Abstract
Numerous options exist for intercalary segmental reconstruction after bone tumor resection. We present the extension of a recently developed surgical two-stage technique that involves insertion of a cement spacer, induction of a membrane, and reconstruction of the defect with cancellous and cortical bone autograft in a 12-year-old child. The boy was referred to our center for treatment of a right femoral diaphyseal Ewing’s sarcoma. The first stage involved resection of the tumor and reconstruction with a locked intramedullary nail and a polymethylmethacrylate cement spacer. Seven months after the initial procedure during which adjuvant chemotherapy was given, the second-stage procedure was performed. The cement was removed and cancellous and cortical bone autograft was grafted in the membrane created around the cement spacer. Touchdown weightbearing was allowed immediately, partial weightbearing was resumed 6 weeks after the operation, and full weightbearing was allowed 4 months later. Successive plain radiographs showed rapid integration of the autograft to the host bone with bone union and cortical reconstitution. The principle of the induced membrane reconstruction seems applicable to intercalary segmental reconstruction after bone tumor resection in children.
Introduction
Bone resection of malignant bone tumors typically leaves large osseous defects [9]. Different techniques have been proposed to reconstruct such bone defects, including diaphyseal endoprostheses [1, 3], allografts [4, 16], bone transport [6], and vascularized autograft [7, 14]. Restoring original bone anatomy and strength to withstand the rigors of use and growth is challenging. Biologic reconstructions usually are favored for young patients with the potential for long-term survival. However, allograft reconstructions and vascularized bone autograft are associated with complications such as fracture, nonunion, infection, and donor site morbidity [4, 7, 14, 16]. In a previous series of 35 adult patients, Masquelet et al. [15] reported the use of an innovative two-stage technique that involves insertion of a cement spacer, induction of a membrane, and reconstruction of the defect with cancellous bone autograft.
We describe the use of this innovative technique with cortical and bone autograft, to achieve an anatomic and functional reconstruction of a 16-cm bone defect after bone tumor femoral resection in a 12-year-old child.
Case Report
A 12-year-old boy was referred in April 2003 for treatment of a right femoral diaphyseal lesion. The patient reported onset of right thigh pain and swelling 4 weeks before the initial visit. He denied any history of trauma. On physical examination, a large, deep, firm mass was palpable in the medial thigh. The mass was painful on palpation. He denied systemic symptoms.
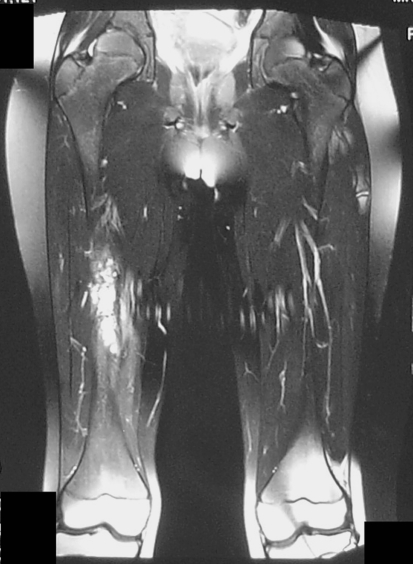
Plain femoral radiographs showed a lytic lesion with erosion of the medial cortex and periosteal reaction (Fig. 1). MRI showed the lesion was 10 cm long, heterogeneous, hyperintense on T2-weighted imaging, and extended into the medial thigh (Fig. 2).
Fig. 1.

A preoperative anteroposterior radiograph of the right femur shows the lytic lesion, cortical erosion, and periosteal reaction.
Fig. 2.
A preoperative T2-weighted coronal MR image shows extension of the tumor into the surrounding soft tissues.
We performed a biopsy of the lesion and Ewing’s sarcoma was diagnosed with histologic and cytogenetic studies. Subsequent CT of the chest and whole-body nuclear scans revealed no metastases.
