Abstract
Unconstrained tripolar hip implants provide an additional bearing using a mobile polyethylene component between the prosthetic head and the outer metal shell. Such a design increases the effective head diameter and therefore is an attractive option in challenging situations of unstable total hip arthroplasties. We report our experience with 54 patients treated using this dual mobility implant in such situations. We ascertained its ability to restore and maintain stability, and examined component loosening and component failure. At a minimum followup of 2.2 years (mean, 4 years; range, 2.2–6.8 years), one hip had redislocated 2 months postoperatively and was managed successfully without reoperation by closed reduction with no additional dislocation. Two patients required revision of the implant because of dislocation at the inner bearing. Technical errors were responsible for these failures. Three patients had reoperations for deep infections. The postoperative radiographs at latest followup showed very satisfactory osseointegration of the acetabular component because no radiolucent line or osteolysis was reported. Use of this unconstrained tripolar design was successful in restoring and maintaining hip stability. We observed encouraging results at short-term followup regarding potential for loosening or mechanical failures.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Dislocation after total hip arthroplasty (THA) remains a troublesome complication and a source of frustration for the patient and the surgeon. The prevalence of reoperation for instability is highly variable and is reportedly approximately 1/3 of the dislocating total hip arthroplasties (THAs) [2, 16, 20, 33, 59]. Because no single operative procedure can uniformly solve the problem of chronic recurrent dislocation, reoperation for unstable THA carries the highest likelihood of failure of any commonly performed reoperation after THA [9, 17].
Revision strategies typically are directed by the identified causes for instability [9, 19] and numerous methods have been described, including reorientation of the implants [17], the use of modular implants [21, 42, 55], elevated rim liners or socket wall addition [10, 13, 14, 41, 43, 46, 57], trochanteric advancement [22, 32], removal of sources of impingement [17], and abductor repair [58]. However, even when the etiology could be clearly identified, limited success rates for stability ranging from 60% to 80% have been reported [2, 3, 17, 22, 37, 44, 45, 49, 53, 55, 59].
Salvage procedures have been proposed when no obvious etiology could be identified or when an attempt at stabilization of the hip using one of the subscribed methods failed. In these cases, use of an allograft to increase the static soft tissue constraints [34, 42, 54], conversion to bipolar arthroplasty [4, 47], or use of constrained devices [3, 38, 50, 51] have been reported. More recently, the use of constraining systems has become the most popular salvage option [45]. However, success to achieve stability is highly variable [3, 26, 50] and depends on the constrained component design [24]. Additionally, such devices raise concerns regarding the potential for increased wear, osteolysis, loosening, and implant disassembly as reports of failure begin to emerge [15, 29, 52, 56, 60].
As an alternative for treatment of recurrently dislocating THAs, unconstrained tripolar hip implants (also called dual-mobility acetabular components) have been proposed. These implants have first been described in France by Bousquet in 1976. Using a large-inside-diameter outer metal shell and a bipolar component, these implants provide an additional bearing and improve range of motion (ROM) before impingement [28]. Therefore, these implants are expected to reduce dislocation.
We therefore ascertained the ability of such an implant to restore and maintain stability, and examined the rates of component loosening and failure.
Materials and Methods
We retrospectively reviewed 54 unconstrained tripolar components implanted in 54 selected patients during revision procedures for unstable THA between March 2000 and June 2005. The dual-mobility component was used to treat instability in three situations: (1) patients in whom no identifiable cause for instability could be identified or corrected; (2) patients in whom prior surgical attempts at stabilization failed; and (3) patients with a marked deficiency of the hip abductors. We advocate the use of a dual-mobility acetabular component instead of a constraining device in such situations, given the previously raised concerns regarding the potential for loosening and failure with constrained implants [29, 52]. There were 35 women and 19 men, and the average age at the time of the revision was 66.5 years (range, 35.7–98.7 years). The mean body mass index was 26.4 kg/m2 (range, 16.5–38.3 kg/m2). Twenty-seven procedures were performed on the right side and 27 were performed on the left side. Primary osteoarthritis was the most common underlying diagnosis at the time of the primary THA (Table 1). Patients included had an average of three dislocations (range, 1–13). The direction of instability was posterior in 40 hips, anterior in five hips, multidirectional in three hips, superior in one hip, and unknown in five hips.
Table 1.
