Abstract
Background
Prior studies of outcomes in systolic and diastolic heart failure (SHF and DHF) are often limited to mortality in hospitalized acute HF patients, and may be confounded by residual bias. In this analysis, we examined long-term mortality and hospitalization in a propensity score matched cohort of ambulatory chronic SHF and DHF patients.
Methods
Of the 7788 patients in the Digitalis Investigation Group trial, 6800 had SHF (ejection fraction ≤45%) and 988 had DHF (ejection fraction >45%). We restricted our analysis to 7617 patients without valvular heart disease: 916 with DHF and 6701 with SHF. Propensity scores for DHF, calculated for each patient by a non-parsimonious multivariable logistic regression model, were used to match 697 DHF with 2091 SHF patients. Matched Cox regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for outcomes for DHF (versus SHF).
Results
During a median 38-month follow-up, Compared with 32% mortality in SHF, 23% of DHF patients died (HR=0.70; 95%CI=0.59-0.84; when DHF is compared with SHF). Respective HR (95%CI) for cardiovascular and HF mortality were 0.60 (0.48-0.74) and 0.56 (0.40-0.79). All-cause hospitalizations occurred in 64% of SHF and 67% of DHF patients (HR=0.99; 95%CI=0.87-1.11). Respective HR (95%CI) for cardiovascular and HF hospitalizations were 0.84 (0.73-0.96) and 0.63 (0.51-0.77).
Conclusions
Despite lower mortality and cardiovascular morbidity, ambulatory DHF patients had similar overall hospitalizations as SHF patients. Ejection fraction should be assessed in all HF patients to guide therapy, with special attention to non-cardiovascular morbidity in DHF patients.
Diastolic heart failure (DHF), defined as clinical heart failure (HF) with normal or near normal left ventricular ejection fraction (LVEF), is common.1-5 With the aging of the US population, the incidence and prevalence of DHF is likely to increase in the coming decades. However, the effects of DHF on mortality and hospitalization have not been adequately defined. In a recent review of outcomes in DHF patients Hogg et al. reported wide variations in mortality and hospitalization in these patients compared to those with systolic heart failure (SHF), defined as clinical HF with low LVEF.6
Results from studies comparing outcomes in SHF and DHF patients are often based on multivariable risk-adjusted mortality in hospitalized HF patients.1, 2, 4, 6, 7 Many of these studies included HF patients with valvular heart disease and arrhythmias (especially atrial fibrillation).4,8-10 On the other hand, community-based studies of outcomes in DHF suffer from small sample sizes.1, 2, 7
Propensity score (PS) methodology is commonly used to reduce the impact of selection bias when comparing exposures and allows for post-risk adjustment evaluation of residual bias using standardized differences between covariates.11-21 The objective of our study was to compare the effect of DHF versus SHF on mortality and hospitalization in a PS-matched cohort of ambulatory chronic HF patients.
METHODS
Study Design
The randomized DIG clinical trial was conducted in the early 1990’s to determine the effects of digoxin on outcomes in HF. DIG enrolled both SHF (LVEF ≤45%) and DHF (LVEF >45%) patients. The detailed design and results of the trial have been published elsewhere.22-24 We conducted a secondary analysis of the DIG dataset obtained from the National Heart, Lung, and Blood Institute, who sponsored the trial.
Patients
In the DIG trial, of the total of 7788 HF patients in normal sinus rhythm, 6,800 had SHF and 988 had DHF. HF was diagnosed by clinical symptoms, signs, and radiographic evidence of pulmonary congestion. Patients were recruited irrespective of their HF etiology or New York Heart Association (NYHA) functional class. For the present analysis, we excluded 171 patients with valvular heart disease as the primary etiology of their HF. Of the remaining 7617 patients, 916 had DHF. We focused our current analysis on a subset of 2788 patients based on a PS matching.
Assessment of Left Ventricular Ejection Fraction
Data on most recent left ventricular ejection fraction (LVEF) were obtained for all DIG participants before enrollment. LVEF was measured at the time of randomization if the prior LVEF was not measured in the six months prior to randomization.25 LVEF was calculated using contrast left ventriculography, radionuclide ventriculography, and two-dimensional echocardiography, in that order of preference.23
Outcomes
The primary outcomes for this analysis were all-cause mortality and all-cause hospitalization during follow up (median: 37.9 months overall; 37.8 months for SHF and 38.1 months for DHF). Secondary outcomes included mortality due to cardiovascular causes and worsening HF, and hospitalizations due to cardiovascular causes, including those due to worsening HF, ventricular arrhythmias / cardiac arrest, supra-ventricular arrhythmias, acute myocardial infarction, unstable angina, and coronary revascularization.
Statistical Analysis
Estimation of PS
Because patients do not randomly develop DHF or SHF, there are significant imbalances in baseline covariate distribution between DHF and SHF patients as is evident from Table 1 (pre-match panel; descriptive analysis based on Chi-square and Wilcoxon rank sum tests, as appropriate). To account for this imbalance, we calculated PS for DHF for each patient using a non-parsimonious multivariable logistic regression model, with consideration of clinically plausible interactions. Covariates in that model included all measured baseline patient characteristics shown in Table 1 (except for LVEF and digoxin use by randomization). The PS for DHF estimates the conditional probability that a patient would have developed DHF given the patient’s measured baseline characteristics.11-20 Chronic kidney disease was defined as a glomerular filtration rate <60 ml/1.73 square meter.26 The model was well calibrated and discriminated effectively between DHF and SHF (c statistic=0.78).
Table 1.
