Abstract
Background
Chronic kidney disease (CKD) is common in systolic heart failure (SHF) and is associated with poor outcomes. It is also associated with underuse of angiotensin-converting enzyme inhibitors (ACEI), yet the effect of these drugs in these (SHF-CKD) patients has not been well studied. The objective of this analysis was to determine if ACEI use was associated with reduction in mortality and hospitalization in SHF-CKD patients.
Methods and Results
Of the 6,800 SHF patients (ejection fraction ≤45%) in the Digitalis Investigation Group trial, 1,707 had CKD (serum creatinine 1.3-2.5 mg/dl for women and 1.5-2.5 mg/dl for men). Propensity scores for ACE inhibitor use were calculated for each of the 1,707 patients and were used to match 104 of the 127 no-ACEI patients with 104 ACEI patients. We estimated the effect of ACEI use on outcomes at 2 years using multivariable-adjusted Cox regression analyses. Overall, 35% died and 67% were hospitalized. Compared with 30% ACEI patients, 39% no-ACEI patients died (adjusted HR=0.58; 95% CI=0.35-0.96; p=0.034). Compared with 64% ACEI patients, 69% no-ACEI patients had hospitalizations due to all causes (adjusted HR=0.69; 95% CI=0.48-0.98; p=0.040).
Conclusion
We observed an association between use of ACEI and reductions in mortality and hospitalization in ambulatory chronic SHF patients with mild to moderate CKD. However, the results of this observational study should be interpreted with caution, and need to be replicated in larger and more recent databases, and confirmed prospectively in well-designed follow-up studies and/or randomized clinical trials.
Keywords: heart failure, chronic kidney disease, ACE inhibitors, mortality, hospitalization
Angiotensin-converting enzyme (ACE) inhibitors reduce mortality and morbidity in patients with systolic heart failure (SHF or clinical heart failure with impaired left ventricular ejection fraction.1, 2 It is also associated with renoprotection and reduction in mortality in patients with chronic kidney disease (CKD).3-5 Despite a theoretical dual benefit from the use of ACE inhibitors in SHF patients with CKD, these drugs are often underused in these patients.6-8 This is particularly important as CKD is common in SHF and is associated with poor outcomes.8, 9
ACE inhibitors has been shown to be associated with reduction in short- and long-term mortality in hospitalized older adults with acute systolic HF and advanced CKD.8, 10 However, the benefit of ACE inhibitors in ambulatory systolic HF patients with mild to moderate CKD has not been well studied.11 In this analysis, we tested the hypothesis that ACE inhibitor use was associated with reduction in mortality and hospitalization in propensity score matched cohort of ambulatory chronic SHF patients with mild to moderate CKD.
Methods
Data source
Using standard protocols, we obtained the DIG dataset from the National Heart, Lung and Blood Institute of the National Institutes of Health. The University of Alabama at Birmingham approved an application for expedited review for the current study.
Patients
The randomized DIG trial, conducted during 1991-1993 in the United States (186 centers) and Canada (116 centers) enrolled 6,800 ambulatory patients with chronic SHF and normal sinus rhythm.12, 13 The objective of the trial was to evaluate the effects of digoxin on mortality and hospitalizations in HF. The DIG protocol encouraged the use of ACE inhibitors in all participants in the absence of specific contraindications or prior intolerance, and over 94% of patients were receiving ACE inhibitors at the time of randomization. Of the 6,800 patients with systolic HF, 1,707 had CKD as define below.
Chronic Kidney Disease
We defined CKD as baseline serum creatinine of 1.5 mg/dl or higher for men and 1.3 mg/dl or higher for women. Patients with serum creatinine 2.5 mg/dl or higher were not enrolled in the DIG trial. We chose to use serum creatinine over estimated glomerular filtration rate (GFR)14 for several reasons. First, in ambulatory care settings most clinicians use serum creatinine, rather than an estimated GFR, to evaluate kidney function. Second, estimated GFR is an unreliable tool to identify CKD in patients in otherwise good health and without CKD.15 Finally, serum creatinine of 1.5 mg/dl or higher for men and 1.3 mg/dl or higher for women has often been used to define CKD in the literature.16-18 In contrast to early stages of CKD, serum creatinine is a more reliable marker of CKD in the later stages.19 Patients included in our analysis had a median estimated GFR of 42 ml/min/1.73 m2.
Outcomes
The primary outcome of the DIG study was all-cause mortality with a mean follow up of 37 months (range 28 to 58 months). All-cause mortality was also the primary outcome of this study, but because few events occurred after the second year, especially in patients not receiving ACE inhibitors (number at risk at 3 and 4 years were 41 and 14, respectively), we restricted our analysis to two-year mortality. We also examined the effect of ACE inhibitor use on 2-year all-cause hospitalization.
