Abstract
Increases in muscular cross-sectional area (CSA) occur in quadriplegics after training, but the effects of neuromuscular electrical stimulation (NMES) along with training are unknown. Thus, we addressed two questions: (1) Does NMES during treadmill gait training increase the quadriceps CSA in complete quadriplegics?; and (2) Is treadmill gait training alone enough to observe an increase in CSA? Fifteen quadriplegics were divided into gait (n = 8) and control (n = 7) groups. The gait group performed training with NMES for 6 months twice a week for 20 minutes each time. After 6 months of traditional therapy, the control group received the same gait training protocol but without NMES for an additional 6 months. Axial images of the thigh were acquired at the beginning of the study, at 6 months (for both groups), and at 12 months for the control group to determine the average quadriceps CSA. After 6 months, there was an increase of CSA in the gait group (from 49.8 ± 9.4 cm2 to 57.3 ± 10.3 cm2), but not in the control group (from 43.6 ± 7.6 cm2 to 41.8 ± 8.4 cm2). After another 6 months of gait without NMES in the control group, the CSA did not change (from 41.8 ± 8.4 cm2 to 41.7 ± 7.9 cm2). The increase in quadriceps CSA after gait training in patients with chronic complete quadriplegia appears associated with NMES.
Introduction
Muscle atrophy is one of many alterations occurring after spinal cord injury; it contributes to various medical complications, such as fractures, deep venous thrombosis [1], cardiovascular deconditioning [5], and early fatigability during neuromuscular electrical stimulation (NMES) [15]. Decrease in average muscle cross-sectional area (CSA) [8], replacement of Type I muscle fibers by Type II fibers [4], infiltration of adipose tissue inside muscles, reduction in the oxidative enzyme level, mitochondria concentration, and number of capillaries are observed in muscles located below the injury level [3, 18, 21].
Producing muscle contractions in paralyzed muscles through NMES of intact peripheral motoneurons [22] can promote muscle hypertrophy [9, 11], muscle endurance increase, and histochemical changes [21]. The positive muscle alterations provided by NMES also can benefit the oxygen consumption [5, 10, 12, 17] and bone mass of individuals with spinal cord injury [2, 6, 19].
Some studies suggest muscle mass increases after functional electrical stimulation (FES) using a cycle-ergometer [19, 23]. However, FES contractions without resistance appear not to prevent muscle atrophy or to increase muscle CSA in the early and chronic phases of spinal cord injury [1, 16]. Thus, these studies suggest the muscle hypertrophy is directly associated with the force produced during electrical stimulation training. The effect of NMES during treadmill gait with body weight support (BWS) therefore is not well established, particularly in patients with complete lesions. An increase in CSA of the thigh after 12 months of treadmill gait training has been observed without using NMES. However, these patients had incomplete spinal cord injuries [14].
Accordingly, we asked the following questions: (1) Does NMES during treadmill gait training increase the quadriceps CSA in complete quadriplegics?; and (2) Is treadmill gait training alone enough to observe an increase in CSA?
Materials and Methods
We recruited 15 patients, all male, with complete quadriplegia (American Spinal Cord Injury Association A). The injury level was C4 to C7. Inclusion criteria were intact lower motor neurons, which are required for muscle contraction under surface electrical stimulation, and a postinjury time of at least 24 months (chronic spinal cord-injured patients). Exclusion criteria were skin damage or ulcers and history of cardiopulmonary disease. We divided the patients into two groups: a gait group with NMES (GG; n = 8) and a control group (CG; n = 7). In the GG, mean age was 32.3 ± 3.5 years; mean body mass was 66.4 ± 8.5 kg; mean height was 176.9 ± 3.5 cm; and mean time postinjury was 64.1 ± 96.2 months. In the CG, mean age was 32.8 ± 3.5 years; mean body mass was 65.5 ± 10.6 kg; mean height was 176.8 ± 8.4 cm; and mean time postinjury was 55.3 ± 10.6 months. There were no differences in demographic data between the groups. We did not perform a priori power analysis; in fact, we had difficulty recruiting patients to participate in the study and therefore used a sample of convenience. The study terminated at 6 months for the GG; after 6 months, the CG was provided an additional 6 months of gait training without NMES. We adhered to all applicable institutional and government regulations concerning the ethical use of human volunteers during this research.