Underlying diagnoses at the time of the primary THA
| Underlying diagnosis | Number of patients |
|---|---|
| Primary osteoarthritis | 36 |
| Posttraumatic arthritis | 2 |
| Congenital dysplasia | 6 |
| Inflammatory arthritis | 2 |
| Osteonecrosis | 3 |
| Femoral neck fracture | 2 |
| Paget’s disease of bone | 1 |
| Little’s disease | 1 |
| Infection | 1 |
| Total | 54 |
At the time of reoperation for instability, 25 of the 54 patients (46%) had previously sustained at least one hip revision procedure (range, 1–8). Thirteen of these patients had the prior revision procedure performed for THA instability (Table 2). All these attempts at stabilization failed. Other prior revision procedures had been performed for aseptic loosening. A trochanteric nonunion was observed in three patients (complication of the primary THA in two and complication of a previous revision procedure for aseptic loosening in one) and contributed to the hip instability. Additional risk factors for dislocation were identified at the time of the reoperation for instability: severe cognitive dysfunction (Alzheimer’s disease) in two patients, medical history of cerebral palsy in one patient, Parkinson’s disease in one patient, Little’s disease in one patient, and old poliomyelitis in one patient.
Table 2.
Prior surgical attempts at stabilization
| Prior surgical attempts at stabilization | Number of patients |
|---|---|
| Socket wall addition or elevated rim liner | 7 |
| Open reduction and impingement sources removal | 3 |
| Cup reorientation | 1 |
| Constrained component | 1 |
| Trochanteric advancement | 1 |
| Total | 13 |
The operative approach at the time of the primary THA was posterolateral in 40 hips (one hip with trochanteric osteotomy) and anterolateral in 14 hips. All procedures were performed by five surgeons (JBH, JPC, VP, GV, OG) at our institution and supervised by one of the two senior surgeons (JBH, JPC). The revision for instability was performed at an average of 8 years (range, 1 month–25 years) after the primary procedure. The revision procedure was performed with the patients under general anesthesia in 42 hips (78%) and under spinal anesthesia in 12 hips (22%). The surgical procedure consisted of an acetabular component revision in 46 cases and acetabular and femoral components revision in eight cases. The operative approach used at the time of the revision for instability was posterolateral in 41 hips and anterolateral in 13 hips.
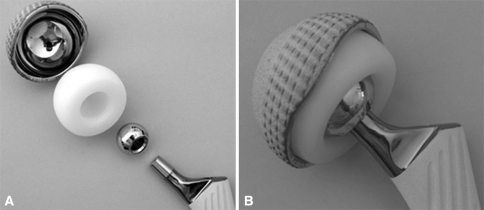
The single-design unconstrained tripolar implant used in our series consists of a large-inside–diameter stainless steel outer shell with a highly polished inner surface that articulates with a mobile ultrahigh-molecular-weight polyethylene bipolar component (Saturne®; Amplitude, Porte du Grand Lyon, Neyron, France). The outer shell is anatomically designed and has superior and posterior lips that are greater than hemispheric and anterior and inferior cutouts that are smaller than hemispheric. To hold the femoral head, the mobile ultrahigh-molecular-weight polyethylene component envelops greater than 50% of the femoral head and its opening diameter is smaller than the femoral head. When reduced, the head is captured in the polyethylene (Fig. 1). The outer metal shell can be either press fit (hydroxyapatite plasma sprayed on titanium coating) or cemented. If necessary (bone stock deficiency or poor bone quality with compromised fixation), this device is available with two additional superior flanges (to secure the press-fit fixation with screws placed through the ileum) and a hook beneath the teardrop (Fig. 2).
Fig. 1A–B.
The photographs show (A) the various components of the unconstrained tripolar device and (B) the implant assembled.
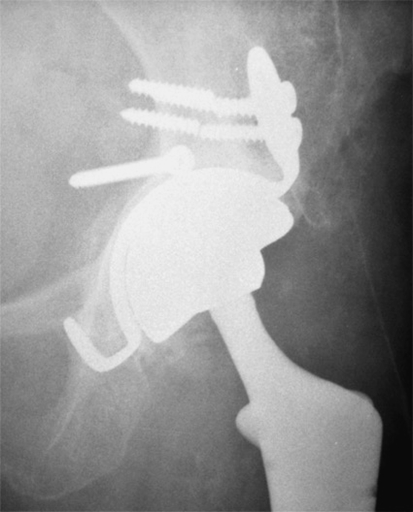
Fig. 2.
The unconstrained tripolar device with additional superior flanges and a hook beneath the teardrop in case of compromised fixation is shown.