Baseline patient characteristics in patients with left ventricular ejection fraction (LVEF) ≤45% and >45%, before and after propensity score matching
| Pre-match | Post-match | |||||
|---|---|---|---|---|---|---|
| SHF | DHF | P | SHF | DHF | P | |
| N = 6701 (88.0%) | N = 916 (12.0%) | N = 2091 (75.0%) | N = 697 (25.0%) | |||
| Age (years), mean (±SD) | 63.4 (±10.9) | 66.6 (±10.2) | <0.0001 | 65.7 (±10.1) | 65.9 (±10.2) | 0.667 |
| Female | 1490 (22.2%) | 367 (40.1%) | <0.0001 | 670 (32.0%) | 217 (31.1%) | 0.673 |
| Non-white | 978 (14.6%) | 130 (14.2%) | 0.803 | 265 (12.7%) | 94 (13.5%) | 0.601 |
| Body mass index, kilogram per square meter, mean (±SD) | 27.1 (±5.2) | 28.9 (±6.2) | <0.0001 | 28.1 (±5.6) | 28.0 (±5.0) | 0.717 |
| Duration of HF (months), median | 30.2 (±36.8) | 26.1 (±32.2) | 0.007 | 26.6 (±33.2) | 27.2 (±33.9) | 0.569 |
| Primary cause of HF | ||||||
| Ischemic | 4803 (71.7%) | 557 (60.8%) | 1446 (69.2%) | 473 (67.9%) | ||
| Hypertensive | 583 (8.7%) | 222 (24.2%) | 306 (14.6%) | 107 (15.4%) | ||
| Idiopathic | 1007 (15.0%) | 104 (11.4%) | <0.0001 | 270 (12.9%) | 92 (13.2%) | 0.946 |
| Alcoholic | 222 (3.3%) | 13 (1.4%) | 29 (1.4%) | 12 (1.7%) | ||
| Others | 86 (1.3%) | 20 (2.2%) | 40 (1.9%) | 13 (1.9%) | ||
| Comorbid conditions | ||||||
| Prior myocardial infarction | 4402 (65.7%) | 480 (52.4%) | <0.0001 | 1278 (61.1%) | 422 (60.5%) | 0.788 |
| Current angina pectoris | 1805 (26.9%) | 280 (30.6%) | 0.022 | 665 (31.8%) | 216 (31.0%) | 0.707 |
| Hypertension | 3043 (45.4%) | 561 (61.2%) | <0.0001 | 1136 (54.3%) | 383 (54.9%) | 0.792 |
| Diabetes mellitus | 1917 (28.6%) | 281 (30.7%) | 0.200 | 643 (30.8%) | 203 (29.1%) | 0.447 |
| Chronic kidney disease | 2993 (44.7%) | 441 (48.1%) | 0.048 | 1009 (48.3%) | 321 (46.1%) | 0.335 |
| Medications | ||||||
| Pre-trial digoxin use | 2968 (44.3%) | 312 (34.1%) | <0.0001 | 768 (36.7%) | 262 (37.6%) | 0.684 |
| Digoxin by randomization | 3349 (50.0%) | 461 (50.3%) | 0.860 | 1052 (50.3%) | 334 (47.9%) | 0.294 |
| ACE inhibitors | 6332 (94.5%) | 795 (86.8%) | <0.0001 | 1896 (90.7%) | 632 (90.7%) | >0.999 |
| Nitrate and hydralazine | 103 (1.5%) | 8 (0.9%) | 0.140 | 20 (1.0%) | 7 (1.0%) | >0.999 |
| Non-potassium sparing diuretics | 5243 (78.2%) | 691 (75.4%) | 0.056 | 1600 (76.5%) | 523 (75.0%) | 0.442 |
| Potassium sparing diuretics | 509 (7.6%) | 71 (7.8%) | 0.842 | 145 (6.9%) | 54 (7.7%) | 0.497 |
| Potassium supplement | 1897 (28.3%) | 238 (26.0%) | 0.147 | 564 (27.0%) | 178 (25.5%) | 0.488 |
| Symptoms and signs of heart failure (past or present) | ||||||
| Dyspnea at rest | 4704 (70.2%) | 627 (68.4%) | 0.282 | 1418 (67.8%) | 482 (69.2%) | 0.491 |
| Dyspnea on exertion | 6382 (95.26%) | 881 (96.2%) | 0.241 | 2002 (95.7%) | 670 (96.1%) | 0.542 |
| Activity limitation | 5128 (76.5%) | 653 (71.3%) | 0.001 | 1529 (73.1%) | 511 (73.3%) | 0.961 |
| Jugular venous distension | 3498 (52.2%) | 429 (46.8%) | 0.002 | 1021 (48.8%) | 325 (46.6%) | 0.336 |
| Third heart sound | 3361 (50.2%) | 299 (32.6%) | <0.0001 | 759 (36.3%) | 259 (37.2%) | 0.683 |
| Pulmonary râles | 4768 (71.2%) | 671 (73.3%) | 0.198 | 1499 (71.7%) | 500 (71.7%) | >0.999 |
| Lower extremity edema | 3471 (51.8%) | 535 (58.4%) | <0.0001 | 1129 (54.0%) | 378 (54.2%) | 0.930 |
| NYHA functional class | ||||||
| I | 891 (13.3%) | 179 (19.5%) | 371 (17.7%) | 122 (17.5%) | ||
| II | 3629 (54.2%) | 532 (58.1%) | <0.0001 | 1224 (58.5%) | 406 (58.2%) | 0.994 |
| III | 2043 (30.5%) | 194 (21.2%) | 464 (22.2%) | 158 (22.7%) | ||
| IV | 138 (2.1%) | 11 (1.2%) | 32 (1.5%) | 11 (1.6%) | ||
| Heart rate (/minute), mean (±SD) | 78.7 (±12.7) | 75.7 (±12.0) | <0.0001 | 76.1 (±12.2) | 76.0 (±12.2) | 0.684 |
| Blood pressure (mm Hg), mean (±SD) | ||||||
| Systolic | 125.8 (±20.0) | 138.0 (±21.3) | <0.0001 | 133.5 (±20.1) | 134.0 (±19.2) | 0.864 |
| Diastolic | 74.9 (±11.3) | 77.2 (±11.2) | <0.0001 | 76.3 (±11.0) | 76.4 (±10.9) | 0.753 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 4509 (67.3%) | 565 (61.7%) | 0.001 | 1360 (65.0%) | 436 (62.6%) | 0.235 |
| Cardiothoracic ratio >0.5 | 4118 (61.5%) | 450 (49.1%) | <0.0001 | 1115 (53.3%) | 360 (51.6%) | 0.457 |
| Laboratory data, mean (±SD) | ||||||
| Serum creatinine (mg/dL), | 1.28 (±0.37) | 1.26 (±0.39) | 0.002 | 1.27 (±0.37) | 1.26 (±0.39) | 0.530 |
| Serum potassium (mEq/L) | 4.34 (±0.44) | 4.34 (±0.42) | 0.870 | 4.34 (±0.43) | 4.35 (±0.42) | 0.667 |
| LVEF (%), mean (±SD) | 28.5 (±8.8) | 55.1 (±7.9) | <0.0001 | 30.7 (±8.5) | 54.1 (±7.7) | <0.0001 |
ACE=Angiotensin-converting enzyme, HF=heart failure, JVP=jugular venous pressure, NYHA=New York heart association
Matching by PS
Matching patients on PS tends to effectively balance the distribution of all measured covariates, thus substantially reducing bias, as is apparent from Table 1 (post-match panel).21, 27 Although PS are frequently used to balance baseline covariates between two treatment groups,18-20 they have also been used to balance baseline covariates between two groups of patients with and without certain comorbidity or risk factors.28-30 Using an SPSS matching macro,31 we matched 697 (76%) of the 916 DHF patients to three unique SHF patients each, for a total of 2,091 matched SHF patients.