Statistical analysis
Propensity Score Analysis
Because patients in the DIG trial were not randomly assigned to receive ACE inhibitor therapy, we used propensity scores to control for selection bias20-24 The propensity score represents the conditional probability of receiving an exposure or therapy given a vector of covariates and is used to adjust for selection bias in observational studies through matching, stratification or direct adjustment.10, 25-27
Estimation of Propensity Score
At first, we compared baseline characteristics of 1,707 patients with SHF and CKD receiving and not receiving ACE inhibitors using Pearson Chi-square tests and Student’s t tests appropriate. We then estimated the propensity scores or probability for the receipt of an ACE inhibitor for each of the 1,707 patients using a non-parsimonious multivariable logistic regression model.21, 28 Covariates in the model included age, sex, race, body mass index, duration of HF, etiology of HF (ischemic, hypertensive, idiopathic, and other), prior myocardial infarction, current angina, hypertension, diabetes, diuretic, potassium-sparing diuretics, combined use of nitroglycerin and hydralazine, pre-trial use of digoxin, limitation in physical activities, New York Heart Association (NYHA) functional class, dyspnea at rest, dyspnea on exertion, third heart sound, elevated jugular venous pressure, pulmonary râles, lower extremity edema, pulmonary congestion, cardiothoracic ratio >0.5, serum creatinine and potassium levels, echocardiographic estimation of left ventricular ejection fraction, and the interaction of age and serum creatinine. This set of covariates was designed to incorporate assessments of all key elements of the ACE inhibitor treatment decision. The model calibrated (Hosmer-Lemeshow test: p = 0.630) and discriminated (area under the ROC curve; C = 0.77) well.
Propensity Score Matching
Propensity score matching allows us to balance the distributions of all baseline characteristics incorporated in our propensity model. Unlike randomized controlled trials in which both measured and unmeasured covariates are expected to be similarly distributed across treatment groups, in propensity matching of observational studies, one can achieve a balanced distribution only of the measured covariates. Therefore, a key assumption in studies using propensity matching is that “hidden” or unmeasured covariates are sufficiently balanced across the matched treatment and control groups so as to not bias our conclusions29 While this assumption cannot be tested directly, we can assess the robustness of our conclusions with sensitivity analyses.
We used a SPSS macro to match patients according to their estimated propensity for receipt of ACE inhibitors.30 In our matching algorithm, we first matched each patient not receiving an ACE inhibitor with another patient receiving ACE inhibitor who had the same 5-digit propensity score. Matched patients were then removed from the file and the above process was then repeated on the remaining file, each time matching by 4-, 3-, 2-, and 1-digit propensity scores. In all, 104 of the 127 patients not receiving ACE inhibitors were matched with 104 patients receiving ACE inhibitors.
Effectiveness of Propensity Score Matching
Before matching (n=1,707), the mean (95% confidence interval) propensity score for patients receiving ACE inhibitors was 0.93990 (0.93635 - 94344) and that for patients not receiving ACE inhibitors was 0.74774 (0.69778 - 0.79769) (p <0.0001). After matching (n=208), the mean propensity score for patients receiving ACE inhibitors was 0.85142 (0.81611 - 88673) and that for those not receiving ACE inhibitors was 0.85121 (0.81571 - 88671) (p=0.993). Table 1 provides details on the balance of these characteristics across the ACE inhibitor and non-ACE inhibitor groups before and after propensity score matching.
Table 1.
Baseline patient characteristics by angiotensin-converting enzyme (ACE) inhibitor use before and after propensity score matching
| Pre-match | Post-match | |||||
|---|---|---|---|---|---|---|
| No ACE Inhibitors | ACE Inhibitors | P value | No ACE Inhibitors | ACE Inhibitors | P value | |
| % or mean (±SD) | N = 127 | N = 1580 | N = 104 | N = 104 | ||
| Age (years) | 69.2 (±10.2) | 68.2 (±9.7) | 0.268 | 68.7 (±10.3) | 68.1 (±9.2) | 0.625 |
| Women | 29 (22.8%) | 375 (23.7%) | 0.914 | 23 (22.1%) | 19 (18.3%) | 0.605 |
| Non-whites | 25 (19.7%) | 268 (17.0%) | 0.463 | 19 (18.3%) | 18 (17.3%) | >0.999 |