In the GG, all individuals began treadmill gait training at an absolute speed of 0.14 m/second, which was increased according to the capacity of each patient (the adjustment in walking speed was based on qualitative gait assessment, which included adequate posture and a usual gait pattern). The highest absolute speed achieved was 0.39 m/second after 6 months. The initial speed normalized by body height was 0.03 m/second and the final normalized speed was 0.09 m/second for all individuals. During gait, partial BWS (30%–50% of body weight reduction) was provided. The level of support was the minimal level required to maintain the upright trunk and heel strike. BWS was provided by a harness suspended from an overhead support, and the support vest allowed free movement of the lower limbs (Fig. 1). Staff physiotherapists helped move the legs through the gait cycle. Training was performed for 6 months, twice a week, for 20 minutes during each session. NMES was delivered by a custom-built, four-channel stimulator. Charge-balanced pulses of 300 ms were delivered at 25 Hz. The maximum intensity of stimulation was 200 V with a load of 1 kΩ in output. NMES was used to provide the stance gait phase through quadriceps muscle activation and the swing phase characterized by ankle dorsiflexion, knee flexion, and hip flexion through withdrawal reflexes elicited by stimulation of the common peroneal nerve. The stimulation unit was triggered by hand switches controlled by the staff. Self-adhesive surface electrodes measuring 5 × 9 cm were placed on the quadriceps and smaller, circular electrodes 3.2 cm in diameter were placed over the common peroneal nerves.
Fig. 1.
Treadmill gait training using NMES and BWS is shown.
In the CG, individuals did not perform gait training during the first 6 months; they only performed conventional physiotherapy without using NMES twice a week. Traditional therapy included passive movements of the hips, knees, and ankles and strengthening of every muscle group involved in the movements (two sets of 30 seconds). Elbow and wrist movements were performed depending on the extent of the injuries. The preserved upper limbs underwent muscular strengthening. During each session, the orthostatic position was maintained for 20 to 30 minutes. After 6 months, we included individuals in the treadmill gait training following the same protocol as GG but without NMES and trained them an additional 6 months. The initial and final achieved velocities (after normalizing by height) were the same as those of the GG (0.03 m/second and 0.09 m/second, respectively).
At the beginning of the study and after 6 months, we performed MRI (2T Prestige™; Elscint, Haifa, Israel) of both thighs for all patients to determine the average CSA of the quadriceps. We determined the femur length, defined as the distance between trochanter major and femoral lateral condyle, on coronal T1-weighted imaging. Subsequently, we acquired 30 axial T1-weighted images of both thighs for all patients (spin echo 9-mm slice thickness and 8-mm gap between each slice; echo time, 20 ms; repetition time, 700 ms; matrix, 230 × 290 mm; field of view, 450 × 450 mm; in-plane pixel size, 1.6 × 2.0 mm). Images were downloaded to compact disk and all slices were analyzed with custom software (semiautomated segmentation; NeuroLine®, Campinas, São Paulo, Brazil) [7]. Images were analyzed by the same investigator (DCCA). The MRI was performed once, the CSA evaluation was performed five times, and the coefficient of variation of repeat assessment of the same image was less than 1.5%. There were no differences in quadriceps values when comparing right and left sides before and after 6 months in the GG and CG. Therefore, results from the right quadriceps were presented. At the time of inclusion in the study, the average CSA values of the GG and CG were similar.
The differences in quadriceps CSA after 6 months of gait training associated with NMES were determined by Wilcoxon test for GG. In the CG, to determine the differences in CSA at the beginning and after 6 months of traditional therapy and after 6 months of gait training without NMES, we used analysis of variance. Results are presented as mean ± standard deviation.
Results
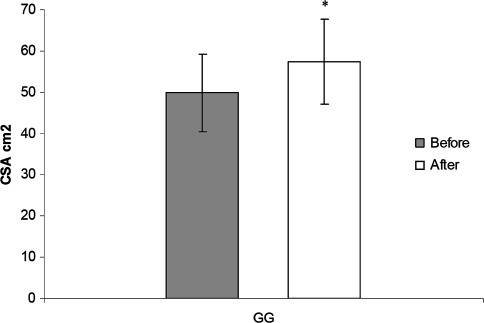
The increase of CSA after 6 months of treadmill gait training was associated with the NMES (Table 1). We observed an increase (p = 0.000007) of 15% in the CSA after 6 months of treadmill gait training, increasing from 49.81 ± 9.36 cm2 to 57.33 ± 10.32 cm2 (Fig. 2).
Table 1.