Evaluation of post-match covariate balance
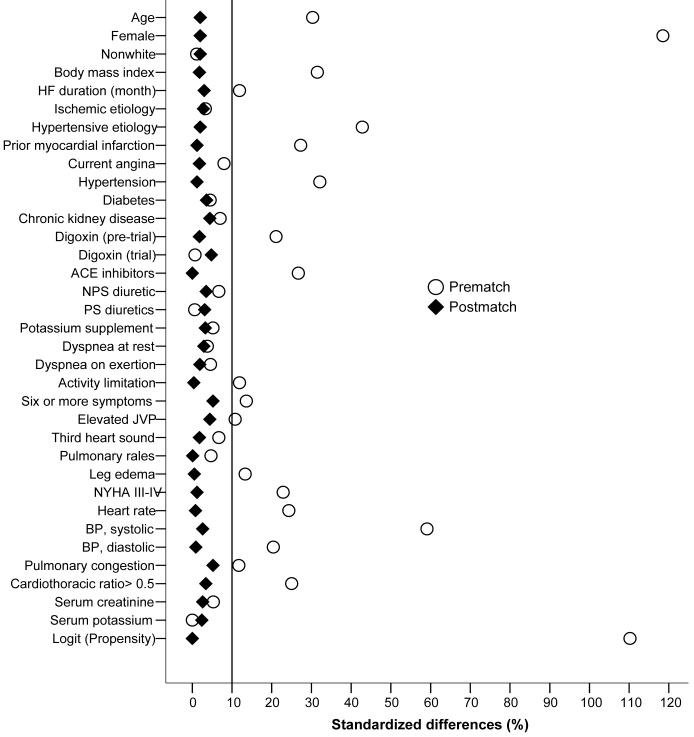
We compared the balance of baseline covariates between SHF and DHF patients before and after matching using standardized differences, which directly describe the observed bias in the means (or proportions) of covariates across two groups, expressed as a percentage of the pooled standard deviation (Figure 1).15, 17, 19, 20 We also used Chi-square and Wilcoxon rank-sum tests as appropriate to compare baseline characteristics (Table 1).
Figure 1.
Absolute standardized differences in covariates between heart failure patients with left ventricular ejection fraction ≤45% and >45%, before and after propensity score matching
Estimating the effect of DHF on outcomes
We used Kaplan-Meier survival analyses to compare various outcomes between patients with SHF and DHF in the matched cohort. We then used Cox proportional hazards models to estimate the association of DHF with various outcomes. We accounted for matching by stratifying the model based on a variable that identified the matched groups.19, 20 We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. We then used multivariable matched Cox regression models to estimate the effect of DHF on various outcomes. Covariates included were the same as those used in the logistic regression model for PS and were entered forward stepwise into the models.
Sensitivity analysis
To address concerns about incomplete matching, we analyzed data from all 7617 patients (pre-match) cohort, using direct regression adjustment for the PS. Then, we estimated the association between DHF and outcomes in bivariate and multivariable Cox regression models adjusting for all covariates used in the PS estimation (forward stepwise entry). Finally, we repeated these analyses using LVEF as a continuous variable.
Subgroup analysis
To examine whether the association of DHF with all-cause mortality was homogeneous across various subgroups of patients, we conducted subgroup analyses in the pre-match cohort.32 We first calculated absolute risks, and then formally tested for first-order interactions in Cox proportional hazards models, entering interaction terms between DHF and the subgroups. All statistical tests were evaluated using a two-tailed 95% confidence level. All data analyses were performed using SPSS for Windows version 14.33
RESULTS
Baseline Patient Characteristics
Before matching, compared with DHF patients, SHF patients were more likely to be young, male, and have longer duration of HF, ischemic heart disease, receive angiotensin-converting enzyme (ACE) inhibitors, and have higher NYHA class symptoms. Patients in the matched cohort had a mean (±SD) age of 65.8 (±10.1) years, 31.8% were women and 12.9% were non-whites.
PS Matching and Covariate Balance
The distributions of baseline covariates between DHF and SHF patients before and after matching are displayed in Table 1 and Figure 1. After matching, standardized differences for all observed covariates were below 10% in absolute value, suggesting substantial improvement in covariate balance between DHF and SHF patients (Figure 1). Mean PS for unmatched and matched SHF (0.0698 vs. 0.1720; p <0.0001) and DHF (0.1718 vs. 0.5250; p <0.0001) patients and a graphical assessment (not shown) indicate that patients excluded in the matching process were at the extremes of the PS distribution, as is usual.
Outcomes
During 38 months of median follow up, 836 (30%) of the 2788 PS matched patients died from all causes, 637 (23%) died from cardiovascular causes, and 262 (9%) died due to worsening HF. During the same follow up period, 1805 patients (64.7%) were hospitalized from all causes, with an average 1.8 hospitalizations for each patient. There were 1375 (49.3%) hospitalizations due to cardiovascular causes, and 684 (24.5%) due to worsening HF.