| Body mass index (kilogram / m2) | 26.2 (±4.5) | 26.7 (±5.1) | 0.289 | 26.4 (±4.5) | 27.5 (±5.5) | 0.102 |
| Duration of heart failure (months) | 28.6 (±30.9) | 32.0 (±39.1) | 0.413 | 29.0 (±31.4) | 25.3 (±33.0) | 0.413 |
| Etiology of heart failure | ||||||
| Ischemic | 95 (74.8%) | 1166 (73.8%) | 77 (74.0%) | 81 (77.9%) | ||
| Hypertensive | 9 (7.1%) | 155 (9.8%) | 0.403 | 9 (8.7%) | 6 (5.8%) | 0.811 |
| Idiopathic | 14 (11.0%) | 190 (12.0%) | 12 (11.5%) | 10 (9.6%) | ||
| Others | 9 (7.1%) | 69 (4.4%) | 6 (5.8%) | 7 (6.7%) | ||
| Comorbid conditions | ||||||
| Prior myocardial infarction | 83 (65.4%) | 1067 (67.5%) | 0.624 | 66 (63.5%) | 68 (65.4%) | 0.885 |
| Current angina pectoris | 40 (31.5%) | 446 (28.2%) | 0.432 | 30 (28.8%) | 35 (33.7%) | 0.550 |
| Hypertension | 74 (58.3%) | 863 (54.6%) | 0.459 | 59 (56.7%) | 53 (51.0%) | 0.487 |
| Diabetes Mellitus | 42 (33.1%) | 543 (34.4%) | 0.846 | 33 (31.7%) | 47 (45.2%) | 0.064 |
| Medications | ||||||
| Digoxin (pre-trial use) | 55 (43.3%) | 688 (43.5%) | >0.999 | 45 (43.3%) | 41 (39.4%) | 0.673 |
| Digoxin (during trial) | 60 (47.2%) | 781 (49.4%) | 0.646 | 48 (46.2%) | 49 (47.1%) | >0.999 |
| Diuretics | 107 (84.3%) | 1379 (87.3%) | 0.336 | 86 (82.7%) | 88 (84.6%) | 0.852 |
| Potassium-sparing diuretics | 16 (12.6%) | 145 (9.2%) | 0.207 | 12 (11.5%) | 14 (13.5%) | 0.834 |
| Nitrates and hydralazine | 33 (26.0%) | 18 (1.1%) | <0.0001 | 13 (12.5%) | 10 (9.6%) | 0.659 |
| Potassium supplement | 44 (34.6%) | 505 (32.0%) | 0.554 | 36 (34.6%) | 28 (26.9%) | 0.293 |
| Symptoms and signs | ||||||
| Dyspnea at rest | 41 (32.3%) | 409 (25.9%) | 0.117 | 32 (30.8%) | 38 (36.5%) | 0.463 |
| Dyspnea on exertion | 100 (78.7%) | 1241 (78.5%) | >0.999 | 84 (80.8%) | 85 (81.7%) | >0.999 |
| Activity limitation | 103 (81.1%) | 1256 (79.5%) | 0.732 | 84 (80.8%) | 86 (82.7%) | 0.858 |
| Elevated jugular venous pressure | 27 (21.3%) | 285 (18.0%) | 0.403 | 21 (20.2%) | 31 (29.8%) | 0.149 |
| Third heart sound | 31 (24.4%) | 461 (29.2%) | 0.308 | 23 (22.1%) | 21 (20.2%) | 0.865 |
| Pulmonary râles | 40 (31.5%) | 317 (20.1%) | 0.004 | 30 (28.8%) | 38 (36.5%) | 0.301 |
| Leg edema | 33 (26.0%) | 385 (24.4%) | 0.683 | 23 (22.1%) | 29 (27.9%) | 0.442 |
| NYHA functional class | ||||||
| I | 13 (10.2%) | 156 (9.9%) | 12 (11.5%) | 9 (8.7%) | ||
| II | 58 (45.7%) | 781 (49.4%) | 0.006 | 48 (46.2%) | 43 (41.3%) | 0.634 |
| III | 44 (34.6%) | 591 (37.4%) | 36 (34.6%) | 40 (38.5%) | ||
| IV | 12 (9.4%) | 52 (3.3%) | 8 (7.7%) | 12 (11.5%) | ||
| Heart rate (per minute) | 83.3 (±13.9) | 78.6 (±12.8) | <0.0001 | 83.2 (±14.4) | 84.3 (±13.1) | 0.576 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 130.5 (±20.8) | 127.7 (±21.6) | 0.160 | 129.5 (±20.8) | 128.5 (±21.1) | 0.725 |
| Diastolic | 76.5 (±10.4) | 74.3 (±11.8) | 0.035 | 75.5 (±9.9) | 76.1 (±12.0) | 0.674 |
| Chest x-ray findings | ||||||
| Pulmonary congestion | 26 (20.5%) | 295 (18.7%) | 0.637 | 20 (19.2%) | 21 (20.2%) | >0.999 |
| Cardiothoracic ratio >0.5 | 85 (66.9%) | 1053 (66.6%) | >0.999 | 66 (63.5%) | 65 (62.5%) | >0.999 |
| Laboratory data | ||||||
| Serum potassium (mEq/L) | 4.4 (±0.48) | 4.4 (±0.47) | 0.782 | 4.39 (±0.45) | 4.43 (±0.43) | 0.616 |
| Serum creatinine (mg/dL), | 1.88 (±0.44) | 1.76 (±0.34) | <0.0001 | 1.81 (±0.40) | 1.83 (±0.39) | 0.757 |
| Estimated GFR (ml/min/1.73 m2) | 39.5 (±10.3) | 41.5 (±8.8) | 0.014 | 40.8 (±9.5) | 40.6 (±9.3) | 0.889 |
| Ejection fraction (%),mean (±SD) | 29.2 (±9.0) | 28.0 (±8.9) | 0.142 | 28.8 (±9.0) | 28.4 (±8.8) | 0.708 |
GFR=glomerular filtration rate, HF=heart failure, JVP=jugular venous pressure, NYHA=New York heart association
To determine if the propensity score matching produced balanced distributions of baseline characteristics across the non-ACE inhibitor and ACE inhibitor groups, we measured covariate imbalance using standardized differences, which describe the observable selection bias remaining after matching. The standardized difference is the difference of the group means (or proportions, in the case of binary covariates) expressed as a percentage of an appropriate (pooled) standard deviation.25, 27 A perfectly balanced covariate will have a standardized difference of 0%. Standardized differences substantially exceeding 10% in absolute value after matching suggest relatively poor balance,25 and indicate the need for additional covariate adjustments (i.e. through regression) in order to develop fair assessments of the treatment effect.