Neoadjuvant chemotherapy was started following the EuroEwing 99 protocol (vincristine, ifosfamide, doxorubicin, and etoposide) [9]. Six months after initial presentation, we resected the lesion and first-stage reconstruction (ie, reconstruction using the cement spacer) was performed. Tumor resection was performed according to principles of management of malignant bone tumors. The tumor was approached medially. A 2-cm cuff of normal tissue was left with the tumor and the biopsy track was left in continuity with the specimen. The femoral vessels were dissected from proximal to distal and small branches ligated. The tumor was circumvented anteriorly at the quadriceps muscle and posteriorly in the posterior compartment. The sciatic nerve was located and protected. We osteotomized the femur proximally, 14 cm below the greater trochanter and distally 10 cm above the knee. The vastus lateralis was dissected from the femur and a margin specimen was sent for histologic evaluation. The measured resection length was 16.4 cm. We reviewed frozen sections of the marrow at the site of proximal and distal resections to ensure the margins were free of tumor before reconstruction. Initial reconstruction was performed with a locked intramedullary nailing technique. The nail was inserted in a standard fashion at the greater trochanter and, after ensuring proper rotation and length, we performed proximal and distal locking using an image intensifier. A polymethylmethacrylate cement spacer was placed around the nail in place of the bone resected. Thermal effects of polymerization were minimized with the use of syringes cut in half to protect surrounding soft tissues and with continuous irrigation with a saline solution. Hemostasis was obtained, drains were positioned anteriorly and posteriorly, and the wound was closed. Postoperative plain radiographs were satisfactory (Fig. 3). Hip and knee mobilization were started immediately postoperatively. Touchdown weightbearing was allowed and adjuvant chemotherapy resumed after the diagnosis was reconfirmed by pathologic evaluation. Histologic response from chemotherapy showed less than 4.5% viable tumor cells and all margins were free of tumor. Early during the postoperative course, an inflammatory reaction to the sutures developed requiring suture excision and lavage of the incision site. The tissue sent for histologic evaluation confirmed foreign body granuloma with chronic dermohypodermitis. Cultures were obtained and were negative. Healing was uneventful afterward.
Fig. 3.
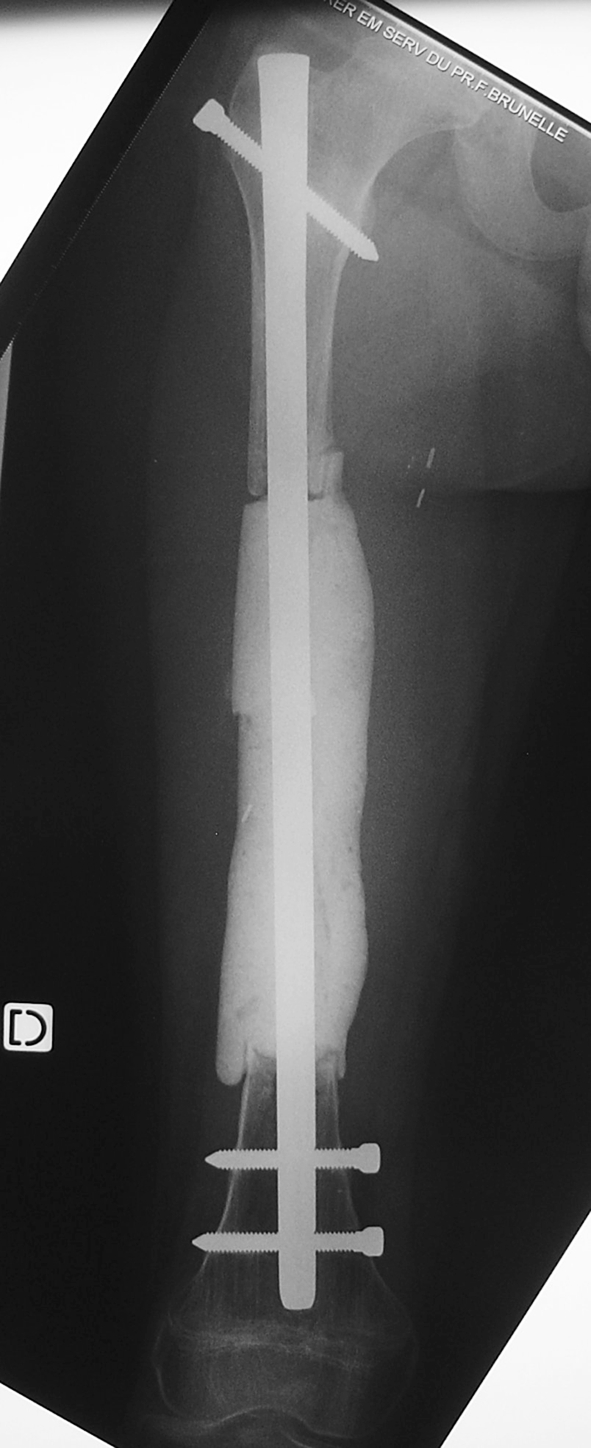
This anteroposterior radiograph was obtained after resection and reconstruction with a nail and cement spacer.
