Abstract
The evaluation and treatment of aggressive bone tumors continue to be diagnostic and therapeutic challenges for orthopaedic surgeons. Despite compelling data regarding the hazards of biopsy, incomplete preoperative evaluation, inappropriate biopsy techniques, and premature surgical interventions continue to compromise optimal treatment of primary bone sarcomas. We retrospectively identified eight patients who had internal fixation of a primary bone sarcoma before referral to an orthopaedic oncology service. Six of the eight patients subsequently underwent amputations and two patients underwent limb salvage for local disease control. Biopsy techniques from referring institutions were highly variable, with only two of seven rendering an accurate diagnosis. The average Musculoskeletal Tumor Society functional score was 10.6 and four of eight patients were disease-free and alive at a minimum followup of 8 months (mean, 26.9 months; range, 8–80 months). Implant violation of primary bone malignancies was associated with frequent high-level amputation for local disease control and low Musculoskeletal Tumor Society functional scores. Common errors in the initial evaluation and treatment included inadequate attention to patient history, incomplete radiographic evaluation, and improper biopsy and surgical techniques, which violated compartmental boundaries.
Level of Evidence: Level IV, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The diagnostic evaluation of pathologic fractures continues to challenge orthopaedic surgeons because of the many diagnostic possibilities, including metastatic and primary tumors of bone. Metastatic tumors of bone are far more common than primary malignancies [8]; however, with vastly different treatment objectives and surgical management, accurate differentiation between these two clinical entities is critical. Multiple diagnostic strategies have been suggested to determine the origin of a suspected metastatic bone lesion of unknown primary tumor [14, 17, 21]. In the absence of a biopsy, however, these strategies do not effectively identify primary bone malignancies. As a result, primary bone sarcomas may be mistaken for metastatic bone disease and treated inappropriately. A single case series of three patients with high-grade primary bone sarcomas initially treated with retrograde intramedullary nails by Spence et al. [22] and an instructional course lecture by Peabody [16] provide the only literature on the subject, with those authors emphasizing thorough preoperative evaluation and diagnostic biopsy before surgical treatment. We presumed failure to adhere to these preoperative recommendations contributed to inadvertent fixation of primary bone sarcomas in a series of patients referred to our institution after internal fixation.
The purposes of our study were (1) to identify and report the critical errors in initial diagnosis and management leading to inadvertent implant violation of these eight primary bone sarcomas with particular attention to simple preventive strategies and (2) to report the definitive salvage treatment measures, functional outcomes, disease control, and survival after inadvertent internal fixation of primary bone sarcomas.
Materials and Methods
We retrospectively reviewed the medical records of eight patients with primary malignancies of bone who had been treated previously for an actual or impending fracture with an internal fixation device from 1994 to 2004. Patients with metastatic osseous lesions, including metastatic sarcomas, were excluded. There were seven women and one man, with an average age of 63 years (range, 43–79 years). Six patients had no medical history of malignancy. One patient had a history of basal cell carcinoma treated with wide local excision and the remaining patient had a history of prostate carcinoma treated with pelvic external beam radiation. In seven patients, the tumor was located in the femur, three in the proximal metaphysis and four in the distal metaphysis. In the remaining patient, the tumor was located in the proximal humeral metaphysis (Table 1). The minimum followup after the index procedure (internal fixation) was 8 months (mean, 27 months; range, 8–80 months); followup was limited by the early death of four patients at 8, 9, 12, and 18 months postoperatively. We had prior Institutional Review Board approval.
Table 1.
