays showed that the deposition of superparamagnetic nanoparticles was significantly higher in the aortic wall of Watanabe rabbits compared to controls (0.64±0.67 versus 0.12±0.08 mg Fe/g tissue, p<0.001) but was of the same order or higher in organs of the reticuloendothelial system including lymph nodes, liver and spleen (0.83±0.48; 1.14±0.73 and 3.34±1.45 mg Fe/g tissue respectively).
Discussion
The central findings of this study comprise: 1) macrophage-laden atherosclerotic plaques can be noninvasively detected using IRON-MRI combined with the systemic administration of MION-47 and 2) the magnitude of the contrast enhancement by IRON-MRI in areas of plaque correlates with the number of macrophages. Because superparamagnetic particles have already completed phase-3 clinical trials(12), the translation of these findings to the clinical realm appears promising.
Previous approaches for imaging of atherosclerotic plaque
After the study of Ruehm et al(7), which reported that superparamagnetic nanoparticles are phagocytosed by macrophages, several studies have employed superparamagnetic contrast agents with the goal to visualize atherosclerotic plaque in animals(8,10,11) and in humans(9,13). In all these studies, superparamagnetic nanoparticles accumulated in regions of atherosclerotic plaque, shortening the local T2-and T2*-relaxation rates, which caused signal loss on MR-images. A fundamental drawback of negative contrast techniques however, is that the agent cannot be distinguished from other sources of signal loss in the image, such as the absence of tissue, motion artifacts, hemorrhage, signal cancellations at water-fat interfaces or calcifications(8,13,14). Therefore, most the previous approaches reported visual findings without providing quantification of SNR/CNR and determined the presence of inflammation within plaque categorically. Consistent with these concerns, in our study, the signal voids in areas of the aortic wall were subtle and could not be exclusively localized due to competing sources of signal voids.
Detection of macrophages in the aortic wall using IRON-MRI
To visualize early atherosclerotic changes with high spatial resolution, a small receiver coil was used in our study at 3T. Hyperlipidemic rabbits exhibited increased wall thickness compared to controls and showed pronounced intimal thickening, associated with lipid-and macrophage-rich plaque formation. Using IRON, a striking positive signal could be readily detected in areas of macrophage-rich plaque on ‘Day 3’, which further increased on ‘Day 6’ after the second injection of 250μmol Fe/Kg MION-47. The increase in positive signal correlated with the macrophage density in areas of plaque, and allowed for detection of macrophages with high sensitivity and specificity. Areas with plaque in Watanabe rabbits exhibited a significantly higher positive signal on IRON-images post contrast, as compared to that of wall thickness-matched areas without plaque in controls. Thus, in contrast to conventional approaches, IRON-MRI can accurately detect superparamagnetic nanoparticles in the vessel wall, independent of partial volumes effects.
Iron-oxide deposition was not present exclusively in the aortic wall of the Watanabe rabbits, but also in other organs of the reticuloendothelial system with high density of macrophages, such as paraaortic lymph nodes and paraspinal ribs. Indeed accumulation can be used to assess the morphological and functional state of these tissues and the number and size of paraaortic lymphnodes may correlate with active inflammation in atherosclerosis. Furthermore, recent studies have introduced alternative techniques for positive contrast magnetic resonance imaging of superparamagnetic nanoparticles(15-17). Although the data on imaging of atherosclerotic plaque with these techniques is still limited, the comparison of IRON to other positive contrast techniques merits further investigation.
Clearly, our study has some limitations. The number of rabbits examined was small and the total dose of MION-47 injected to the animals was 500μmol/kg, which exceeds the currently approved clinical dose. Although human plaques are expected to be more advanced than the early atherosclerotic changes observed in Watanabe rabbits(9,13) the optimal human dose will clearly have to be established in further studies. Furthermore, a repeated injection scheme was performed in order to reduce the per-session administrated dose for toxicity reasons and to simultaneously prolong the total duration, during which macrophages are exposed to superparamagnetic nanoparticles. This repeated administration and scanning protocol did not allow the evaluation of the clearance of the superparamagnetic nanoparticles, which would necessitate serial MR-imaging over longer time periods (up to 30 days). However, the aim of our study was to establish a proof-of-principle for the ability of IRON to quantify inflamed atherosclerotic plaque. The current findings warrant further studies in which IRON-MRI is used to study the clearance pattern of the contrast agents. Furthermore, as an important next step, the relation between local T2* measurements and NER needs to be established. As part of the present protocol, additional T2* measurements would have significantly increased overall scanning time and the duration of anaesthesia for the rabbits. For these reasons, quantitative T2* measurements were not obtained, which is a limitation. The positive contrast on IRON-images originated from superparamagnetic nanoparticles, which have been engulfed by macrophages in inflamed plaques of the hyperlipidemic animals. However, other sources of iron-oxide particles contained for instance in areas of intraplaque haemorrhage in rupture-prone lesions(25) may also give rise to positive signal on baseline IRON-images. Furthermore, while the degree of background suppression can be chosen by the parameter settings of the IRON pre-pulse, sub-optimal magnetic field shimming or differences in the T1 of the background tissue may additionally lead to positive signal on IRON images as discussed in (18). This should be considered for the correct interpretation of pre-and post-contrast IRON images. Atherosclerotic plaques were imaged in the aortic wall and not in moving coronary arteries. Furthermore, imaging was performed using a dedicated carotid coil, and although rabbit aortas are similar in size to human coronaries(26), the distance between the coil and human coronaries versus rabbit aortas is substantially larger than the distance to the rabbit aorta studied here, which may result in a reduced spatial resolution secondary to an SNR loss in human studies. From a technical standpoint however, IRON can easily be combined with whole-heart imaging(27) that incorporates sophisticated motion-compensation strategies. Because the present study was conducted on a human MR-system, a translation to human studies seems feasible.