One month after completion of adjuvant chemotherapy (7 months after resection of the tumor), we performed the second stage of the reconstruction. The patient was placed prone and a large right posterior corticocancellous iliac crest autograft was harvested. After the wound was closed, the patient was positioned supine and we harvested a large strut cortical autograft 1 cm longer than the defect from the medial surface of the left tibia; the tibia was marked with electric cautery, the angles were predrilled to avoid crack propagation, and the bone was harvested with an oscillating saw. No prophylactic stabilization was provided. The wound was closed and reconstruction of the right femur was started. The cement spacer was approached easily with minimal dissection using the previous incision. The membrane covering the cement was opened along the spacer and the first few centimeters on the proximal and distal part of the femur with minimal dissection around the membrane. The prior reconstruction proved stable with no movement evident between the bone ends and the nail, and the nail was retained. Bone was decorticated on the medial side at the junction with the spacer to facilitate removal of the cement and to produce local autogenous bone graft. The cement was easily removed. Care was taken to leave the surrounding soft tissues attached to the external side of the membrane. The margins of the resected femur were freshened until bleeding bone was obtained. The tibial strut graft was embedded in the proximal and distal femoral ends on the medial side. We positioned the remaining tibial graft on the distal and lateral part of the reconstruction, and the iliac autograft was used to fill the remaining osseous defect throughout its length. The membrane was left open and soft tissues closed after insertion of a suction drain. The postoperative radiographs showed satisfactory positions of the grafts (Fig. 4).
Fig. 4.

This anteroposterior radiograph was obtained after the second-stage reconstruction with tibial strut autograft and corticocancellous iliac crest autograft.
Intermittent passive mobilization of the hip and knee was started immediately after surgery as was touchdown weightbearing. The patient was discharged 15 days postoperatively, at which time supervised physiotherapy was performed at home. Partial weightbearing was resumed 6 weeks after the operation with full weightbearing 4 months later.
The patient was followed at 6-months intervals the first 3 years and every year thereafter. He remained asymptomatic and tumor-free. He walked with no discernable limp and had no pain. His only concern was the hypertrophic thigh scar and he requested plastic surgery for revision. Hip and knee ranges of motion were normal. His right limb was 1 cm shorter than the left. Successive plain radiographs showed rapid integration of the autograft to the host bone with bone union and cortical reconstitution at 1 (Fig. 5) and 2 years postoperatively (Fig. 6). At last followup, he was 14 years 9 months old, weighed 49 kg, and was 165 cm tall.
Fig. 5.