Matched Pairs Mortality
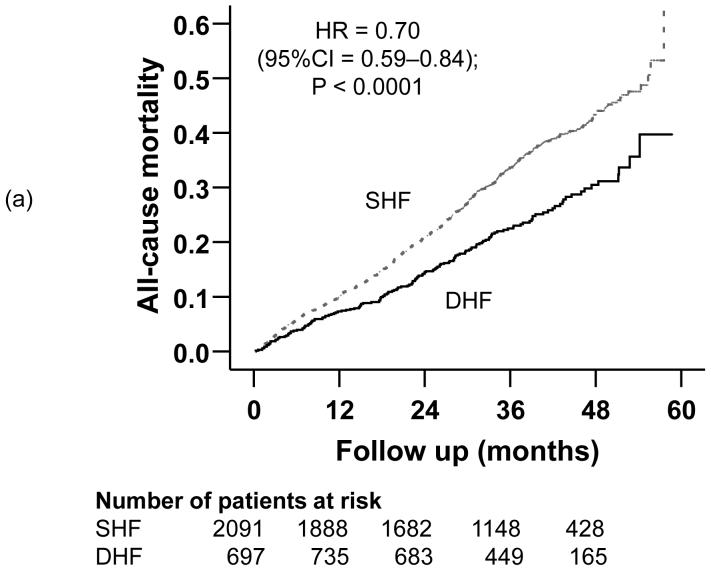
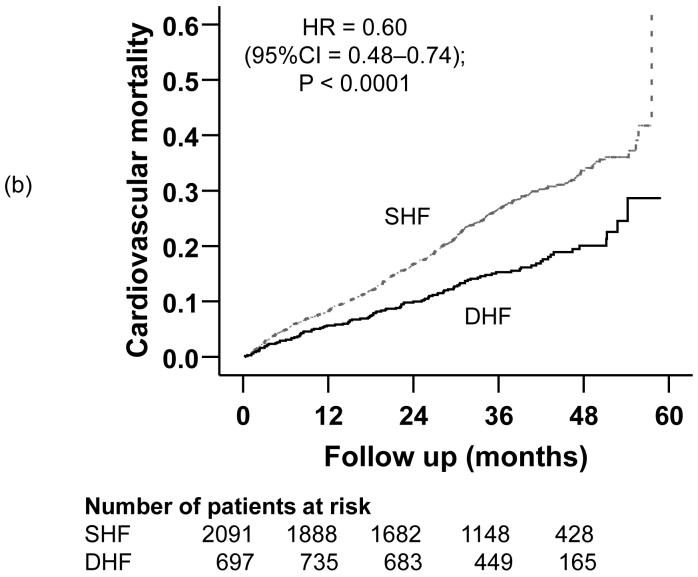
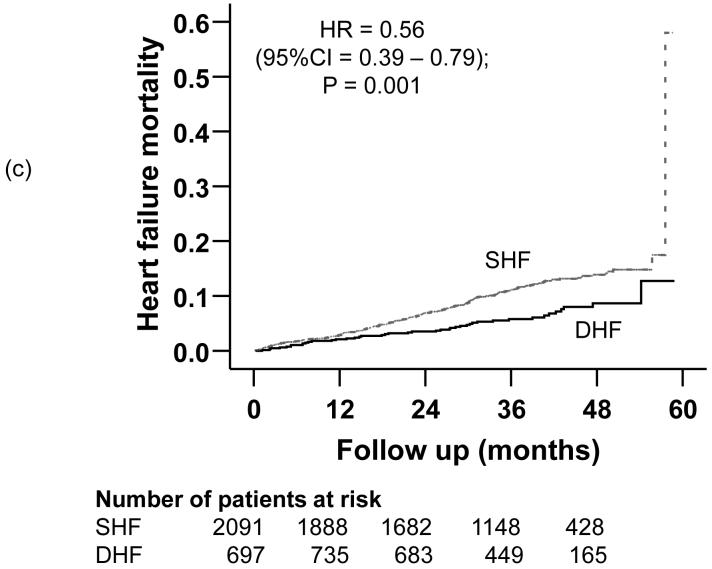
Compared with 32% mortality in SHF patients during a median follow up of 37.8 months, 23% of DHF patients died during a median follow up of 38.1 months (hazard ratio {HR} =0.70; 95% confidence interval {CI} =0.59-0.84); p <0.0001; Table 2). Death due to cardiovascular causes occurred in 25% of SHF and 17% of DHF patients (HR =0.60; 95% CI =0.48-0.74); p <0.0001; Table 2). HF mortality occurred in 11% of SHF and 6% of DHF patients (matched pairs HR =0.56; 95% CI =0.39-0.79); p =0.001; Table 2). Kaplan-Meier plots for all-cause, cardiovascular and HF mortality for SHF and DHF patients are displayed in Figures 2a, 2b, and 2c. The associations between DHF and various causes of mortality remained essentially unchanged after multivariable adjustment for covariates (Table 2).
Table 2.
Matched hazard ratios (HR) and 95% confidence intervals (CI) for mortality in ambulatory patients with diastolic heart failure (DHF) compared with systolic heart failure (SHF)
| % event (median follow up) | Absolute SHF-DHF risk difference | Unadjusted HR for DHF (95% CI) | Adjusted* HR for DHF (95% CI) | ||
|---|---|---|---|---|---|
| SHF (n=2091) | DHF (n=697) | ||||
| All-cause mortality | 32.2% (38 months) | 23.2% (38 months) | -9.0% | 0.70 (0.59 - 0.84); P<0.0001 | 0.73 (0.61 - 0.89); P=0.002 |
| Cardiovascular mortality | 25.3% (38 months) | 17.1% (38 months) | -8.2% | 0.60 (0.48 - 0.74); P<0.0001 | 0.60 (0.47 - 0.75); P<0.0001 |
| Heart failure mortality | 10.5% (38 months) | 6.2% (38 months) | -4.3% | 0.56 (0.39 - 0.79); P=0.001 | 0.58 (0.39 - 0.85); P=0.006 |
Adjusted for matching, and the following covariates, which were entered forward stepwise: age, female sex, non-white race, body mass index, duration and etiology of heart failure, past myocardial infarction, current angina, hypertension, diabetes, pre-trial use of digoxin, digoxin use during trial, use of angiotensin-converting enzyme inhibitors, combined use of hydralazine and nitrates, use of non-potassium sparing diuretics, potassium-sparing diuretics, potassium supplements, dyspnea at rest, dyspnea on exertion, activity limitation, New York Heart Association class, distended jugular vein, third heart sound, pulmonary râles, lower extremity edema, presence of 6 or more symptoms or signs, heart rate, blood pressure (systolic and diastolic), serum creatinine and potassium levels, pulmonary congestion and cardiothoracic ratio >0.5 by chest x-ray, and propensity score for left ventricular ejection fraction >45%.
Figure 2.