Survival Analysis
After assessing the adequacy of our propensity match, we constructed survival curves to describe the 208 matched patients by receipt of ACE inhibitors using Kaplan-Meier estimates and assessed statistical significance based on the log-rank test. In plotting the survival curves, we first estimated mean 2-year unadjusted survival times for patients stratified by receipt or non-receipt of ACE inhibitors. The association of ACE inhibitor therapy with all-cause, 2-year mortality was determined using bivariate Cox proportional hazard regression analyses. We then used a multivariable Cox proportional hazard model to determine the risk of 2-year mortality adjusted by propensity scores and other covariates. The covariates in the model included those used in the propensity score model. Use of digoxin during the trial was also included in the model. Age, sex, race, and key covariates with >10% post-match standardized differences, namely, body mass index, duration of HF, current angina, hypertension, diabetes, use of potassium supplement, dyspnea at rest, elevated jugular venous pressure, pulmonary râles, edema, and NYHA class III-IV were forced into the model. All other covariates were entered in a forward stepwise fashion. A similar approach was use to examine the effect of ACE inhibitors on 2-year all-cause hospitalizations. We also repeated our analysis in the pre-match cohort of patients using a similar approach. All statistical tests were evaluated using a two-tailed 95% confidence level. Analyses were performed using SPSS for Windows (Release 13).31
Results
Patient characteristics
The mean (±SD) age of the 208 propensity matched patients with SHF and CKD was 68.4 (±9.8) years. Forty two (20.2%) were female and 37 (17.8%) were non-white. Table 1 compares baseline characteristics of 208 propensity score matched patients with CKD by the receipt of ACE inhibitor. Matching reduced the standardized differences for almost all prognostically important variables below 10% in absolute value, including age, sex, race, serum creatinine, and left ventricular ejection fraction. Post-match standardized differences for a few covariates, including diabetes, dyspnea at rest, elevated jugular venous pressure, and NYHA class, exceeded 10% in absolute value, indicating relatively weak balance, and prompting subsequent additional adjustments for these covariates in our Cox regression models.
ACE Inhibitor Use and All-Cause Mortality
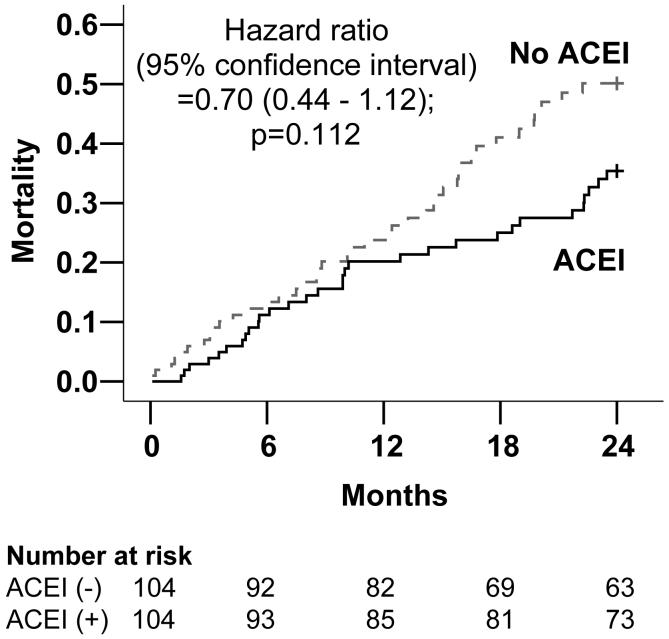
Overall, 72 patients (34.6%) died from all causes during a 2-year follow up. Compared with 31 (29.8%) deaths in patients receiving ACE inhibitors, 41 (39.4%) of those not receiving ACE inhibitors died (9.6% absolute risk reduction; Chi-square p =0.145). Figure 1 displays Kaplan-Meier plots for unadjusted 2-year cumulative mortality for patients receiving versus not receiving ACE inhibitor therapy. Unadjusted mean survival was 45 days longer for patients receiving ACE inhibitors: 613 (95% confidence interval 572 to 655) days for versus 568 (95% confidence interval 523 to 614) days for those not receiving ACE inhibitors (log rank test p = 0.136).
Figure 1.