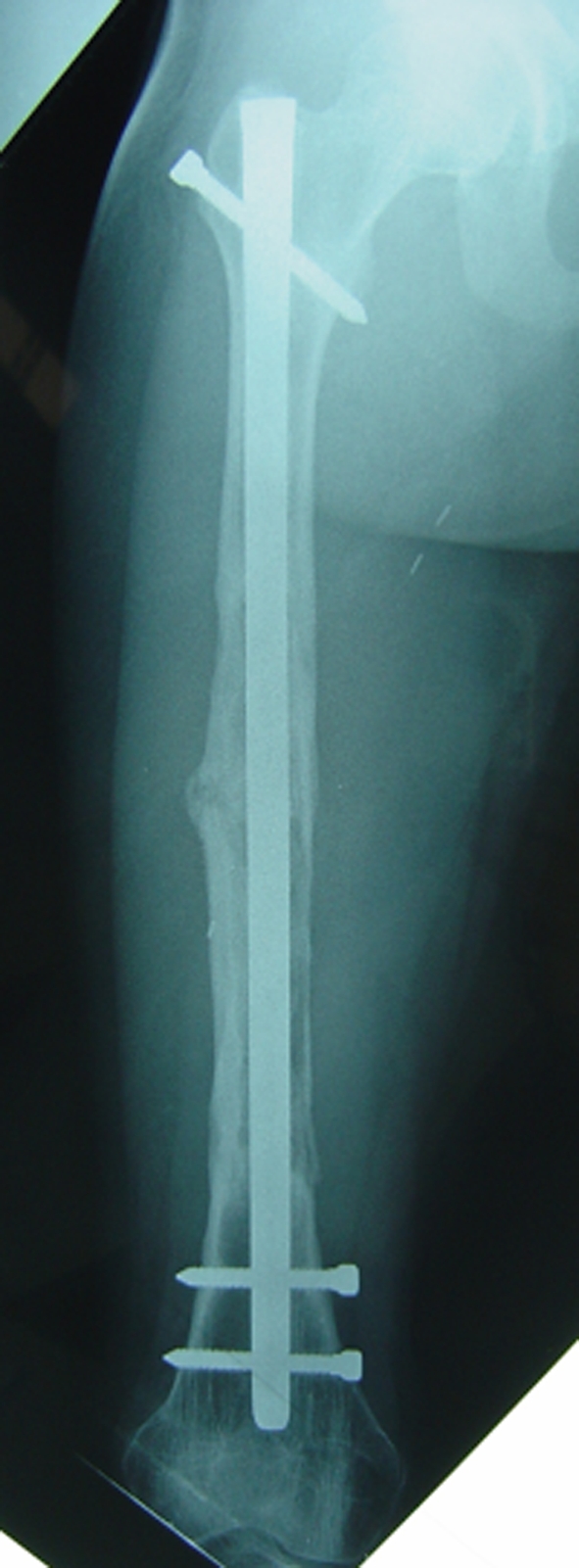
An anteroposterior radiograph obtained 1 year postoperatively is shown.
Fig. 6A–B.
(A) Anteroposterior and (B) lateral radiographs obtained 2 years postoperatively show osseous integration, excellent corticalization, and restoration of the original anatomy of the femur.
Discussion
In a previous series of 35 adult patients, Masquelet et al. reported the use of the induced membrane for treating bone defects using cancellous bone autograft [15]. In the current report, we describe an extension of their technique with cortical and cancellous bone autograft to achieve anatomic reconstruction of a large bone defect after femoral resection of a bone tumor in a 12-year-old child.