Forty-three unconstrained tripolar implants were inserted without cement and the average cup size was 53 mm (range, 46–64 mm). An implant with additional superior flanges and a hook beneath the teardrop was used in three patients in whom we judged biologic fixation unlikely to succeed. Eleven acetabular implants were cemented and the average cup size was 51 mm (range, 44–60 mm); seven were cemented into an acetabular antiprotrusio cage, two were cemented into a secure cementless acetabular shell inserted previously, and two were cemented into the bony acetabulum.
Postoperative protection included an abduction pillow for immediate postoperative immobilization in all cases treated with a posterior approach. An abduction brace also was used at the discretion of the surgeon (five hips).
At the time of the latest followup, the surviving patients were asked to return for clinical and radiographic evaluations. Clinical and radiographic assessments were performed by four individuals (JBH, JPC, VP, OG) among the operating surgeons. These observers were not blinded to the results. Patients who were unable to return for followups were interviewed by telephone. For patients who died during the study period, information regarding function of the THA was obtained from interviews of family members with particular attention to the occurrence of dislocation.
Four patients, who died during the study period, were followed for a minimum of 5 months (mean, 31 months; range, 5–79 months). Three of them had a well-functioning and stable THA at the time of death and died of an unrelated cause. The fourth patient died 16 months after the index revision for instability and underwent a reoperation because of dislocation at the inner bearing 3 months before he died. Fifty patients were alive at the time of the latest followup. Among them, 39 patients (78%) returned for clinical and radiographic evaluations. Ten patients (20%) were unable to return and were interviewed by telephone. One patient (2%) was lost to followup and was last evaluated 30 months postoperatively. No additional dislocation or reoperation was reported for this patient. The minimum clinical followup for the living patients at the end of the study period was 26 months (mean, 47.5 months; range, 26–81 months). The minimum radiographic followup was 25 months (mean, 45.4 months; range, 25–72 months).
We assessed outcomes using the Harris hip score [31]. The radiographic evaluation focused on signs of loosening of the implants at the bone-implant interface, osteolysis, and heterotopic ossification. The presence of the metallic bipolar system inside the metallic shell made measurement of wear unreliable. Anteroposterior and lateral radiographs of the involved hip were reviewed. On the acetabular side, migration was evaluated using the criteria of Massin et al. [40], and radiolucent lines were recorded on the basis of the three acetabular zones described by DeLee and Charnley [18]. For femoral components, subsidence was measured using the method of Loudon and Charnley [39]. The femur was divided into seven zones as described by Gruen et al. [27] to assess radiolucent lines and osteolysis. Loosening of the femoral components inserted without cement was classified according to the criteria of Engh et al. [23]. Loosening of the femoral components inserted with cement was categorized according to the classification of Harris et al. [31].
For patients with a previously placed femoral or acetabular component retained at the time of the revision for instability, radiolucent lines present at the time of the revision were recorded as old, whereas those first noted at the latest followup were recorded as new. New or progressive osteolysis was recorded in a similar fashion when cystic bone loss was at least 10 mm in length. Radiographic grading of heterotopic ossification was reported using the Brooker classification [12].
At latest followup, the changes in the Harris hip scores from preoperatively were evaluated using the Student’s paired t test. Significance was determined using a 95% confidence level.
Results
The unconstrained tripolar component restored stability (no dislocation or subluxation) in 51 of the 54 patients (94.5%). Three patients (5.5%) experienced redislocation. All of these patients had only the acetabular component revised. One patient (an 80-year-old woman with poliomyelitis residuals) sustained a posterior dislocation 2 months postoperatively as she was picking up an object from the floor while sitting in a high chair. She was successfully managed nonoperatively with closed reduction and the hip remained stable at the latest followup without using any hip guide brace or reoperation. The two remaining patients presented with intraprosthetic dislocation at the inner bearing; the metal head had dislodged from the polyethylene component. In the first case (an 84-year-old man with severe Parkinson’s disease and dementia), the failure occurred after a fall 13 months after the index revision for hip instability. The patient was extremely frail and reoperation with a Girdlestone procedure was performed. This patient died 3 months after this second reoperation. In the second case (a 52-year-old active man), the failure occurred 35 months after the index revision. A reoperation was performed with prosthetic head and mobile polyethylene component exchange. Wear at the chamfer of the mobile polyethylene component was observed at the time of the reoperation. The hip remained stable at latest followup.