Kaplan-Meier plots for all-cause mortality
Table 2 demonstrates that among propensity matched patients, ACE inhibitor use was associated with a non-significant 30% relative reduction in the risk of unadjusted all-cause mortality (unadjusted hazard ratio, 0.70; 95% confidence interval, 0.44 to 1.12). Adjustment for covariates made the association stronger and statistically significant (adjusted hazard ratio, 0.59; 95% confidence interval, 0.36 to 0.97). The relationship remained essentially unchanged after additional adjustment for propensity scores. When we examined the effect of ACE inhibitors on mortality for the entire follow up (34 months median), use of ACE inhibitor was associated with a 23% non-significant reduction in mortality (adjusted hazard ratio, 0.77; 95% confidence interval, 0.50 to 1.18). Among the 1,707 pre-match patients, use of ACE inhibitor was associated with a significant 31% relative reduction in all-cause mortality (unadjusted hazard ratio, 0.69; 95% confidence interval, 0.51 to 0.92). The association became weaker and lost significance after adjustment for propensity score; however, remained essentially unchanged after adjustment for covariates.
Table 2.
Crude and adjusted hazard ratios (95% confidence intervals) for 2-year all-cause mortality for ambulatory chronic heart failure patients with systolic dysfunction and chronic kidney disease by use of angiotensin-converting enzyme (ACE) inhibitors
| Hazard ratio (95% confidence interval) | P values | |
|---|---|---|
| Pre-match (n=1,707) | 0.69 (0.51 - 0.92) | 0.016 |
| Pre-match: Adjusted for propensity scores | 0.83 (0.59 - 1.17) | 0.292 |
| Pre-match: Adjusted* for covariates | 0.66 (0.49 - 0.90) | 0.008 |
| Post-match (n=208) | 0.70 (0.44 - 1.12) | 0.112 |
| Post-match: Adjusted** for covariates | 0.59 (0.36 - 0.97) | 0.039 |
| Post-match: Adjusted** for covariates and propensity scores | 0.58 (0.35 - 0.96) | 0.034 |
Covariates in the final model included age, sex, race, diabetes, pulmonary râles, NYHA class III-IV, pre-trial use of digoxin, diastolic blood pressure, serum creatinine, cardiothoracic ration >0.50, and number of symptoms and signs of heart failure.
Covariates in the final model included age, sex, race, body mass index, duration of heart failure, angina, hypertension, diabetes, use of potassium supplement, dyspnea at rest, elevated jugular venous pressure, pulmonary râles, edema, NYHA class III-IV, diastolic blood pressure, serum creatinine, and number of symptoms and signs of heart failure.
ACE Inhibitor Use and All-Cause Hospitalization
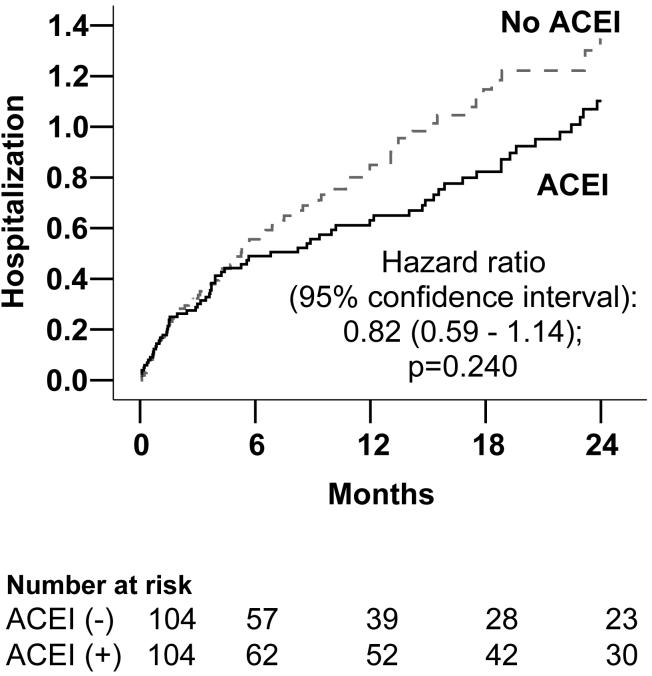
Overall, 139 (66.8%) patients were hospitalized from all causes during the 2-year follow up. Compared with 64.4% (67 / 104) of patients hospitalized among those receiving ACE inhibitors, 69.2% (41 / 104) of those not receiving ACE inhibitors were hospitalized (4.5% absolute reduction; Chi-square p =0.462). Figure 2 displays Kaplan-Meier plots for unadjusted 2-year cumulative all-cause hospitalization for patients receiving versus not receiving ACE inhibitors. Cumulative survival free from hospitalization for patients receiving ACE inhibitors was 33% (versus 26% for those not receiving these drugs). This represents 54 additional mean days free from hospitalization for patients receiving ACE inhibitors: 401 (95% confidence interval 343 to 459) days versus 347 (95% confidence interval 291 to 403) days for those not receiving ACE inhibitors (log rank test p = 0.136).
Figure 2.