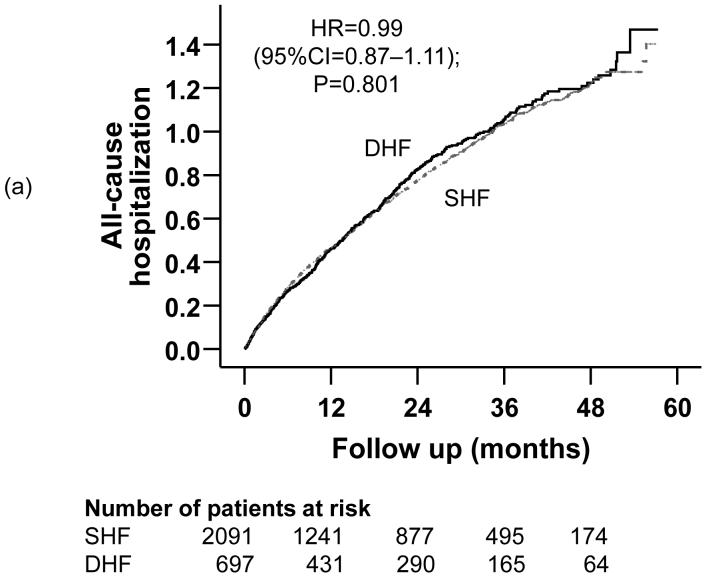
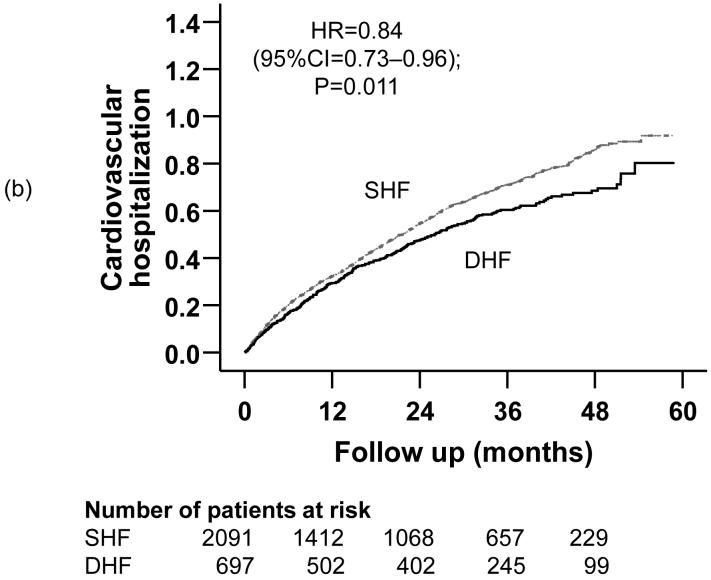
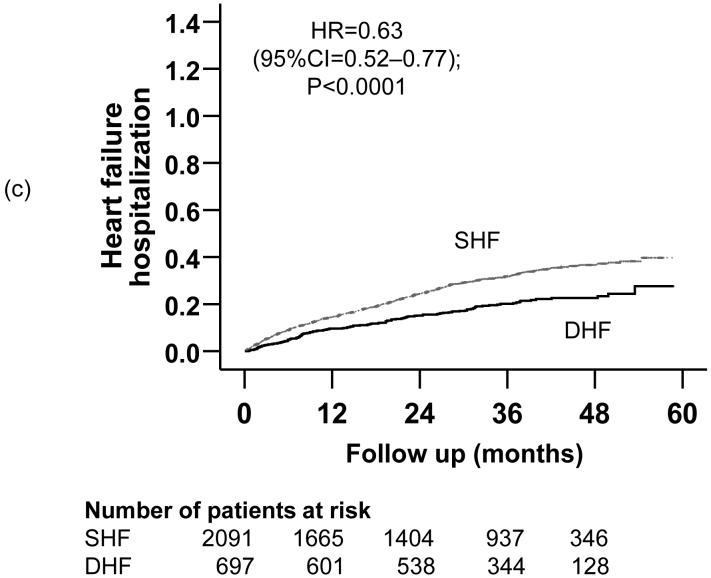
Kaplan-Meier plots demonstrating cumulative risk of mortality due to (a) all causes, (b) cardiovascular causes, and (c) heart failure, in ambulatory patients with systolic heart failure (SHF) and diastolic heart failure (DHF)
Multivariable Risk-Adjusted Mortality
In the pre-match cohort of 7617 patients, compared with 35% of SHF patients, 23% of DHF patients died (unadjusted HR =0.62; 95% CI =0.54-0.71; p <0.0001). Adjustment for PS alone (adjusted HR =0.76; 95% CI =0.66-0.88; p <0.0001) or multivariable adjustment for other covariates (adjusted HR = 0.82; 95% CI = 0.71 - 0.95; p =0.008) weakened the association, but it remained significant. The association remained significant when LVEF was used as a continuous variable (adjusted HR =0.983; 95% CI =0.980 - 0.987; p <0.0001).
Matched Pairs All-Cause Hospitalizations
Compared with 64% all-cause hospitalizations in SHF patients during a median 44.8 months of follow up, 66% of DHF patients were hospitalized from all causes during a median follow up of 48 months (HR =0.99; 95% CI =0.87-1.11). Kaplan-Meier plots for time to first hospitalization due to all causes are displayed in Figure 3a. The association between DHF and all-cause hospitalization remained essentially unchanged after multivariable covariate adjustment (Table 3). There was no difference in the number of hospitalizations between the two groups (mean, 1.8 for each group, p =0.906).
Figure 3.
Kaplan-Meier plots demonstrating cumulative risk of first hospitalizations due to (a) all causes, (b) cardiovascular causes, and (c) worsening heart failure, in ambulatory patients with systolic heart failure (SHF) and diastolic heart failure (DHF)
Table 3.