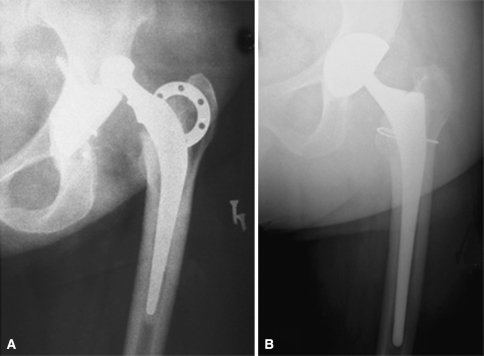
At last followup we identified no patients with acetabular component loosening or osteolysis. The acetabular implant showed satisfactory osseointegration, as no radiolucent line was reported. In one patient in whom the tripolar implant was cemented into a cage secured with screws, the screws broke (Fig. 3). However, no evidence of cup loosening was reported at latest followup. Radiolucent lines around a cementless femoral component were observed in one patient (Zones 1 and 2) without evidence of component loosening. Heterotopic bone formation (Grade 1) was observed in four patients. The trochanteric nonunion persisted in the three patients with a preoperative nonunion. In these cases, the trochanteric fixation at the time of the revision had failed.
Fig. 3.
The dual mobility socket had been cemented into a cage secured with screws at the time of the revision for instability. At latest followup, no evidence of cup loosening was reported although the screws had broken.
The Harris hip score improved from preoperatively to the last postoperative followup (mean preoperative score, 68.8, range, 11–98 to 83.7, range, 50–100).
In addition to recurrence of instability in three patients, additional postoperative complications were deep venous thrombosis in three patients (without pulmonary embolism) and incomplete sciatic nerve palsy in one patient (with a mild neurologic deficit at latest followup). Five patients (9.2%) required subsequent reoperations: two for intraprosthetic dislocations (as mentioned previously) and three for deep infections at an average of 11 months (range, 5–19 months) after index revision. Surgical débridement with removal of the components was performed in these infected hips. All of these patients had multiple surgical procedures of the involved hip. Only one of these three patients underwent delayed reimplantation of a new unconstrained tripolar implant 3 months later, after intravenous antibiotic therapy. This patient remained stable at latest followup.
Discussion
Surgery usually is recommended after a second THA dislocation [20, 49]. Three categories of surgical procedures have been described [45]: nonrevision reoperation, revision procedures, and salvage procedures. The type of strategy elected to address instability depends on the etiology, and the reoperation is more likely successful when a cause for instability is identified [17, 59]. However, no single procedure can uniformly solve the problem of instability, and limited success rates have been reported with any type of procedure [9]. The use of an unconstrained tripolar implant to manage unstable THAs is an attractive option. Encouraging results have been reported with the use of such implants in primary THAs to prevent instability [30], but only one report focused on the use of such implants during revision procedures for unstable THAs has been published [35]. Limited information regarding the durability of such implants in terms of preventing additional dislocation, loosening of components, and potential for mechanical failure is available. In this study, we evaluated the ability of a single-design dual-mobility acetabular component to restore stability to the unstable THA.
Limitations of the study include the fact that all data were reviewed retrospectively. Twenty percent of the patients did not return for clinical and radiographic evaluations and were interviewed by telephone. The radiographic followup was complete for 78% of the patients. Clinical and radiographic measures were performed by four individuals who were not blinded to the results. Such measurements are subject to intraobserver and interobserver variations although we made no such assessment.
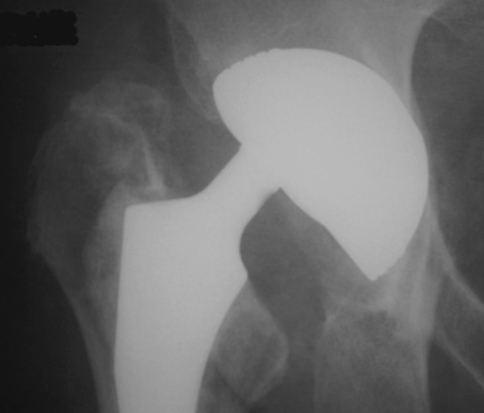
We found the unconstrained tripolar implant was successful in restoring a stable durable THA in challenging clinical situations (Fig. 4). The success rate for obtaining and maintaining a stable THA was 94.5% at an average 4-year followup for unstable THAs. In such a design, the interposition of a mobile polyethylene component provides a dramatic increase in the effective head diameter and allows greater ROM before the femoral neck impinges on the metal shell [28]. In addition, the femoral head must travel a greater distance before subluxating or dislocating. This successful result of stability is in agreement with a previously reported study in which Bousquet’s dual-mobility implant was used to treat recurrent instability [35].
Fig. 4A–B.
(A) The photographs show a failed attempt at stabilization with the addition of a socket wall. (B) This challenging situation was managed successfully using an unconstrained tripolar acetabular component.