Kaplan-Meier plots for all-cause hospitalization
Table 3 demonstrates that among propensity matched patients, ACE inhibitor use was associated with a non-significant 18% relative reduction in the risk of unadjusted all-cause hospitalization (unadjusted hazard ratio, 0.82; 95% confidence interval, 0.59 to 1.14). Adjustment for covariates made the association stronger (adjusted hazard ratio, 0.70; 95% confidence interval, 0.49 to 1.01, p=0.054). After further adjustment for propensity score, the relationship remained essentially unchanged (adjusted hazard ratio 0.69; 95% confidence interval 0.48-0.98, p=0.04). Among the 1,707 pre-match patients, use of ACE inhibitor was associated with a significant 26% relative reduction in all-cause hospitalization (unadjusted hazard ratio, 0.74; 95% confidence interval, 0.60 to 0.92). The association became weaker and lost significance after adjustment for propensity score; however, remained essentially unchanged after adjustment for covariates.
Table 3.
Crude and adjusted hazard ratios (95% confidence intervals) for 2-year all-cause hospitalization for ambulatory chronic heart failure patients with systolic dysfunction and chronic kidney disease by use of ACE inhibitors
| Hazard ratio (95% confidence interval) | P values | |
|---|---|---|
| Pre-match (n=1,707) | 0.74 (0.60 - 0.92) | 0.007 |
| Pre-match: Adjusted for propensity scores | 0.83 (0.65 - 1.06) | 0.128 |
| Pre-match: Adjusted* for covariates | 0.77 (0.61 - 0.96) | 0.019 |
| Post-match (n=208) | 0.82 (0.59 - 1.14) | 0.240 |
| Post-match: Adjusted** for covariates | 0.70 (0.49 - 1.01) | 0.054 |
| Post-match: Adjusted** for covariates and propensity scores | 0.69 (0.48 - 0.98) | 0.040 |
Covariates in the final model included age, sex, race, diabetes, pre-trial use of digoxin, use of potassium supplement, dyspnea on exertion, pulmonary râles, edema, NYHA class III-IV, diastolic blood pressure, serum creatinine, and cardiothoracic ratio >0.50.
Covariates in the final model included age, sex, race, body mass index, duration of heart failure, angina, hypertension, diabetes, use of potassium supplement, dyspnea at rest, elevated jugular venous pressure, pulmonary râles, edema, NYHA class III-IV, diastolic blood pressure, and serum creatinine
Discussion
In ambulatory chronic SHF patients with mild to moderate CKD, ACE inhibitor use was associated with significant reduction in risk-adjusted all-cause mortality and all-cause hospitalizations. These results are important as CKD is common in HF, is associated with poor prognosis,32 and underuse of ACE inhibitors.6, 7, 33 However, our data indicate that such an approach is likely to be detrimental.
Comparison with other published studies
We noted a 9.6% reduction in absolute risk of all-cause mortality associated with ACE inhibitor use. This compares favorably with the 4.5% absolute reduction in all-cause death in SHF patients in the SOLVD trial (39.7% deaths in patients receiving placebo versus 35.2% for those receiving enalapril).34 Compared to the SOLVD trial, patients in our analysis were older (mean age 68 years versus 61 years in SOLVD) and sicker (46% had NYHA class III-IV versus 33% in SOLVD). In addition, they had higher mean serum creatinine (1.8 mg/dL versus 1.2 mg/dL in SOLVD). Treatment effects are often known to depend on severity or stage of the disease, or other comorbidities.35 In an elderly (mean age 79 years) cohort of hospitalized acute SHF patients with advanced CKD (mean serum creatinine 2.9 mg/dL), use of ACE inhibitor was associated with a 31% absolute reduction in mortality.8 Therefore, the findings of our study are consistent with other published reports, and are mechanistically plausible. SHF patients with CKD comprise a high-risk segment of the SHF population, and ACE inhibitors likely confer added benefit to these patients through their dual renoprotective and cardioprotective properties.
Clinical Implications
National HF guidelines and manufacturers’ package inserts of commonly used ACE inhibitors do not identify CKD as a contraindication to ACE inhibitor use.1, 8, 36 However, due to the paucity of outcomes data in this population, coupled with concerns about an increased risk for worsening renal function and hyperkalemia,37, 38 the guidelines alert clinicians to be cautious when using ACE inhibitors in patients with significant CKD1, 36 Short- and long-term survival benefits of ACE inhibitors in hospitalized older adults with acute SHF and advanced CKD have been documented in the literature.8, 10 Our analysis demonstrates that in ambulatory patients with chronic mild to moderate SHF and mild to moderate CKD, ACE inhibitor use was associated with reduced mortality and hospitalization. Because there are no randomized clinical trials of ACE inhibitors in SHF patients with CKD, analyses of existing databases using methods such as propensity score analysis will likely provide cumulative evidence for use of ACE inhibitors in these patients. However, because these patients may be at risk for hyperkalemia, serum potassium levels should be closely monitored during initiation and titration of ACE inhibitor therapy.
Even though many clinicians interpret a rise in serum creatinine in response to ACE inhibitor therapy as an indicator of renal damage, the renoprotective properties of ACE inhibitors is well documented in the literature.3-5 In one study, serum creatinine dropped to baseline after ACE inhibitor therapy was discontinued after 6 years suggesting that there was no permanent structural damage to the kidneys.38 In deed, many nephrologists compare a rise in serum creatinine in response to ACE inhibitor therapy to bradycardia in response to a beta-blockers and consider that a marker of effectiveness of ACE inhibitor therapy.