Matched hazard ratios (HR) and 95% confidence intervals (CI) for hospitalizations in ambulatory patients with diastolic heart failure (DHF) compared with systolic heart failure (SHF)
| % event (median follow up) | Absolute SHF-DHF risk difference | Unadjusted HR for DHF (95% CI) | Adjusted* HR for DHF (95% CI) | ||
|---|---|---|---|---|---|
| SHF (n=2091) | DHF (n=697) | ||||
| All causes | 64.0% (45 months) | 66.9% (48 months) | 2.9 | 0.99 (0.87- 1.11); P=0.801 | 1.00 (0.88 - 1.13); P=0.976 |
| Cardiovascular causes | 50.5% (63 months) | 45.9% (75 months) | -4.6 | 0.84 (0.73 - 0.96); P=0.011 | 0.85 (0.74 - 0.98); P=0.023 |
| Worsening heart failure | 26.6% (86 months) | 18.2% (90 months) | -8.4% | 0.63 (0.52 - 0.77): P<0.0001 | 0.60 (0.48 - 0.74); P<0.0001 |
| Ventricular arrhythmias/cardiac arrest | 3.7% (95 months) | 1.4% (96 months) | -2.3% | 0.39 (0.18 - 0.84); P=0.016 | 0.37** (0.17 - 0.80); P=0.011 |
| Supra-ventricular arrhythmias | 4.1% (97 months) | 5.5% (95 months) | 1.2% | 1.28 (0.82 - 1.98); p=0.273 | 1.27** (0.82 - 1.97); P=0.295) |
| Acute myocardial infarction | 6.6% (94 months) | 5.9% (95 months) | -0.7% | 0.87 (0.59 - 1.28); P=0.481 | 0.88** (0.59 - 1.29); P=0.503 |
| Unstable angina | 13.0% (80 months) | 15.8% (90 months) | 2.8% | 1.07 (0.81 - 1.42); P=0.631 | 1.07** (0.81 - 1.41); P=0.644 |
| Coronary revascularization | 2.5% (94 months) | 3.6% (95 months) | 1.1% | 1.27 (0.67 - 2.41); P=0.468 | 1.18** (0.61 - 2.26); P=0.625 |
Adjusted for same covariates as in Table 2.
Adjusted for matching and propensity scores only
Multivariable Risk-Adjusted All-cause Hospitalization
In the pre-match cohort of 7617 patients, compared with 66% of SHF patients, 67% of DHF patients were hospitalized from all causes (unadjusted HR =0.97; 95% CI =0.89-1.05; p =0.405). The association remained essentially unchanged after adjustment for PS alone (adjusted HR =1.02; 95% CI =0.93-1.12; p =0.673), multivariable covariate adjustment (adjusted HR = 1.08; 95% CI = 0.99 - 1.18; p =0.096), or when LVEF was used as a continuous variable (adjusted HR =0.999; 95% CI =0.997-1.002; p =0.582).
Cardiovascular Hospitalizations
Compared with 51% of SHF patients who were hospitalized due to cardiovascular causes during a median follow up of 63 months, 46% of DHF patients were hospitalized from cardiovascular causes during a median follow up of 75 months (HR =0.84; 95% CI =0.73-0.96). Kaplan-Meier plots for time to first hospitalization due to cardiovascular causes are displayed in Figure 3b. The association between DHF and all-cause hospitalization remained essentially unchanged after multivariable covariate adjustment (Table 3). In the pre-match cohort of 7617 patients, unadjusted, PS adjusted and multivariable adjusted HR (95% CI) for cardiovascular hospitalization, when DHF was compared with SHF, were respectively 0.82 (0.74-0.91; p <0.0001), 0.89 (0.80-0.99; p =0.026) and 0.93 (0.84-1.04; p =0.201).
Heart Failure Hospitalizations
Compared with 27% of SHF patients who were hospitalized due to worsening HF during a median follow up of 86 months, 18% of DHF patients were hospitalized due to worsening HF during a median follow up of 90 months (matched pairs HR = 0.63; 95% CI = 0.52 - 0.77). Kaplan-Meier plots for time to first HF hospitalization are displayed in Figure 3c. The association between DHF and all-cause hospitalization remained essentially unchanged after multivariable covariate adjustment (Table 3). In the pre-match cohort of 7617 patients, unadjusted, PS adjusted and multivariable adjusted HR (95% CI) for HF hospitalization, when DHF was compared with SHF, were respectively 0.55 (0.47-0.64; p <0.0001), 0.65 (0.56-0.77; p =0.026) and 0.69 (0.59-0.81; p <0.0001).
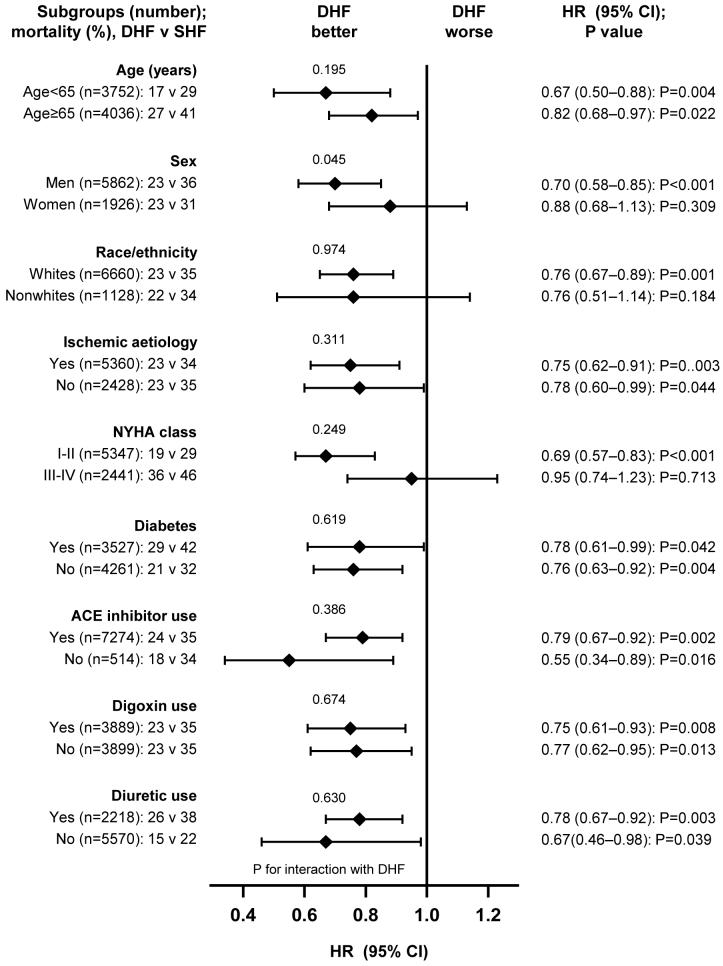
Subgroup Analysis
Associations between DHF and reduced all-cause mortality were observed in various subgroups of patients (Figure 4). DHF patients had better prognosis, regardless of age, sex, race, HF etiology, NYHA functional class, diabetes, or therapy with ACE inhibitors, diuretics or digoxin. There were no significant interactions between DHF and any of these subgroups, except for female sex (p =0.045).
Figure 4.