Options using biologic or nonbiologic materials are available for reconstruction of diaphyseal bone defects after tumor resection. Diaphyseal endoprostheses [1, 3], allografts [4, 16], bone transport [6], and vascularized autograft [7, 14] are some of the preferred methods. Nonbiologic materials provide immediate reconstruction but the potential for high implant failure and additional operations is likely [1, 3]. We believe reconstructions such as endoprostheses should be reserved for patients with poor survival because they allow for immediate weightbearing. However, biologic reconstructions usually are favored for young patients with long-term survival. Allograft reconstructions are attractive; they allow mechanical and biologic reconstruction during the same procedure. Nonetheless, they are associated with high rates of complications, including fracture, nonunion, and infection, and their long-term survival is a concern for children [4, 16]. Use of a vascularized bone autograft is an attractive option because it allows reconstruction of a bony defect and the autograft may enlarge with time and growth. These reconstructions are technically demanding and also have complications such as stress fracture, malunion, and donor site morbidity [7, 14]. Moreover, the transported fibula is unlikely to enlarge sufficiently in width to equal that of the original bone, especially so with femoral replacement, and we usually prefer this method of reconstruction for young children who are less likely to experience complications. The incidences of the most prevalent complications were reported in a review of possible options of intercalary segmental reconstruction after bone tumor resection [11]. Vascular fibular autografts are not recommended in weightbearing bones and the fracture rates range from 30% to 50% in the upper nonweightbearing extremity; the infection rates after intercalary allograft reconstruction range from 12% to 14%, the rates of allograft fractures range from 9% to 19%, and the rates of allograft-host junction nonunion range from 17% to 50% [11]. However, the majority of series report reconstruction in adult patients and the rate of complications may differ in children.
The technique we presented offers advantages and disadvantages over other types of reconstruction. The main disadvantage is that it is a two-step procedure with the associated risks of secondary anesthesia and hospitalization. Although a single-stage procedure with resection and reconstruction is ideal, in an unpublished series of 12 children who had diaphyseal resection and reconstruction without induced membrane, a secondary operation was necessary for all patients: eight had secondary bone grafting, three were treated for mechanical complications, and three had infectious complications. Other potential disadvantages are limited bone to harvest in very young children and pain, prolonged limp, hematoma, and infection associated with graft harvesting [2, 5]. The technique we presented is straightforward, the biologic reconstruction is separated in time from the tumor resection, and deleterious effects of chemotherapy agents on bone healing are avoided [13]. Use of an intramedullary device for initial reconstruction allows early weightbearing. This is particularly important in young patients with lack of compliance who render fragile reconstructions such as free vascularized autograft inadequate. This approach allows early discharge and rapid return to social activities. Although the timing of progression of weightbearing after the second stage is based on clinical examination and followup radiographs, it remains arbitrary and more cases are required to define a precise protocol. Surrounding the intramedullary device with bone autograft generated a cylindrical reconstruction that mimicked the original femoral shape. The possible complications, such as implant failure, infection, and nonunion, from this reconstruction method are similar to those after bone autograft reconstruction. One should consider exchanging the locking bolts at the time of reconstruction if any sign of fatigue, such as deformation or fracture of the locking bolts, appears on the preoperative radiographs. Preventive exchange of the locking bolts in the absence of fatigue signs may be considered.
The membrane induced by the reaction to the cement offers the necessary conditions for segmental bone loss repair to occur: prevention of soft tissue protrusion in the defect; a scaffold for osteoconduction; maintenance of adequate vascularization in the defect; and creation of a closed space in which osteogenic cells and substances are retained [12].
Animal studies show the membrane is richly vascularized by numerous small capillaries and with high concentrations of growth factors (vascular endothelial growth factor, transforming growth factor-β1), osteoblast precursors (CBFA1+), and osteoinductive (bone morphogenetic protein-2) factors when compared with control subjects [17, 18]. Moreover, the membrane induced by cement shows an absence of inflammatory cells [18], as opposed to membrane induced by silicone interposition [10] which may prevent deleterious graft resorption. Although bone resorption along with new bone formation are prerequisites to bone incorporation and remodeling, animal studies suggest the absence of inflammatory cells in this membrane [18] and that may minimize the amount of resorption. In our patient we could detect no resorption radiographically. The effects of chemotherapy and radiotherapy on the induced membrane are unknown.