Despite longer followup of our patients being required to document long-term fixation, this design showed very satisfactory osseointegration because no evidence of cup loosening or a radiolucent line around the cup was observed at an average radiographic followup of 45.4 months. Cementation of an unconstrained tripolar implant into an acetabular antiprotrusio cage or into an existing secure cementless acetabular shell was also a viable solution.
Two patients had revision of the unconstrained tripolar implant because of intraprosthetic dislocation at the inner bearing. Despite such a failure occurring during the short-term period, it is typically a medium- to long-term complication of dual-mobility implants. Cold-flow failure and wear of the capturing area of the polyethylene component related to impingement of the prosthetic femoral neck against the chamfer are responsible for this complication [5, 25, 36]. The use of a femoral component with a thin Morse taper (10/12 Morse taper) and a highly polished neck to reduce abrasive wear is recommended to prevent this complication. In our case series, this complication occurred in patients who had only the acetabular component revised, and technical errors have been identified to explain the occurrence of such a failure. We believe such mechanical failures could have been avoided with an appropriate technique. The first case occurred in an 84-year-old frail man with severe Parkinson’s disease 13 months after the index revision and intraprosthetic dislocation occurred after a fall (Fig. 5). At the time of the reoperation, no wear was observed at the mobile polyethylene component chamfer, and careful analysis of the postoperative radiographs at the time of the index revision for instability showed evidence of incomplete seating of the prosthetic metal head into the mobile polyethylene component. The second case occurred 35 months after the revision for instability in a 58-year-old man. Wear at the chamfer of the mobile polyethylene component was seen at the time of the second revision in this active man. At the time of the index revision for instability, the femoral component (with a large Morse taper) had been retained and the tripolar unconstrained implant had been inserted with a 22-mm femoral head. The poor head-to-neck ratio was responsible for early impingement and wear at the chamfer and finally dislocation at the inner bearing. In this particular case (large Morse taper), a 28-mm femoral head should have been used to improve the head/neck ratio and delay impingement at the neck-chamfer area and avoid accelerated polyethylene wear.
Fig. 5.
The photograph shows a dislocation at the inner bearing of the unconstrained tripolar device.
Other salvage procedures have been proposed in such challenging clinical situations. Bipolar hemiarthroplasty has been proposed with satisfactory results for stability, but concerns have been raised regarding the potential for poor functional results related to recalcitrant groin pain possibly leading to conversion to a THA [4, 47]. Constrained implants have become the most popular option to manage unstable THA [45]. However, their ability to restore stability is design-dependent. The constrained tripolar design (Omnifit® Constrained Acetabular System; previously Osteonics, Allendale, NJ; currently Stryker Orthopedics, Mahwah, NJ) reportedly has a success rate as high as 94% at 10 years followup [11], whereas that of the locking ring design is 71.1% at 10.7 years followup [8]. However, the success of the constrained tripolar design must be balanced against the theoretical possibility of increased transmission of stress to the implant-bone or implant-cement interface leading to loosening because of decreased ROM and early impingement. A short-term study supported these concerns and a 14% rate of radiolucent lines around the acetabular component at 3.2 years average followup was reported [52]. Additionally, there are possible adverse effects on polyethylene wear and osteolysis resulting from early impingement, thinner polyethylene, and increased number of bearing surfaces. Moreover, the complexity of the mechanisms of constrained components involving many parts raises concerns regarding the potential for mechanical failure as reports began to emerge [15, 29, 60]. It therefore seems logical to suggest caution in the use of these constraining devices [9, 15, 52].
Although the benefit of increased stability from a jumbo femoral head may outweigh the risk for increased wear and osteolysis, the unconstrained tripolar design used in our patients raises concerns about the potential for excessive wear at the two bearing surfaces. In such a design, the outer diameter of the mobile polyethylene component represents the effective head diameter and the global ROM is not influenced by the diameter of the prosthetic head used. Use of a 22-mm prosthetic metal head is advocated because it allows for preservation of the polyethylene thickness while providing the same global ROM [28]. In addition, in such a design, the motion is supposed to occur preferentially at the inner bearing and limited polyethylene wear at the outer bearing can be expected. Such an assumption was documented in a laboratory study of retrieved polyethylene inserts [1]. The use of a different design of unconstrained tripolar component has been proposed and successful outcomes for stability have been reported [6, 7, 48]; in this design, the bipolar liner is covered with a polished Co-Cr shell and articulates a polyethylene outer liner that accepts a jumbo-sized head (either a custom-made liner locked into a stable well-positioned acetabular shell or an all-polyethylene cemented cup). Although the use of highly cross-linked polyethylene is advocated with such a design [7], the thickness of the polyethylene (only 4 to 7 mm with metal shells) raises concern regarding potential for excessive wear. One case of fracture of the polyethylene has been reported [7]. The interposition of a mobile polyethylene component between the prosthetic femoral head and an outer metal shell has the advantage of a greater polyethylene thickness and greater effective head diameter.