Summary data for eight patients with inadvertently instrumented primary sarcomas of bone
| Patient | Age (years) | Gender | Diagnosis | Stage | Location | Initial procedure | Definitive surgery | Followup (months) | Status | MSTS score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 79 | Female | High-grade pleomorphic undifferentiated sarcoma | III | Right distal femur | RIMN | Hip disarticulation | 22 | DF/A | 11.5 (38.3%) |
| 2 | 66 | Female | Dedifferentiated chondrosarcoma | IIb | Left proximal humerus | PS | Megaprosthesis | 31 | DF/A | 20 (66.7%) |
| 3 | 43 | Female | Telangiectatic osteosarcoma | IIb | Left distal femur | RIMN | Hip disarticulation | 35 | DF/A | 7.5 (25%) |
| 4 | 63 | Female | Dedifferentiated chondrosarcoma | IIb | Right distal femur | RIMN | Megaprosthesis | 12 | M/D | 11.5 (38.3%) |
| 5 | 52 | Female | Mesenchymal chondrosarcoma | III | Left proximal femur | RIMN | Hip disarticulation | 8 | M/D | 7.5 (25%) |
| 6 | 66 | Female | Malignant giant cell tumor | III | Right proximal femur | PS | Hemipelvectomy | 9 | M/D | 6.5 (21.7%) |
| 7 | 71 | Male | High-grade pleomorphic undifferentiated sarcoma | IIb | Right proximal femur | AIMN | Hemipelvectomy | 18 | M/D | 10.5 (35%) |
| 8 | 64 | Female | Dedifferentiated chondrosarcoma | IIb | Right distal femur | RIMN | Hip disarticulation | 80 | DF/A | 10 (33.3%) |
MSTS = Musculoskeletal Tumor Society; RIMN = retrograde intramedullary nail; AIMN = antegrade intramedullary nail; PS = plate and screw construct; DF = disease-free; M = metastatic disease; A = alive; D = dead.
From the records, we noted relevant demographic and clinical data, including patient age, gender, medical history of malignancy, and tumor location. We recorded the history of present illness and the initial radiographic evaluation and interpretation. The initial presenting complaint was a fall associated with acute fracture in six patients, one of whom reported antecedent pain. One patient presented with localized extremity pain without fracture and another patient presented with extremity pain and sustained an atraumatic fracture while undergoing inpatient preoperative evaluation for biopsy. The initial radiographic evaluation consisted only of radiographs of the involved extremity in seven patients (Fig. 1). One patient had radiographs, MRI of the extremity, and a whole-body technetium-99 bone scan. Clinical suspicion of a pathologic fracture was documented before initial treatment in five cases.
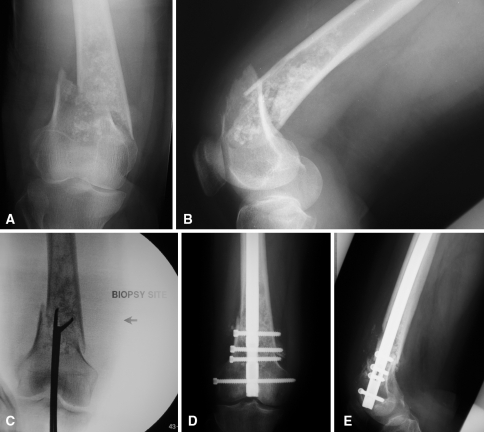
Fig. 1A–E.
Preoperative (A) anteroposterior and (B) lateral radiographs of a 65-year-old woman with a pathologic fracture of the right distal femur show a large intramedullary lesion with ill-defined borders, diffuse endosteal scalloping, and cartilaginous matrix production at the level of the fracture. (C) An intraoperative fluoroscopic image shows a joint-violating intramedullary biopsy of the chondrosarcoma. Postoperative (D) anteroposterior and (E) lateral radiographs show stabilization of the fracture with a retrograde intramedullary nail, violating the chondrosarcoma and contaminating the proximal femoral canal.
We recorded the biopsy technique, including timing, surgical approach, and use of frozen-section analysis at initial biopsy, initial histopathologic impression, and initial treatment modalities for each patient. In five patients, tissue was obtained for histopathologic analysis at the time of internal fixation using various methods: conventional incisional biopsy (one), curettings through the trochlear notch before retrograde nail insertion (two), and intramedullary reamings (two). Intraoperative frozen-section analysis was used in two patients, reported as “cellular elements with atypia” in one and “fibrous histiocytoma versus giant cell tumor” in the other. In two additional patients, the biopsy tissue was obtained during a secondary procedure at the referring institution, one prompted by progressive lucency around the implant and the other by persistent pain and swelling at the incision site. In the remaining patient, no biopsy tissue was obtained at the referring facility and open biopsy was performed at our institution.