Quadriceps cross-sectional area of each individual in the gait group
| Gait group | Level of injury | Cross-sectional area (cm2) | |
|---|---|---|---|
| Before | After | ||
| 1 | C5 | 60.2 | 73.3 |
| 2 | C4 | 59.1 | 69.3 |
| 3 | C5 | 57.2 | 60.7 |
| 4 | C4 | 38.9 | 53.0 |
| 5 | C4 | 47.5 | 51.4 |
| 6 | C5 | 48.5 | 52.8 |
| 7 | C7 | 34.5 | 41.1 |
| 8 | C6 | 52.5 | 56.9 |
| Mean | 49.8 | 57.3 | |
| Standard deviation | 9.4 | 10.3 | |
Fig. 2.
The graph shows the increase of quadriceps CSA after 6 months of gait training with NMES in the GG. The bars represent mean values and standard deviations, and the asterisk indicates different (p = 0.000007) initial values and values after 6 months.
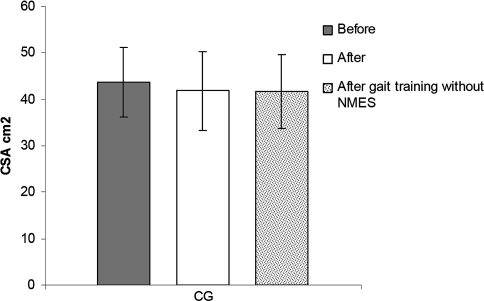
The gait training without NMES did not promote any change in muscle mass compared with the 6-month period of traditional therapy (Table 2). In the CG, the CSA did not differ (p = 0.08) between baseline and after 6 months, going from 43.60 ± 7.56 cm2 to 41.81 ± 8.45 cm2. The results at the beginning of gait training between the GG and the CG (49.81 ± 9.36 cm2 versus 41.81 ± 8.45 cm2) showed a tendency to be different (p = 0.05). After another 6 months training of all individuals in the CG with treadmill gait training and BWS but without NMES, there were no changes (p = 0.09) in the quadriceps CSA (41.81 ± 8.45 cm2 versus 41.75 ± 7.87 cm2, respectively) (Fig. 3). We noted mixed results in the CG: some patients had an increase in CSA and others had a decrease.
Table 2.
Quadriceps cross-sectional area of each individual in the control group
| Control group | Level of injury | Cross-sectional area (cm2) | ||
|---|---|---|---|---|
| Before | After | After gait training without NMES | ||
| 1 | C5 | 46.2 | 44.2 | 41.9 |
| 2 | C7 | 55.8 | 53.2 | 57.3 |
| 3 | C6 | 38.6 | 35.8 | 36.9 |
| 4 | C6 | 39.9 | 36.8 | 38.1 |
| 5 | C5 | 40.5 | 36.9 | 32.8 |
| 6 | C5 | 33.9 | 32.7 | 39.9 |
| 7 | C7 | 50.3 | 53.0 | 45.3 |
| Mean | 43.6 | 41.8 | 41.7 | |
| Standard deviation | 7.6 | 8.4 | 7.9 | |
NMES = neuromuscular electrical stimulation.
Fig. 3.
The graph shows no differences in the quadriceps CSA before, after 6 months without gait training, and after 6 months of gait training without NMES in the CG. The bars represent mean values and standard deviations.
Discussion
An increase in CSA of muscle has been observed in previous studies reporting rehabilitation regimens. However, it is not clear if the use of NMES during gait in individuals with chronic quadriplegia is necessary to promote improvements in muscle CSA or if gait by itself, by promoting mechanical load on the musculoskeletal system, is beneficial. We therefore raised two questions: Does NMES promote an increase of quadriceps CSA in complete quadriplegics, and is treadmill gait training even without NMES sufficient to promote any improvement in muscle compared with inactivity?
We note several study limitations: (1) The lack of a longitudinal followup prevented us from observing if the CSA increase is maintained with time and if the observed increase produces considerable physiologic effects avoiding secondary health issues. (2) The small samples owing to the difficulty of recruiting individuals with spinal cord injuries willing to participate may prevent application of our data to the general population of individuals with spinal cord injuries. The importance of the clinical changes to the muscles will be obtained from longitudinal studies with larger sample sizes making the statistical analysis possible. (3) The small sample also prevented creation of a third group in which only NMES would be performed without gait. Despite the limitations, our study produced some preliminary results to evaluate the effects of gait training, with and without NMES, in quadriceps CSA in patients with complete quadriplegia. (4) When analyzing the results for each subject after gait training without NMES, we noted mixed results among individuals in the CG as some had an increase in CSA and others had a decrease. Therefore, a study with a larger sample size is necessary to draw definitive conclusions.