Results of our study support use of unconstrained tripolar hip implants for treatment of unstable THAs; this design was very efficient in restoring and maintaining hip stability. Unlike constraining systems, it did not raise any concerns regarding early loosening resulting from increased transmission of stresses or mechanical failures, provided an appropriate technique is used. The design presented in this study involves few parts compared with the complexity of the mechanisms of tripolar constrained components. Despite longer followup being required, this has become the preferred implant in our current approach for surgical treatment of an established unstable THA.
Acknowledgments
We thank Professor Jean-Paul Carret for his contribution to this study.
Footnotes
One or more of the authors (OG, VP, JBH) certifies that he has or may receive payments or benefits from a commercial entity (Amplitude, 01707 Neyron, France) related to this work.
Each author certifies that his institution either has waived or does not require approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Adam P, Farizon F, Fessy MH. [Dual articulation retentive acetabular liners and wear: surface analysis of 40 retrieved polyethylene implants][in French]. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:627–636. [DOI] [PubMed]
- 2.Ali Khan MA, Brakenbury PH, Reynolds IS. Dislocation following total hip replacement. J Bone Joint Surg Br. 1981;63:214–218. [DOI] [PubMed]
- 3.Anderson MJ, Murray WR, Skinner HB. Constrained acetabular components. J Arthroplasty. 1994;9:17–23. [DOI] [PubMed]
- 4.Attarian DE. Bipolar arthroplasty for recurrent total hip instability. J South Orthop Assoc. 1999;8:249–253. [PubMed]
- 5.Aubriot JH, Lesimple P, Leclercq S. [Study of Bousquet’s non-cemented acetabular implant in 100 hybrid total hip prostheses (Charnley type cemented femoral component): average 5-year follow-up][in French]. Acta Orthop Belg. 1993;59(suppl 1):267–271. [PubMed]
- 6.Beaule PE, Roussignol X, Schmalzried TP, Udomkiat P, Amstutz HC, Dujardin FH. [Tripolar arthroplasty for recurrent total hip prosthesis dislocation][in French]. Rev Chir Orthop Reparatrice Appar Mot. 2003;89:242–249. [PubMed]
- 7.Beaule PE, Schmalzried TP, Udomkiat P, Amstutz HC. Jumbo femoral head for the treatment of recurrent dislocation following total hip replacement. J Bone Joint Surg Am. 2002;84:256–263. [DOI] [PubMed]
- 8.Berend KR, Lombardi AV Jr, Mallory TH, Adams JB, Russell JH, Groseth KL. The long-term outcome of 755 consecutive constrained acetabular components in total hip arthroplasty examining the successes and failures. J Arthroplasty. 2005;20(suppl 3):93–102. [DOI] [PubMed]
- 9.Berry DJ. Unstable total hip arthroplasty: detailed overview. Instr Course Lect. 2001;50:265–274. [PubMed]
- 10.Bradbury N, Milligan GF. Acetabular augmentation for dislocation of the prosthetic hip: a 3 (1–6)-year follow-up of 16 patients. Acta Orthop Scand. 1994;65:424–426. [DOI] [PubMed]
- 11.Bremner BR, Goetz DD, Callaghan JJ, Capello WN, Johnston RC. Use of constrained acetabular components for hip instability: an average 10-year follow-up study. J Arthroplasty. 2003;18(suppl 1):131–137. [DOI] [PubMed]
- 12.Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed]
- 13.Cobb TK, Morrey BF, Ilstrup DM. The elevated-rim acetabular liner in total hip arthroplasty: relationship to postoperative dislocation. J Bone Joint Surg Am. 1996;78:80–86. [DOI] [PubMed]
- 14.Cobb TK, Morrey BF, Ilstrup DM. Effect of the elevated-rim acetabular liner on loosening after total hip arthroplasty. J Bone Joint Surg Am. 1997;79:1361–1364. [DOI] [PubMed]
- 15.Cooke CC, Hozack W, Lavernia C, Sharkey P, Shastri S, Rothman RH. Early failure mechanisms of constrained tripolar acetabular sockets used in revision total hip arthroplasty. J Arthroplasty. 2003;18:827–833. [DOI] [PubMed]