We defined index treatment as the initial surgical treatment for the actual or impending pathologic fracture before referral and included internal fixation with either an intramedullary device (six) or a plate and screw construct (two). All six intramedullary devices involved femoral lesions and four of the six were retrograde nails used to stabilize the distal femur. Plates and screws were used in two patients, one in the proximal femur and the other in the proximal humerus.
Subsequent to presentation at our institution, stage of disease and final diagnoses, based on permanent histopathology from the definitive resection or amputation, were determined. We recorded definitive medical and surgical treatments, complications, and followup from time of the index surgical procedure. Musculoskeletal Tumor Society (MSTS) functional scores [5] were calculated to assess posttreatment function, and patient survival, local recurrence, and metastatic disease were recorded. Tumors were staged using the MSTS staging system [6], and all patients were considered to have extracompartmental disease resulting from compartmental contamination at their index procedure. Thus, five patients presented with Stage IIB disease and three patients presented with Stage III disease. The final pathologic diagnoses were dedifferentiated chondrosarcoma (three), mesenchymal chondrosarcoma (one), malignant fibrous histiocytoma (two), osteosarcoma (one), and malignant giant cell tumor (one). We compared these final diagnoses with initial pathologic impressions from biopsy. In seven of eight patients, the initial pathologic impression was suspicious for sarcoma; however, an accurate histopathologic diagnosis was made in only two patients. In the remaining patient, “fracture healing reaction” was the initial pathologic diagnosis based on analysis of intramedullary reamings after acute fracture.
Definitive surgical treatment was defined as the procedure required for local tumor control and included amputation or wide local resection with limb salvage. Amputation was performed in six patients: four hip disarticulations and two hemipelvectomies. Limb salvage was performed in two patients: one proximal humeral resection with endoprosthetic reconstruction and one femoral resection with total femur endoprosthetic reconstruction. The patient with the total femur megaprosthesis refused amputation after retrograde intramedullary nailing of a distal femoral dedifferentiated chondrosarcoma. There were two surgical complications for which additional surgery was performed. One patient had an acute postoperative infection after hip disarticulation and was treated successfully with irrigation, débridement, and intravenous antibiotics. The second patient had anterior subluxation of a proximal humeral prosthesis 9 months postoperatively effectively treated with open relocation, capsular reconstruction, and subscapularis advancement. Including initial treatment and secondary procedures, patients underwent an average of 2.9 operative procedures (range, 2–4 procedures). Adjuvant external beam radiation was administered to one patient after the initial procedure and before presentation to our institution. Adjuvant chemotherapy was administered to four patients after referral.
Results
We identified four critical errors: inadequate attention to patient history, incomplete radiographic evaluation, improper biopsy techniques and histopathologic interpretation, and surgical techniques that violated compartmental boundaries (Table 2). Three of eight patients had a documented clinical history suspicious for pathologic fracture; however, only one patient had any additional radiographic or clinical assessment. The initial radiographic evaluation was considered inadequate in seven patients, consisting of radiographs of the affected extremity only. Initial biopsy errors occurred in all referred patients, including the failure to biopsy the lesion before initial treatment (three), use of an intraarticular biopsy technique (three), intraoperative biopsy without frozen-section analysis (three), failure to properly interpret intraoperative frozen section (two), and use of intramedullary reamings as biopsy material (two). Initial treatment errors occurred in all eight patients, including violation of soft tissue compartments and/or the knee in all patients. All eight patients were surgically treated before establishing a diagnosis and three patients were treated without an attempted biopsy.
Table 2.