Hazard ratios (HR) and 95% confidence interval (CI) for all-cause mortality in subgroups of patients with diastolic heart failure (DHF) compared with systolic heart failure (SHF) (ACE=angiotensin-converting enzyme; NYHA=New York Heart Association)
DISCUSSION
The key findings of our study are that in a PS-matched cohort of ambulatory chronic HF patients, compared with SHF patients, DHF patients had reduced risk of mortality, and cardiovascular and HF hospitalizations, but similar all-cause hospitalizations. These findings are important, as with the aging of the US population, the prevalence of HF in general, and that of DHF in particular, is expected to increase in the coming decades, with significant implications for health care utilization and costs.
Potential Mechanistic Explanation
DHF patients also share similar clinical and pathophysiologic characteristics with SHF patients, including reduction in exercise capacity, neurohormonal activation, and impairment of quality of life.3, 24, 34 35, 36 Despite these similarities, DHF was associated with reduced mortality and cardiovascular morbidity. Even though, diastolic function was not specifically measured, most HF patients with normal or near normal have abnormal DHF.37 Recent evidence that a significant proportion of DHF patients eventually develop SHF suggests that DHF may represent an earlier stage in the natural history of HF and thus better prognosis.38, 39 However, do not know why overall hospitalization was similar for both SHF and DHF patients. It is possible that due to the advanced nature of the SHF patients, they had more competing cardiovascular causes for hospitalization than those with DHF.
Comparison with Prior Studies
The most commonly studied outcome in community-based comparisons of DHF patients (versus SHF) is all-cause mortality, which has yielded varying results.1, 2, 7 In the Framingham Heart Study, 51% of the 73 patients had DHF (LVEF >50%).1 During a median follow-up of 6.2 years, DHF patients had an unadjusted annual mortality of 8.7% (versus 18.9% in SHF). This mortality difference lost significance after adjustment for age and sex.1 In the Cardiovascular Health Study, 4.9% of the 5532 persons ≥65 years had HF 2. Of these, 78% had DHF (LVEF ≥45%) and 22% had SHF (LVEF <45%), who had annual mortality of about 10% and 15%, respectively. Compared with healthy controls, LVEF >55%, 45-55% and <45% were associated with respectively 48%, 140% and 88% higher risk of death.2
In the Olmstead County study, LVEF was known in137 of the 216 incident HF patients and 43% had DHF (LVEF ≥50%).5 In that study, the 4 year unadjusted mortality rate was about 50% for both DHF and SHF patients. After adjustment for age, sex, NYHA class, and coronary artery disease, DHF patients had a non-significant 20% lower risk of death (adjusted relative risk =0.80; p =0.369). Curtis et al. have also reported an overall inverse association between LVEF and mortality among participants in the DIG trial.40 However, propensity analysis and inclusion of hospitalizations as outcomes distinguish our study from theirs.
Clinical Implications
We noted that the overall hospitalization rates of patients with DHF and SHF patients were similar, suggesting that DHF patients may suffer excess morbidity from non-cardiovascular causes relative to the SHF patients. This is likely to negatively impact health care resource utilization, especially as the prevalence of HF increases with the aging of the US population. Many of these non-cardiac conditions are chronic and studies need to determine if a chronic disease management model would be effective in reducing hospitalization due to those non-cardiac causes. In addition, we also noted a non-significant increased incidence of supraventricular arrhythmias, unstable angina, and coronary revascularization in HF patients with PSF. This might be due to a higher prevalence of subclinical ischemic heart disease and presence of viable myocardium in DHF than commonly believed.41 Future studies should investigate these associations in DHF.
Strengths and Limitations
The strengths of our study are (1) use of PS matching; (2) a relatively large number of DHF patients (n=697) compared with Framingham Heart Study (n=37),1 Cardiovascular Health Study (n=170),2 and Rochester Epidemiology Project (n=83)5; (3) validation of HF diagnosis by cardiologist DIG investigators; (4) exclusion of patients with atrial fibrillation and valvular heart disease; and (5) examination of multiple outcomes including hospitalizations not reported before.
Our study has several limitations. First, women and elderly HF patients were underrepresented, which is reflected in the lower overall mortality rates in our analysis. Second, HF patients with atrial fibrillation and valvular heart disease were excluded. Although this was useful to avoid misclassification of DHF patients, it limits generalizability. Third, we combined LVEF >55% and 45-55% as DHF. However, overall mortality for these patients are similar (23% each).40 Fourth, confounding due to unmeasured covariates is possible. However, an unmeasured confounder must be unrelated to all the measured covariates and also be correlated with DHF and outcomes. Finally, exclusion of patients during matching compromises generalizability. However, only patients at the extremes of PS were excluded, leaving patients with similar PS to be matched. This increased the internal validity of our study. This is important as only strong and internally valid associations should precede studies of external validity.42
Conclusions
In conclusion, compared with SHF, ambulatory chronic DHF patients have lower mortality, and cardiovascular and HF hospitalization, but similar overall hospitalizations, suggesting disproportionately higher non-cardiovascular comorbidity and hospitalizations in these patients. LVEF should be measured in all HF patients, for risk stratification and to guide therapy, with special attention to the assessment and management of non-cardiovascular comorbidities in those with DHF. SHF patients should be treated with an ACE inhibitor (or an angiotensin receptor blocker if intolerant to an ACE inhibitor), approved beta-blockers, and digoxin.19, 43, 44 Diuretics should be used with caution for symptomatic patients with fluid overload.20 Evidence-based therapy for DHF is limited. However, recent data suggest that digoxin and candesartan may be beneficial in reducing HF hospitalizations in such patients.45, 46 Future studies needs to focus on reduction in non-cardiovascular and non-HF hospitalizations in DHF patients.
Acknowledgements
The Digitalis Investigation Group (DIG) study was conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the DIG Investigators. This manuscript has been reviewed by NHLBI for scientific content and consistency of data interpretation with previous DIG publications and significant comments have been incorporated prior to submission for publication.
Grant support: Ali Ahmed is supported by the National Institutes of Health through grants from the National Institute on Aging (1-K23-AG19211-04) and the National Heart, Lung, and Blood Institute (1-R01-HL085561-01 and P50-HL077100).
Appendix
Addendum
Two recent reports by Bhatia et al and Owan et al, bothbased on hospitalized HF patients, published after this manuscript was accepted, reported variable outcomes in DHF, compared with SHF patients.47,48 Using data from the Mayo Clinical hospitals in Olmstead County, MO, Owan et al reported that DHF was associated with somewhat better survival (adjusted HR 0.96; 95%CI 0.92-1.00).48 Using data from 103 hospitals in Ontario, Canada, Bhatia et al reported similar 1-year survival for DHF compared to SHF patients (adjusted HR 1.13; 95% CI 0.96-1.36, when SHF compared with DHF).47 Neither of these hospital-based studies, however, used PS matching to reduce imbalances in baseline covariates between DHF and SHF groups.48
Contributor Information
Ali Ahmed, University of Alabama at Birmingham (UAB) and VA Medical Center (VAMC), Birmingham, AL.