- 16.Coventry MB. Late dislocations in patients with Charnley total hip arthroplasty. J Bone Joint Surg Am. 1985;67:832–841. [PubMed]
- 17.Daly PJ, Morrey BF. Operative correction of an unstable total hip arthroplasty. J Bone Joint Surg Am. 1992;74:1334–1343. [PubMed]
- 18.DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 19.Dorr LD, Wan Z. Causes of and treatment protocol for instability of total hip replacement. Clin Orthop Relat Res. 1998;355:144–151. [DOI] [PubMed]
- 20.Dorr LD, Wolf AW, Chandler R, Conaty JP. Classification and treatment of dislocations of total hip arthroplasty. Clin Orthop Relat Res. 1983;173:151–158. [PubMed]
- 21.Earll MD, Fehring TK, Griffin WL, Mason JB, McCoy T, Odum S. Success rate of modular component exchange for the treatment of an unstable total hip arthroplasty. J Arthroplasty. 2002;17:864–869. [DOI] [PubMed]
- 22.Ekelund A. Trochanteric osteotomy for recurrent dislocation of total hip arthroplasty. J Arthroplasty. 1993;8:629–632. [DOI] [PubMed]
- 23.Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990;257:107–128. [PubMed]
- 24.Etienne G, Ragland PS, Mont MA. Use of constrained acetabular liners in total hip arthroplasty. Orthopedics. 2005;28:463–469; quiz 470–471. [DOI] [PubMed]
- 25.Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility: a twelve-year follow-up study. Int Orthop. 1998;22:219–224. [DOI] [PMC free article] [PubMed]
- 26.Goetz DD, Capello WN, Callaghan JJ, Brown TD, Johnston RC. Salvage of total hip instability with a constrained acetabular component. Clin Orthop Relat Res. 1998;355:171–181. [DOI] [PubMed]
- 27.Gruen TA, McNeice GM, Amstutz HC. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;141:17–27. [PubMed]
- 28.Guyen O, Chen QS, Bejui-Hugues J, Berry DJ, An KN. Unconstrained tripolar hip implants: effect on hip stability. Clin Orthop Relat Res. 2007;455:202–208. [DOI] [PubMed]
- 29.Guyen O, Lewallen DG, Cabanela ME. Modes of failure of Osteonics constrained tripolar implants: a retrospective analysis of forty-three failed implants. J Bone Joint Surg Am. 2008;90:1553–1560. [DOI] [PubMed]
- 30.Guyen O, Pibarot V, Vaz G, Chevillotte C, Carret JP, Bejui-Hugues J. Unconstrained tripolar implants for primary total hip arthroplasty in patients at risk for dislocation. J Arthroplasty. 2007;22:849–858. [DOI] [PubMed]
- 31.Harris WH, McCarthy JC Jr, O’Neill DA. Femoral component loosening using contemporary techniques of femoral cement fixation. J Bone Joint Surg Am. 1982;64:1063–1067. [PubMed]
- 32.Kaplan SJ, Thomas WH, Poss R. Trochanteric advancement for recurrent dislocation after total hip arthroplasty. J Arthroplasty. 1987;2:119–124. [DOI] [PubMed]
- 33.Kristiansen B, Jorgensen L, Holmich P. Dislocation following total hip arthroplasty. Arch Orthop Trauma Surg. 1985;103:375–377. [DOI] [PubMed]
- 34.Lavigne MJ, Sanchez AA, Coutts RD. Recurrent dislocation after total hip arthroplasty: treatment with an Achilles tendon allograft. J Arthroplasty. 2001;16(suppl 1):13–18. [DOI] [PubMed]
- 35.Leclercq S, el Blidi S, Aubriot JH. [Bousquet’s device in the treatment of recurrent dislocation of a total hip prosthesis: apropos of 13 cases][in French]. Rev Chir Orthop Reparatrice Appar Mot. 1995;81:389–394. [PubMed]
- 36.Lecuire F, Benareau I, Rubini J, Basso M. [Intra-prosthetic dislocation of the Bousquet dual mobility socket][in French]. Rev Chir Orthop Reparatrice Appar Mot. 2004;90:249–255. [DOI] [PubMed]
- 37.Lind M, Krarup N, Petersen LG, Mikkelsen S, Horlyck E. Acetabular revision for recurrent dislocations: results in 14 cases after 3 years of follow-up. Acta Orthop Scand. 2002;73:291–294. [DOI] [PubMed]