Although questions remain regarding the role of the preserved membrane, its use for reconstruction of diaphyseal bone defects after resection of bone tumors is attractive in children. The procedure is technically simple. On plain radiographs, the appearance of the bone generated is similar to that of the original bone. We believe the technique merits further investigation to better define its use compared with other methods of reconstruction.
Acknowledgments
We thank Dr. Carl L. Stanitski, (Medical University of South Carolina Charleston, SC) for his wise and helpful comments.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the reporting of this case report, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Abudu A, Carter SR, Grimer RJ. The outcome and functional results of diaphyseal endoprostheses after tumour excision. J Bone Joint Surg Br. 1996;78:652–657. [PubMed]
- 2.Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84:716–720. [DOI] [PubMed]
- 3.Aldlyami E, Abudu A, Grimer RJ, Carter SR, Tillman RM. Endoprosthetic replacement of diaphyseal bone defects: long-term results. Int Orthop. 2005;29:25–29. [DOI] [PMC free article] [PubMed]
- 4.Alman BA, De Bari A, Krajbich JI. Massive allografts in the treatment of osteosarcoma and Ewing sarcoma in children and adolescents. J Bone Joint Surg Am. 1995;77:54–64. [DOI] [PubMed]
- 5.Chen YC, Chen CH, Chen PL, Huang IY, Shen YS, Chen CM. Donor site morbidity after harvesting of proximal tibia bone. Head Neck. 2006;28:496–500. [DOI] [PubMed]
- 6.Dormans JP, Ofluoglu O, Erol B, Moroz L, Davidson RS. Case report: reconstruction of an intercalary defect with bone transport after resection of Ewing’s sarcoma. Clin Orthop Relat Res. 2005;434:258–264. [DOI] [PubMed]
- 7.El-Gammal TA, El-Sayed A, Kotb MM. Reconstruction of lower limb bone defects after sarcoma resection in children and adolescents using free vascularized fibular transfer. J Pediatr Orthop B. 2003;12:233–243. [DOI] [PubMed]
- 8.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–120. [PubMed]
- 9.EURO-EWING 99: European Ewing Tumour Working Initiative of National Groups. Available at: http://controlled-trials.com/ISRCTN61438620/61438620. Accessed September 11, 2008.
- 10.Freund R, Wolff TW, Freund B. Silicone block interposition for traumatic bone loss. Orthopedics. 2000;23:795, 799, 802, 804. [DOI] [PubMed]
- 11.Fuchs B, Ossendorf C, Leerapun T, Sim FH. Intercalary segmental reconstruction after bone tumor resection. Eur J Surg Oncol. 2008 Jan 11 [Epub ahead of print]. [DOI] [PubMed]
- 12.Gugala Z, Gogolewski S. Regeneration of segmental diaphyseal defects in sheep tibiae using resorbable polymeric membranes: a preliminary study. J Orthop Trauma. 1999;13:187–195. [DOI] [PubMed]
- 13.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. [DOI] [PubMed]
- 14.Hsu RW, Wood MB, Sim FH, Chao EY. Free vascularised fibular grafting for reconstruction after tumour resection. J Bone Joint Surg Br. 1997;79:36–42. [DOI] [PubMed]
- 15.Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft [in French]. Ann Chir Plast Esthet. 2000;45:346–353. [PubMed]
- 16.Ozaki T, Hillmann A, Bettin D, Wuisman P, Winkelmann W. Intramedullary, antibiotic-loaded cemented, massive allografts for skeletal reconstruction: 26 cases compared with 19 uncemented allografts. Acta Orthop Scand. 1997;68:387–391. [DOI] [PubMed]
- 17.Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22:73–79. [DOI] [PubMed]
- 18.Viateau V, Guillemin G, Calando Y, Logeart D, Oudina K, Sedel L, Hannouche D, Bousson V, Petite H. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: an ovine model. Vet Surg. 2006;35:445–452. [DOI] [PubMed]