Application of NMES during treadmill gait, in our study, effectively increased quadriceps CSA in patients with complete quadriplegia, whereas patients who performed treadmill gait training without NMES did not have changes in quadriceps muscle area. The 15% increase in quadriceps CSA confirms previous studies that showed muscle increase in chronic spinal cord-injured patients using NMES in other modalities of therapy [3, 19]. Two studies suggest NMES does not reverse muscle atrophy in chronic spinal cord-injured patients [1, 16] but can promote moderate increases after muscle contractions against some external load [20, 21]. Scremin et al. [23] observed an increase of 31% in the CSA of the rectus femoris after 98.1 ± 9.1 sessions of FES cycle-ergometer training with progressive resistance as shown on CT. Mohr et al. reported an increase of 12% in CSA of the thigh after FES cycle-ergometer with progressive load for 12 months three times a week [19].
The effect of gait on muscle CSA in spinal cord-injured patients was reported by few studies, which contrasts our results [14, 24]. In one study, there was an increase of 4.9% in thigh muscle area and 8.2% in the lower leg after 12 months of gait training (three times a week, totaling 144 sessions) without NMES; however, this study included only incomplete spinal cord injuries, paraplegia and quadriplegia [14]. A reversal of muscle atrophy in acute patients with incomplete quadriplegia (American Spinal Cord Injury Association B and C) after 48 sessions of treadmill gait training twice a week also was reported [13]. Stewart et al. [24] reported an increase in size of Types I and IIa fibers with restoration of the normal size of muscle fibers after 6 months of gait (68 sessions) without NMES beginning with 65% ± 3% and ending with 23% ± 5% of BWS in individuals with incomplete (American Spinal Cord Injury Association C) paraplegia and quadriplegia.
We observed an increase in muscle mass during gait with partial BWS in complete chronic quadriplegics was associated with NMES. The inability to find increases in muscle CSA after gait training without NMES was possibly the result of insufficient mechanical load imposed on paralyzed muscles (reduction of 30%–50% of body weight during gait) associated with severity of the spinal cord lesion (complete and extensive lesions). Although the data at the beginning of gait training with and without NMES tended to be different, it has been reported that muscles of subjects with chronic spinal cord injuries can improve after exercise [3, 14, 19, 22]. This fact was observed in subjects with low CSA values in the gait group, who also benefited from the training. Therefore, the atrophied muscles also can improve with exercise; however, the improvement depends on the type and intensity of exercise, which can vary among subjects.
The increase in muscle area after gait without NMES observed in some studies probably was related to the injury level, residual motor function (patients with incomplete lesions), and the capacity of patients with incomplete lesions to achieve a higher intensity level of training compared with patients with complete cervical lesions (included in this study).
Therefore, the increase of CSA we observed in complete quadriplegic subjects after gait training was associated with the NMES, and the treadmill gait without NMES did not promote any benefit to the muscle mass when the mean values of the CG were analyzed.
Acknowledgments
We thank the students and the subjects for their participation in the project and the FAPESP.