- 38.Lombardi AV Jr, Mallory TH, Kraus TJ, Vaughn BK. Preliminary report on the S-ROM constraining acetabular insert: a retrospective clinical experience. Orthopedics. 1991;14:297–303. [PubMed]
- 39.Loudon JR, Charnley J. Subsidence of the femoral prosthesis in total hip replacement in relation to the design of the stem. J Bone Joint Surg Br. 1980;62:450–453. [DOI] [PubMed]
- 40.Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration: an experimental study. J Arthroplasty. 1989;4:245–251. [DOI] [PubMed]
- 41.McCollum DE, Gray WJ. Dislocation after total hip arthroplasty: causes and prevention. Clin Orthop Relat Res. 1990;261:159–170. [PubMed]
- 42.McGann WA, Welch RB. Treatment of the unstable total hip arthroplasty using modularity, soft tissue, and allograft reconstruction. J Arthroplasty. 2001;16(suppl 1):19–23. [DOI] [PubMed]
- 43.Mogensen B, Arnason H, Jonsson GT. Socket wall addition for dislocating total hip: report of two cases. Acta Orthop Scand. 1986;57:373–374. [DOI] [PubMed]
- 44.Morrey BF. Instability after total hip arthroplasty. Orthop Clin North Am. 1992;23:237–248. [PubMed]
- 45.Morrey BF. Results of reoperation for hip dislocation: the big picture. Clin Orthop Relat Res. 2004;429:94–101. [DOI] [PubMed]
- 46.Olerud S, Karlstrom G. Recurrent dislocation after total hip replacement: treatment by fixing an additional sector to the acetabular component. J Bone Joint Surg Br. 1985;67:402–405. [DOI] [PubMed]
- 47.Parvizi J, Morrey BF. Bipolar hip arthroplasty as a salvage treatment for instability of the hip. J Bone Joint Surg Am. 2000;82:1132–1139. [DOI] [PubMed]
- 48.Ries MD, Wiedel JD. Bipolar hip arthroplasty for recurrent dislocation after total hip arthroplasty: a report of three cases. Clin Orthop Relat Res. 1992;278:121–127. [PubMed]
- 49.Robbins GM, Masri BA, Garbuz DS, Greidanus N, Duncan CP. Treatment of hip instability. Orthop Clin North Am. 2001;32:593–610, viii. [DOI] [PubMed]
- 50.Shapiro GS, Weiland D, Sculco TP, Padgett DE, Pellicci PM. The use of a constrained acetabular component for recurrent dislocation. Instr Course Lect. 2001;50:281–287. [PubMed]
- 51.Shapiro GS, Weiland DE, Markel DC, Padgett DE, Sculco TP, Pellicci PM. The use of a constrained acetabular component for recurrent dislocation. J Arthroplasty. 2003;18:250–258. [DOI] [PubMed]
- 52.Shrader MW, Parvizi J, Lewallen DG. The use of a constrained acetabular component to treat instability after total hip arthroplasty. J Bone Joint Surg Am. 2003;85:2179–2183. [DOI] [PubMed]
- 53.Soong M, Rubash HE, Macaulay W. Dislocation after total hip arthroplasty. J Am Acad Orthop Surg. 2004;12:314–321. [DOI] [PubMed]
- 54.Stromsoe K, Eikvar K. Fascia lata plasty in recurrent posterior dislocation after total hip arthroplasty. Arch Orthop Trauma Surg. 1995;114:292–294. [DOI] [PubMed]
- 55.Toomey SD, Hopper RH Jr, McAuley JP, Engh CA. Modular component exchange for treatment of recurrent dislocation of a total hip replacement in selected patients. J Bone Joint Surg Am. 2001;83:1529–1533. [DOI] [PubMed]
- 56.Tufescu TV, Dust W. Failure of a new constrained acetabular insert: a report of 2 cases. J Arthroplasty. 2004;19:238–239. [DOI] [PubMed]
- 57.Watson P, Nixon JR, Mollan RA. A prosthesis augmentation device for the prevention of recurrent hip dislocation: a preliminary report. Clin Orthop Relat Res. 1991;267:79–84. [PubMed]
- 58.Weber M, Berry DJ. Abductor avulsion after primary total hip arthroplasty: results of repair. J Arthroplasty. 1997;12:202–206. [DOI] [PubMed]
- 59.Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982;64:1295–1306. [PubMed]
- 60.Yun AG, Padgett D, Pellicci P, Dorr LD. Constrained acetabular liners: mechanisms of failure. J Arthroplasty. 2005;20:536–541. [DOI] [PubMed]