Gilbert J. Perry, University of Alabama at Birmingham (UAB) and VA Medical Center (VAMC), Birmingham, AL.
Jerome L. Fleg, National Heart, Lung, and Blood Institute, Bethesda, MD.
Thomas E. Love, Case Western Reserve University, Cleveland OH.
David C. Goff, Jr, Wake Forest University, Winston-Salem, NC.
Dalane W. Kitzman, Wake Forest University, Winston-Salem, NC.
References
- 1.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 2.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–9. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Roseman JM, Duxbury AS, Allman RM, DeLong JF. Correlates and outcomes of preserved left ventricular systolic function among older adults hospitalized with heart failure. Am Heart J. 2002;144:365–72. doi: 10.1067/mhj.2002.124058. [DOI] [PubMed] [Google Scholar]
- 5.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–9. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 6.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 7.Senni M, Redfield MM. Heart failure with preserved systolic function. A different natural history? J Am Coll Cardiol. 2001;38:1277–82. doi: 10.1016/s0735-1097(01)01567-4. [DOI] [PubMed] [Google Scholar]
- 8.Pernenkil R, Vinson JM, Shah AS, Beckham V, Wittenberg C, Rich MW. Course and prognosis in patients > or = 70 years of age with congestive heart failure and normal versus abnormal left ventricular ejection fraction. Am J Cardiol. 1997;79:216–9. doi: 10.1016/s0002-9149(96)00719-9. [DOI] [PubMed] [Google Scholar]
- 9.Varadarajan P, Pai RG. Prognosis of congestive heart failure in patients with normal versus reduced ejection fractions: results from a cohort of 2,258 hospitalized patients. J Card Fail. 2003;9:107–12. doi: 10.1054/jcaf.2003.13. [DOI] [PubMed] [Google Scholar]
- 10.Lenzen MJ, Scholte Op, Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, et al. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25:1214–20. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 12.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Asso. 1984;79:516–524. [Google Scholar]
- 13.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–63. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 15.D’Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 16.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–33. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 17.Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–98. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed A, Centor RM, Weaver MT, Perry GJ. A propensity score analysis of the impact of angiotensin-converting enzyme inhibitors on long-term survival of older adults with heart failure and perceived contraindications. Am Heart J. 2005;149:737–43. doi: 10.1016/j.ahj.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Rich MW, Love TE, Lloyd-Jones DM, Aban IB, Colucci WS, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006;27:178–86. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed A, Husain A, Love TE, Gambassi G, Dell’italia LJ, Francis GS, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. European Heart Journal. doi: 10.1093/eurheartj/ehi890. doi:10.1093/eurheartj/ehi890. Advance Access published online on May 18, 2006. Eur Heart J 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–70. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 22.The Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–33. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 23.Collins JF, Egan D, Yusuf S, Garg R, Williford WO, Geller N. Overview of the DIG trial. Control Clin Trials. 2003;24:269S–276S. doi: 10.1016/s0197-2456(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J. 2006;151:444–50. doi: 10.1016/j.ahj.2005.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Digitalis Investigation Group . Protocol: Trial to evaluate the effect of digitalis on mortality n heart failure. NHLBI; 1991. [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 27.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2006 doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 28.Feng D, D’Agostino RB, Silbershatz H, Lipinska I, Massaro J, Levy D, et al. Hemostatic state and atrial fibrillation (the Framingham Offspring Study) Am J Cardiol. 2001;87:168–71. doi: 10.1016/s0002-9149(00)01310-2. [DOI] [PubMed] [Google Scholar]
- 29.Kubal C, Srinivasan AK, Grayson AD, Fabri BM, Chalmers JA. Effect of risk-adjusted diabetes on mortality and morbidity after coronary artery bypass surgery. Ann Thorac Surg. 2005;79:1570–6. doi: 10.1016/j.athoracsur.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed A, Ali M, Lefante CM, Mullick MSI, Kinney FC. Secondary diagnosis of depression and nursing home admission in ambulatory older adults hospitalized for heart failure: A propensity score analysis. Am J Geri Psych. 2006 doi: 10.1097/01.JGP.0000209639.30899.72. In Press. [DOI] [PubMed] [Google Scholar]
- 31.Levesque R. Macros. In: Levesque R, editor. SPSS® Programming and Data Management, 2nd Edition: A Guide for SPSS® and SAS® Users. SPSS; Chicago, Illinois: 2005. available for download at http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 32.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005;365:176–86. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 33.SPSS . SPSS for Windows, Rel. 14. SPSS Inc.; Chicago, IL: 2006. [Google Scholar]
- 34.Cohn JN, Johnson G, Veterans Administration Cooperative Study Group Heart failure with normal ejection fraction. The V-HeFT Study. Circulation. 1990;81:III48–53. [PubMed] [Google Scholar]
- 35.Badgett RG, Lucey CR, Mulrow CD. Can the clinical examination diagnose left-sided heart failure in adults? JAMA. 1997;277:1712–9. [PubMed] [Google Scholar]
- 36.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–93. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 37.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–82. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 38.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 39.Cahill JM, Ryan E, Travers B, Ryder M, Ledwidge M, McDonald K. Progression of preserved systolic function heart failure to systolic dysfunction -- a natural history study. Int J Cardiol. 2006;106:95–102. doi: 10.1016/j.ijcard.2004.12.096. [DOI] [PubMed] [Google Scholar]
- 40.Curtis JP, Sokol SI, Wang Y, Rathore SS, Ko DT, Jadbabaie F, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 41.Isoyama S. Interplay of hypertrophy and myocardial ischemia. In: Lorell BH, Grossman W, editors. Diastolic Relaxation of the Heart. Kluwer Academic Publishers; Boston: 1992. p. 203. [Google Scholar]
- 42.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. Bmj. 2001;323:42–6. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 44.Brophy JM. Rehabilitating digoxin. Eur Heart J. 2006;27:127–9. doi: 10.1093/eurheartj/ehi686. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: The ancillary Digitalis Investigation Group trial. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.106.628347. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 47.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Eng J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 48.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Eng J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]