Strengths and Limitations
A key strength of our study is that we used propensity scores to specify a matched subgroup of patients within a nonrandomized cohort. Propensity score matching, in combination with additional regression-based adjustments, allowed us to substantially reduce the impact of selection bias due to all observed baseline characteristics in Table 1.
Several key limitations must be acknowledged. First, propensity methods do not account for bias due to unmeasured or hidden covariates. The results of our study are sensitive to potential hidden covariates (normal deviate =1.19, two-tailed p value =0.234).29 However, sensitivity analysis cannot determine if such a hidden covariate existed. If such a hidden covariate existed, it would only explain away our findings if it correlated with both receipt of ACE inhibitor and clinical outcomes, and if it was not strongly correlated with any of the other variables used in the propensity model. Of note, the sensitivity analysis was based on our propensity-matched “unadjusted” results, and not based on “additional multivariable adjusted” results.
Another potential limitation of our study is that baseline characteristics were not perfectly balanced in the propensity matched cohorts. This most likely reflects the large number of variables considered for the propensity score analysis and the relatively small number of matched pairs. Nonetheless, it is worth noting that more patients receiving ACE inhibitors had diabetes, dyspnea at rest, elevated jugular venous pressure, and higher NYHA classes (Table 1), which might have increased the risk of adverse events for these patients. When we adjusted for these covariates in the multivariable model, the association between ACE inhibitor use and favorable outcomes became stronger. Exclusion of unmatched patients might be considered a potential limitation. Compared with matched patients receiving ACE inhibitors (n=104), unmatched patients receiving ACE inhibitors (n=1476) had significantly higher propensity to receive these drugs (0.94613 versus 0.85142 for unmatched patients; p <0.0001). As higher propensity or probability of receiving ACE inhibitors might be marker for better outcomes, inclusion of unmatched patients in our analysis would have inflated our findings. Therefore, while exclusion of unmatched patients might have compromised to some degree the generalizability of our findings, it has added internal validity to our results.
Patients with a serum creatinine level of 2.5 mg or higher and atrial fibrillation were excluded from the DIG trial. In addition, DIG participants tended to be younger than most HF patients treated in clinical practice, and women and minorities were under-represented. Studies are also needed to assess the safety and efficacy of ACE inhibitors in patients with HF and preserved left ventricular systolic function.
Conclusions
In ambulatory patients with chronic systolic mild to moderate HF and mild to moderate CKD, use of ACE inhibitors was associated with significant reductions in risk-adjusted all-cause mortality and all-cause hospitalization. The results of this study, based on observational data, are mechanistically plausible and consistent with previous reports. Therefore, these hypothesis-generating finding also provide interim evidence of potential benefits of ACE inhibitors in SHF patients with CKD. However, life-saving therapy with ACE inhibitors is probably being withheld on the basis of questionable evidence. These cumulative evidence of the beneficial effects of ACE inhibitors in SHF patients with CKD calls for a randomized clinical trial of ACE inhibitors in these patients.
Acknowledgement
“The Digitalis Investigation Group (DIG) study was conducted and supported by the National Heart Lung and Blood Institute (NHLBI) in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.
The authors wish to thank David Warnock, MD, Division of Nephrology, Department of Medicine, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama for his review of the manuscript and his excellent constructive comments.
Funding/Support: Dr. Ahmed is supported by grants 1-K23-AG19211-04 from the National Institutes of Health / National Institute on Aging and 1-R01-HL085561-01 from the National Institutes of Health / National Heart Lung and Blood Institute.
Footnotes
Preliminary results based on this analysis were presented at the Scientific Sessions of the 2004 American Heart Association national meeting in New Orleans, LA.
Contributor Information
Ali Ahmed, Department of Medicine, School of Medicine, and Department of Epidemiology, School of Public Health, and Center for Heart Failure Research, University of Alabama at Birmingham, and VA Medical Center, Birmingham, Alabama; USA.
Thomas E. Love, Case Western Reserve University - MetroHealth Medical Center, Department of Medicine, School of Medicine and Department of Operations, Weatherhead School of Management, Case Western Reserve University, Cleveland, Ohio; USA.
Xuemei Sui, Center for Data Management, the Cooper Institute, Dallas, Texas, USA.
Michael W. Rich, Department of Medicine, School of Medicine, Washington University, St Louis, Missouri, USA.