Critical errors in the initial evaluation and treatment
| Patient | Clinical history error | Radiologic error | Biopsy error | Treatment error |
|---|---|---|---|---|
| 1 | Failure to document ± pain before fracture | Radiographs only | No biopsy at index procedure | (1) Treatment without diagnosis; (2) treatment without biopsy; (3) violation of knee |
| 2 | Failure to document ± pain before fracture | Radiographs only | No biopsy at index procedure | (1) Treatment without diagnosis; (2) treatment without biopsy; (3) soft tissue contamination |
| 3 | Failure to document ± pain before fracture | Radiographs only | No biopsy at index procedure | (1) Treatment without diagnosis; (2) treatment without biopsy; (3) violation of knee |
| 4 | Failure to evaluate suspicious history (atraumatic fracture) | Radiographs only | (1) Failure to properly interpret intraoperative frozen section analysis; (2) biopsy violation of knee | (1) Treatment without diagnosis; (2) violation of knee |
| 5 | Failure to document ± pain before fracture | Radiographs only | (1) Intraoperative biopsy without intraoperative frozen section analysis; (2) biopsy violation of knee | (1) Treatment without diagnosis; (2) violation of knee |
| 6 | Failure to evaluate suspicious history (pain preceding fracture) | Radiographs, MRI, bone scan obtained and inappropriately interpreted | Failure to properly interpret intraoperative frozen section analysis | (1) Treatment without diagnosis; (2) soft tissue contamination |
| 7 | Failure to evaluate suspicious history (pain preceding fracture) | Radiographs only | (1) Intraoperative biopsy without intraoperative frozen section analysis; (2) reamings as biopsy specimen | (1) Treatment without diagnosis; (2) soft tissue contamination |
| 8 | Failure to document ± pain before fracture | Radiographs only | (1) Intraoperative biopsy without intraoperative frozen section analysis; (2) reamings as biopsy specimen; (3) biopsy violation of knee | (1) Treatment without diagnosis; (2) violation of knee |
Four of the eight patients were alive and free of apparent disease at last followup with one in full remission from metastatic disease, but inadvertent internal fixation of primary osseous sarcomas resulted in high-level amputations, multiple surgical procedures, and low MSTS functional scores. Six of eight patients underwent amputations for local disease control: four hip disarticulations and two hemipelvectomies. Three of the four patients with distal femoral lesions had hip disarticulations, a considerably more radical procedure than typically is indicated for high-grade malignancies of the distal femur, the majority of which can be treated successfully with limb-sparing surgery or transfemoral amputation. Additionally, these patients had an average of 2.9 procedures, which is unnecessarily high for a series of patients who should be diagnosed and treated successfully with one biopsy and a definitive procedure. The mean MSTS functional score of all patients at last clinical followup was 10.6 (range, 6.5–20). The only patient with reasonable recovery of function, with an MSTS functional score of 20, was the patient with proximal humeral reconstruction. The average functional score for the seven patients with lower extremity tumors was 9.3. Four of the eight patients died, with a mean survival of 12.8 months (range, 8–21 months). Two patients who died presented with Stage IIB disease, one of whom had disseminated disease develop after amputation and systemic chemotherapy. The other two patients who died had Stage III disease at presentation. One patient with metastatic disease to the lungs at referral had a complete response to chemotherapy and remained disease-free at 22 months followup. There were no observed local recurrences.
Discussion
Existing literature regarding inadvertent internal fixation of primary osseous sarcomas emphasizes the need for thorough preoperative evaluation and diagnostic biopsy before surgical intervention [16, 22]. However, we suspected an analysis of a series of eight patients undergoing inadvertent internal fixation would reveal failure to adhere to these principles. Through analysis of this patient series and identification of critical errors, we formulated a clear and effective prevention strategy for referring physicians.
The major limitation of our study was the small series size, which precluded statistical analyses. As this was a retrospective study, our review of medical records and radiographs from outside institutions was limited to those made available by the referring physician and patient and was not uniform across the series. Followup was limited by the early death of four patients and the loss of one patient at 22 months resulting from relocation to a foreign country. Despite these limitations, we believe important conclusions can be drawn from this series that could positively affect physician decision making.