Footnotes
One or more of the authors (ACJ) has received funding from Grants 2005/53530-0, 2003/05856-9, and 1996/12198-2 from Fundação de Amparo à Pesquisa do Estado de São Paulo.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Baldi JC, Jackson RD, Moraille R, Mysiw WJ. Muscle atrophy is prevented in patients with acute spinal cord injury using functional electrical stimulation. Spinal Cord. 1998;36:463–469. [DOI] [PubMed]
- 2.Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81:1090–1098. [DOI] [PubMed]
- 3.Bickel CS, Slade JM, Haddad F, Adams GR, Dudley GA. Acute molecular responses of skeletal muscle to resistance exercise in able-bodied and spinal cord-injured subjects. J Appl Physiol. 2003;94:2255–2262. [DOI] [PubMed]
- 4.Burnham R, Martin T, Stein R, Bell G, Maclean I, Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord. 1997;35:86–91. [DOI] [PubMed]
- 5.Carvalho DC, de Cassia ZM, Sereni JM, Cliquet A. Metabolic and cardiorespiratory responses of tetraplegic subjects during treadmill walking using neuromuscular electrical stimulation and partial body weight support. Spinal Cord. 2005;43:400–405. [DOI] [PubMed]
- 6.Carvalho DC, Garlipp CR, Bottini PV, Afaz SH, Moda MA, Cliquet A Jr. Effect of treadmill gait on bone markers and bone mineral density of quadriplegic subjects. Braz J Med Biol Res. 2006;39:1357–1363. [DOI] [PubMed]
- 7.Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59:1061–1069. [DOI] [PubMed]
- 8.Castro MJ, Apple DF Jr, Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross- sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80:373–378. [DOI] [PubMed]
- 9.Crameri RG, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29:104–111. [DOI] [PubMed]
- 10.de Carvalho DC, Martins CL, Cardoso SD, Cliquet A. Improvement of metabolic and cardiorespiratory responses through treadmill gait training with neuromuscular electrical stimulation in quadriplegic subjects. Artif Organs. 2006;30:56–63. [DOI] [PubMed]
- 11.Dudley GA, Castro MJ, Rogers S, Apple DF Jr. A simple means of increasing muscle size after spinal cord injury: a pilot study. Eur J Appl Physiol Occup Physiol. 1999;80:394–396. [DOI] [PubMed]
- 12.Faghri PD, Glaser RM, Figoni SF. Functional electrical stimulation leg cycle ergometer exercise: training effects on cardiorespiratory responses of spinal cord injured subjects at rest and during submaximal exercise. Arch Phys Med Rehabil. 1992;73:1085–1093. [PubMed]
- 13.Giangregorio LM, Hicks AL, Webber CE, Phillips SM, Craven BC, Bugaresti JM, McCartney N. Body weight supported treadmill training in acute spinal cord injury: impact on muscle and bone. Spinal Cord. 2005;43:649–657. [DOI] [PubMed]
- 14.Giangregorio LM, Webber CE, Phillips SM, Hicks AL, Craven BC, Bugaresti JM, McCartney N. Can body weight supported treadmill training increase bone mass and reverse muscle atrophy in individuals with chronic incomplete spinal cord injury? Appl Physiol Nutr Metab. 2006;31:283–291. [DOI] [PubMed]
- 15.Gordon T, Mao J. Muscle atrophy and procedures for training after spinal cord injury. Phys Ther. 1994;74:50–60. [DOI] [PubMed]
- 16.Greve J, Muszkat R, Schmidt B, Chiovatto J, Barros T, Batistella L. Functional electrical stimulation (FES): muscle histochemical analysis. Paraplegia. 1993;31:764–770. [DOI] [PubMed]
- 17.Hooker SP, Figoni SF, Rodgers MM, Glaser RM, Mathews T, Suryaprasad AG, Gupta SC. Physiologic effects of electrical stimulation leg cycle exercise training in spinal cord injured persons. Arch Phys Med Rehabil. 1992;73:470–476. [PubMed]
- 18.Modlesky CM, Bickel CS, Slade JM, Meyer RA, Cureton KJ, Dudley GA. Assessment of skeletal muscle mass in men with spinal cord injury using dual-energy x-ray absorptiometry and magnetic resonance imaging. J Appl Physiol. 2004;96:561–565. [DOI] [PubMed]
- 19.Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61:22–25. [DOI] [PubMed]
- 20.Pacy PJ, Hesp R, Halliday DA, Katz D, Cameron G, Reeve J. Muscle and bone in paraplegic patients and the effect of functional electrical stimulation. Clin Sci. 1988;75:481–487. [DOI] [PubMed]
- 21.Ragnarsson KT. Physiologic effects of functional electrical stimulation-induced exercises in spinal cord-injured individuals. Clin Orthop Relat Res. 1988;233:53–63. [PubMed]
- 22.Ragnarsson KT, Pollack S, O’Daniel W Jr, Edgar R, Petrofsky J, Nash MS. Clinical evaluation of computerized functional electrical stimulation after spinal cord injury: a multi-center pilot study. Arch Phys Med Rehabil. 1988;69:672–677. [PubMed]
- 23.Scremin AM, Kurta L, Gentili A, Wiseman B, Perell K, Kunkel C, Scremin OU. Increasing muscle mass in spinal cord injured person with a functional electrical stimulation exercise program. Arch Phys Med Rehabil. 1999;80:1531–1536. [DOI] [PubMed]
- 24.Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS, Phillips SM. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 2004;30:61–68. [DOI] [PubMed]