References
- 1.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation. Endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 13; doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.Garg R, Yusuf S, Collaborative Group on ACE Inhibitor Trials Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA. 1995 May 10;273:1450–1456. [PubMed] [Google Scholar]
- 3.Ruggenenti P, Perna A, Remuzzi G. ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol. 2001 Dec;12:2832–2837. doi: 10.1681/ASN.V12122832. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F, Carbarns IR, Maschio G, et al. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group Long-term progression of chronic renal insufficiency in the AIPRI Extension Study. Kidney Int Suppl. 1997 Dec;63:S63–66. [PubMed] [Google Scholar]
- 5.Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ. 2004 Oct 9;329:828. doi: 10.1136/bmj.38237.585000.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bart BA, Gattis WA, Diem SJ, O’Connor CM. Reasons for underuse of angiotensin-converting enzyme inhibitors in patients with heart failure and left ventricular dysfunction. Am J Cardiol. 1997;79:1118–1120. doi: 10.1016/s0002-9149(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 7.Frances CD, Noguchi H, Massie BM, Browner WS, McClellan M. Are we inhibited? Renal insufficiency should not preclude the use of ACE inhibitors for patients with myocardial infarction and depressed left ventricular function. Arch Intern Med. 2000;160:2645–2650. doi: 10.1001/archinte.160.17.2645. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed A, Kiefe CI, Allman RM, Sims RV, DeLong JF. Survival benefits of angiotensin-converting enzyme inhibitors in older heart failure patients with perceived contraindications. J Am Geriatr Soc. 2002 Oct;50:1659–1666. doi: 10.1046/j.1532-5415.2002.50457.x. [DOI] [PubMed] [Google Scholar]
- 9.Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006 Feb 7;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed A, Centor R, Weaver MT, DeLong JF. A propensity score analysis of the impact of ACE inhibitors on 4-year survival of older adults with heart failure and perceived contraindications. Am Heart J. 2005;149:737–743. doi: 10.1016/j.ahj.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 11.Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med. 2003 Jun 3;138:917–924. doi: 10.7326/0003-4819-138-11-200306030-00013. [DOI] [PubMed] [Google Scholar]
- 12.The Digitalis Investigation Group Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996 Feb;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 13.The Digitalis Investigation Group The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997 Feb 20;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999 Mar 16;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004 Dec 21;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 16.Chae CU, Albert CM, Glynn RJ, Guralnik JM, Curhan GC. Mild renal insufficiency and risk of congestive heart failure in men and women > or =70 years of age. Am J Cardiol. 2003 Sep 15;92:682–686. doi: 10.1016/s0002-9149(03)00822-1. [DOI] [PubMed] [Google Scholar]
- 17.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004 May;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 18.Shlipak MG, Fried LF, Stehman-Breen C, Siscovick D, Newman AB. Chronic renal insufficiency and cardiovascular events in the elderly: findings from the Cardiovascular Health Study. Am J Geriatr Cardiol. 2004 Mar-Apr;13:81–90. doi: 10.1111/j.1076-7460.2004.02125.x. [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39:S1–266. [PubMed] [Google Scholar]
- 20.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 21.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997 Oct 15;127:757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 22.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999 Aug 15;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 24.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002 Oct 15;137:693–695. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 25.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001 Apr;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 26.Brener SJ, Lytle BW, Casserly IP, Schneider JP, Topol EJ, Lauer MS. Propensity analysis of long-term survival after surgical or percutaneous revascularization in patients with multivessel coronary artery disease and high-risk features. Circulation. 2004 May 18;109:2290–2295. doi: 10.1161/01.CIR.0000126826.58526.14. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J. 2006 Jan;27:178–186. doi: 10.1093/eurheartj/ehi687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Love TE. [Last access date: June 4, 2005];Doing Propensity Analysis: The Basics and Matching. Available at http://www.chrp.org/propensity/
- 29.Rosenbaum PR. Observational Studies. Springer-Verlag; New York: 2002. [Google Scholar]
- 30.Levesque R. Macro. In: Levesque R, editor. SPSS® Programming and Data Management, 2nd Edition. A Guide for SPSS® and SAS® Users. 2nd Edition. SPSS Inc; Chicago, IL: [Last access date: June 4, 2005]. Available online at: http://www.spss.com/spss/data_management_book.htm. [Google Scholar]
- 31.SPSS for Windows, Rel. 13. SPSS Inc.; Chicago: 2005. [computer program]. Version. [Google Scholar]
- 32.Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM. Renal function, digoxin therapy, and heart failure outcomes: evidence from the digoxin intervention group trial. J Am Soc Nephrol. 2004 Aug;15:2195–2203. doi: 10.1097/01.ASN.0000135121.81744.75. [DOI] [PubMed] [Google Scholar]
- 33.Houghton AR, Cowley AJ. Why are angiotensin converting enzyme inhibitors underutilised in the treatment of heart failure by general practitioners? Int J Cardiol. 1997 Mar;59:7–10. doi: 10.1016/s0167-5273(96)02904-x. [DOI] [PubMed] [Google Scholar]
- 34.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991 Aug 1;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 35.Rothwell PM. Treating individuals 2. Subgroup analysis in randomised controlled trials: importance, indications, and interpretation. Lancet. 2005 Jan 8;365:176–186. doi: 10.1016/S0140-6736(05)17709-5. [DOI] [PubMed] [Google Scholar]
- 36.Swedberg K, Cleland J, Dargie H, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005 Jun;26:1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed A. Use of angiotensin-converting enzyme inhibitors in patients with heart failure and renal insufficiency: how concerned should we be by the rise in serum creatinine? J Am Geriatr Soc. 2002 Jul;50:1297–1300. doi: 10.1046/j.1532-5415.2002.50321.x. [DOI] [PubMed] [Google Scholar]
- 38.Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]