We identified four critical errors that preceded inappropriate fixation of primary osseous sarcomas. These included insufficient attention to key elements of the clinical history, inadequate radiographic evaluation, inappropriate biopsy methods, and treatment modalities that violated compartmental boundaries. The clinical history may provide clues to a pathologic fracture, as was the case in three of the patients in this series and in two of three patients in a series by Spence et al. [22] with primary sarcomas undergoing retrograde nailing of the femur. It is possible the initial treating physicians in our series suspected a pathologic process; however, assuming a diagnosis of metastatic carcinoma presumptively they proceeded with internal fixation. With an average patient age of 63 years (range, 43–79 years), our patient population is one in which secondary malignancies are more common than primary bone tumors [16, 17, 21]. However, a medical history of nonmetastatic cancer was present in only two patients and current literature supports the use of a diagnostic biopsy even when a history of carcinoma exists [16, 21, 22]. In a study of 50 patients with a known history of carcinoma presenting with a solitary bone lesion, Clayer and Duncan [2] found a new tumor diagnosis in nine patients (18%), leading them to recommend biopsy of all new solitary bone lesions in patients with a history of carcinoma. Rougraff et al. [17] recommend biopsy if a specific diagnostic strategy of laboratory tests and radiographs do not uncover a metastatic origin in a destructive bone lesion. They reported an 85% success rate in detecting a primary tumor with a diagnostic strategy, including history, physical examination, laboratory analysis, radiographs of the involved bone, whole-body bone scan, chest radiograph, and CT of the chest, abdomen, and pelvis. In addition to the diagnostic protocol described, we suggest additional local imaging studies such as MRI or CT to better characterize the primary pathologic lesion when an isolated metastatic lesion is encountered or a primary sarcoma is suspected. In the rare event that a primary malignancy is not detected with these diagnostics, a biopsy should be performed. Seven of eight patients in this series had inadequate preoperative local imaging of the pathologic lesion and no patients in this series received the diagnostic protocol described by Rougraff et al. [17]. Therefore, a high clinical suspicion of pathologic fractures followed by comprehensive imaging studies and laboratory evaluation will avoid the first two critical errors detected in this series.
The third identified error was the failure to perform a technically appropriate and diagnostic biopsy before surgical intervention. The biopsy is a critical aspect of the diagnostic process, presenting multiple opportunities for surgical and judgment error as highlighted by the myriad of biopsy-related problems in this series. The complications, errors, and deleterious effects related to biopsy of primary malignant sarcomas were highlighted by Mankin et al. [11, 12]. These investigations revealed a fivefold increased rate of biopsy-related problems in referring hospitals versus treating centers. Moreover, poor biopsy techniques resulted in unnecessary amputations (4.5% in 1982; 5.5% in 1996), high complication rates (5% in 1982; 10.1% in 1996), and altered patient outcomes (8.5% in 1982; 10.1% in 1996). Despite these admonitions, inadequate and poorly performed biopsies continue, as seen in our series (Fig. 1C). We consider the biopsy a diagnostic procedure, to be considered separately from definitive treatment but using surgical approaches that do not unnecessarily violate intraarticular or soft tissue compartments; biopsy should provide a definitive histopathologic diagnosis before additional treatment. In certain circumstances, with the collaborative effort of a skilled musculoskeletal pathologist and an orthopaedic oncologist, a definitive diagnosis can be rendered through frozen-section analysis, allowing for definitive treatment at the time of biopsy. Finally, canal reamings are not appropriate biopsy material because the act of reaming may damage the cellular elements, complicating pathologic diagnosis, and may result in intramedullary and systemic tumor dissemination, particularly to the lungs through embolic phenomena [7, 15, 25, 27]. Therefore, six patients in our series (four of whom did not have metastasis at referral) potentially were exposed to malignant emboli through intramedullary reaming at the index procedure. Two of these four patients with Stage IIB tumors had metastatic disease develop at 9 and 10 months postoperatively. Although it is possible micrometastases were present at diagnosis, it is equally plausible metastatic disease was introduced by canal reaming and concomitant tumor embolization. The clinical impact of this potential iatrogenic upstaging is substantial because the stage at diagnosis is a strong prognostic indicator in most high-grade sarcomas [23].