Conclusion
IRON MRI is combined with superparamagnetic nanoparticles to generate positive contrast for atherosclerotic plaque macrophage imaging. Using this methodology, areas of macrophage-rich plaques are highlighted and the magnitude of enhancement is significantly related to the number of macrophages as confirmed by histology. Thus, the proposed method may be valuable for the non-invasive evaluation of macrophage-rich, vulnerable plaques in humans and may be useful to monitor therapeutic interventions in atherosclerosis
Acknowledgments
This study was partially supported by the following NIH Grants: 1 K08 EB004922-01, R01 HL084186, R01 HL61912, R24 CA92782 and by the Donald W. Reynolds Foundation.
List of abbreviations
- MRI
Magnetic resonance imaging
- MRA
Magnetic resonance angiography
- IRON
Inversion Recovery with ON-resonant water suppression
- MION
Monocrystalline iron-oxide nanoparticle
- TR/TE/FA
Repetition-time/echo-time/flip-angle
- FOV
Field-of-view
- SNR/CNR
Signal-to-noise and contrast-to-noise-ratio
- NER
Normalized enhancement ratio
- ROC
Receiver operating characteristics
- AUC
Area under the curve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 5.Amirbekian V, Lipinski MJ, Briley-Saebo KC, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc Natl Acad Sci U S A. 2007;104:961–6. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botnar RM, Buecker A, Wiethoff AJ, et al. In vivo magnetic resonance imaging of coronary thrombosis using a fibrin-binding molecular magnetic resonance contrast agent. Circulation. 2004;110:1463–6. doi: 10.1161/01.CIR.0000134960.31304.87. [DOI] [PubMed] [Google Scholar]
- 7.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–22. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz SA, Taupitz M, Wagner S, et al. Iron-oxide-enhanced magnetic resonance imaging of atherosclerotic plaques: postmortem analysis of accuracy, inter-observer agreement, and pitfalls. Invest Radiol. 2002;37:405–11. doi: 10.1097/00004424-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Trivedi RA, Mallawarachi C, UK-I JM, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–6. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 10.Hyafil F, Laissy JP, Mazighi M, et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: relationship between signal loss and macrophage infiltration. Arterioscler Thromb Vasc Biol. 2006;26:176–81. doi: 10.1161/01.ATV.0000194098.82677.57. [DOI] [PubMed] [Google Scholar]
- 11.Yancy AD, Olzinski AR, Hu TC, et al. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: critical determinants of atherosclerotic plaque labeling. J Magn Reson Imaging. 2005;21:432–42. doi: 10.1002/jmri.20283. [DOI] [PubMed] [Google Scholar]
- 12.Anzai Y, Piccoli CW, Outwater EK, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228:777–88. doi: 10.1148/radiol.2283020872. [DOI] [PubMed] [Google Scholar]
- 13.Kooi ME, Cappendijk VC, Cleutjens KB, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–8. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Han MH, Moon WK, et al. Cervical Lymph Node Metastases: MR Imaging of Gadofluorine M and Monocrystalline Iron Oxide Nanoparticle-47 in a Rabbit Model of Head and Neck Cancer. Radiology. 2006;241:753–762. doi: 10.1148/radiol.2413051979. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham CH, Arai T, Yang PC, McConnell MV, Pauly JM, Conolly SM. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med. 2005;53:999–1005. doi: 10.1002/mrm.20477. [DOI] [PubMed] [Google Scholar]
- 16.Bakker CJ, Seppenwoolde JH, Vincken KL. Dephased MRI. Magn Reson Med. 2006;55:92–7. doi: 10.1002/mrm.20733. [DOI] [PubMed] [Google Scholar]
- 17.Seppenwoolde JH, Viergever MA, Bakker CJ. Passive tracking exploiting local signal conservation: the white marker phenomenon. Magn Reson Med. 2003;50:784–90. doi: 10.1002/mrm.10574. [DOI] [PubMed] [Google Scholar]
- 18.Stuber M, Gilson WD, Schar M, et al. Positive contrast visualization of iron oxide-labeled stem cells using inversion-recovery with ON-resonant water suppression (IRON) Magn Reson Med. 2007;58:1072–7. doi: 10.1002/mrm.21399. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa K, Sugawara D, Goto J, et al. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–6. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 20.Shen T, Weissleder R, Papisov M, Bogdanov A, Jr, Brady TJ. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 21.Wunderbaldinger P, Josephson L, Weissleder R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug Chem. 2002;13:264–8. doi: 10.1021/bc015563u. [DOI] [PubMed] [Google Scholar]
- 22.Korosoglou G, Gilson WD, Schar M, et al. Hind limb ischemia in rabbit model: T2-prepared versus time-of-flight MR angiography at 3 T. Radiology. 2007;245:761–9. doi: 10.1148/radiol.2452062067. [DOI] [PubMed] [Google Scholar]
- 23.Bulte JW, Arbab AS, Douglas T, Frank JA. Preparation of magnetically labeled cells for cell tracking by magnetic resonance imaging. Methods Enzymol. 2004;386:275–99. doi: 10.1016/S0076-6879(04)86013-0. [DOI] [PubMed] [Google Scholar]
- 24.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 25.Altaf N, MacSweeney ST, Gladman J, Auer DP. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke. 2007;38:1633–5. doi: 10.1161/STROKEAHA.106.473066. [DOI] [PubMed] [Google Scholar]
- 26.Tousoulis D, Xenakis C, Tentolouris C, et al. Effects of vitamin C on intracoronary L-arginine dependent coronary vasodilatation in patients with stable angina. Heart. 2005;91:1319–23. doi: 10.1136/hrt.2004.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48:1946–50. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]