Failure to limit compartmental exposure at the index surgical procedure was the final identified error in the management of these patients. Ill-advised procedures such as intramedullary or plate fixation can cause compartmental contamination equal to or worse than an improper biopsy. Major surgical errors in this series included violation of the knee with retrograde intramedullary nail placement, intramedullary canal contamination and potential systemic dissemination associated with reaming and nail placement, and soft tissue contamination through other surgical approaches (Fig. 1D–E). Such errors compromise surgical options, making local and systemic tumor control more difficult, and may necessitate high-level amputation. We therefore recommend noninvasive stabilization of pathologic fractures using casts, splints, and immobilizers until a definitive diagnosis is determined.
Inadvertent internal fixation of high-grade osseous sarcomas resulted in high mortality in our clinical series, with only four surviving patients at an average of 26.9 months followup. Because the diagnoses were variable, expected survival would be guided by histopathologic diagnoses and stage at presentation. Despite the particularly poor prognosis associated with dedifferentiated chondrosarcomas [4, 9, 13, 23], two of the three patients with this diagnosis were alive without disease. Although a somewhat surprising finding, long-term followup would be necessary to make additional comment on survival impact in this series. There were no local recurrences, which may be partially attributable to the early death of half of the patients in this series, and the high-level amputations performed to confidently achieve local control. The average MSTS functional scores were markedly lower than those reported for patients with primary sarcomas undergoing limb salvage surgery [1, 3, 10, 18–20, 24, 26], which again is likely attributable to the disproportionate number of high-level amputations in this series. Without adequate preoperative imaging and staging studies, it is not possible to retrospectively determine what the appropriate definitive procedure for local control would have been for each patient in this series. However, the three patients with tumors of the distal femur who ultimately underwent high-level amputations would likely have been treated with a limb salvage procedure or a transfemoral amputation had they presented to our institution before implant violation. Additionally, an average of 2.9 surgical procedures was performed per patient. Under optimal circumstances, each of these patients would undergo one diagnostic biopsy and one definitive procedure. Our patients thus were exposed to the risks, cost, and morbidity of additional surgical procedures and anesthesia because of inappropriate initial evaluation and treatment. This leads us to conclude the inadvertent fixation of primary osseous sarcomas results in high mortality, the need for high-level amputations, additional procedures, and resultant low MSTS scores. Given this, we emphasize early diagnosis and prevention of these unfortunate events.
Our small series showed inadvertent fixation of high-grade primary osseous sarcomas was associated with poor survival, high-level amputations, low MSTS functional scores, and additional procedures. We recommend a vigilant preoperative assessment of all patients with suspected pathologic fractures with comprehensive clinical and radiographic evaluations. When biopsy is necessary for diagnosis, this should be performed by a surgeon familiar with biopsy principles in association with a skilled pathologist. Noninvasive stabilization is recommended with early referral to an orthopaedic oncologist for biopsy and definitive surgical treatment. In the unfortunate event of internal fixation of a primary bone sarcoma, we recommend a multidisciplinary approach and judicious referral to an orthopaedic oncologist for definitive management.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Agarwal M, Anchan C, Shah M, Puri A, Pai S. Limb salvage surgery for osteosarcoma: effective low-cost treatment. Clin Orthop Relat Res. 2007;459:82–91. [DOI] [PubMed]
- 2.Clayer M, Duncan W. Importance of biopsy of new bone lesions in patients with previous carcinoma. Clin Orthop Relat Res. 2006;451:208–211. [DOI] [PubMed]
- 3.Davis AM, Sennik S, Griffin AM, Wunder JS, O’Sullivan B, Catton CN, Bell RS. Predictors of functional outcomes following limb salvage surgery for lower-extremity soft tissue sarcoma. J Surg Oncol. 2000;73:206–211. [DOI] [PubMed]
- 4.Dickey ID, Rose PS, Fuchs B, Wold LE, Okuno SH, Sim FH, Scully SP. Dedifferentiated chondrosarcoma: the role of chemotherapy with updated outcomes. J Bone Joint Surg Am. 2004;86:2412–2418. [PubMed]
- 5.Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed]
- 6.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed]
- 7.Giannoudis PV, Tzioupis C, Pape HC. Fat embolism: the reaming controversy. Injury. 2006;37(suppl 4):S50–S58. [DOI] [PubMed]
- 8.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. [DOI] [PubMed]
- 9.Grimer RJ, Gosheger G, Taminiau A, Biau D, Matejovsky Z, Kollender Y, San-Julian M, Gherlinzoni F, Ferrari C. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060–2065. [DOI] [PubMed]
- 10.Ham SJ, Schraffordt Koops H, Veth RP, van Horn JR, Molenaar WM, Hoekstra HJ. Limb salvage surgery for primary bone sarcoma of the lower extremities: long-term consequences of endoprosthetic reconstructions. Ann Surg Oncol. 1998;5:423–436. [DOI] [PubMed]
- 11.Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64:1121–1127. [PubMed]
- 12.Mankin HJ, Mankin CJ, Simon MA. The hazards of the biopsy, revisited. Members of the Musculoskeletal Tumor Society. J Bone Joint Surg Am. 1996;78:656–663. [DOI] [PubMed]
- 13.Mitchell AD, Ayoub K, Mangham DC, Grimer RJ, Carter SR, Tillman RM. Experience in the treatment of dedifferentiated chondrosarcoma. J Bone Joint Surg Br. 2000;82:55–61. [DOI] [PubMed]
- 14.Osteen RT, Kopf G, Wilson RE. In pursuit of the unknown primary. Am J Surg. 1978;135:494–497. [DOI] [PubMed]
- 15.Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89:1421–1426. [DOI] [PubMed]
- 16.Peabody T. The rodded metastasis is a sarcoma: strategies to prevent inadvertent surgical procedures on primary bone malignancies. Instr Course Lect. 2004;53:657–661. [PubMed]
- 17.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin: a prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75:1276–1281. [DOI] [PubMed]
- 18.Schreiber D, Bell RS, Wunder JS, O’Sullivan B, Turcotte R, Masri BA, Davis AM. Evaluating function and health related quality of life in patients treated for extremity soft tissue sarcoma. Qual Life Res. 2006;15:1439–1446. [DOI] [PubMed]
- 19.Serletti JM, Carras AJ, O’Keefe RJ, Rosier RN. Functional outcome after soft-tissue reconstruction for limb salvage after sarcoma surgery. Plast Reconstr Surg. 1998;102:1576–1583; discussion 1584-1585. [DOI] [PubMed]
- 20.Sharma S, Turcotte RE, Isler MH, Wong C. Cemented rotating hinge endoprosthesis for limb salvage of distal femur tumors. Clin Orthop Relat Res. 2006;450:28–32. [DOI] [PubMed]
- 21.Simon MA, Bartucci EJ. The search for the primary tumor in patients with skeletal metastases of unknown origin. Cancer. 1986;58:1088–1095. [DOI] [PubMed]
- 22.Spence GM, Dunning MT, Cannon SR, Briggs TW. The hazard of retrograde nailing in pathological fractures: three cases involving primary musculoskeletal malignancy. Injury. 2002;33:533–538. [DOI] [PubMed]
- 23.Staals EL, Bacchini P, Bertoni F. Dedifferentiated central chondrosarcoma. Cancer. 2006;106:2682–2691. [DOI] [PubMed]
- 24.Tunn PU, Pomraenke D, Goerling U, Hohenberger P. Functional outcome after endoprosthetic limb-salvage therapy of primary bone tumours: a comparative analysis using the MSTS score, the TESS and the RNL index. Int Orthop. 2007 Aug 15 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 25.Ulrich C, Burri C, Worsdorfer O, Heinrich H. Intraoperative transesophageal two-dimensional echocardiography in total hip replacement. Arch Orthop Trauma Surg. 1986;105:274–278. [DOI] [PubMed]
- 26.van Loon CJ, Veth RP, Pruszczynski M, Wobbes T, Lemmens JA, van Horn J. Chondrosarcoma of bone: oncologic and functional results. J Surg Oncol. 1994;57:214–221. [DOI] [PubMed]
- 27.Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36:202–207. [DOI] [PubMed]