Abstract
Hydroxyapatite coatings for THA promote bone ongrowth, but bone and coating are exposed to stress shielding-driven osteoclastic resorption. We asked: (1) if the resorption of hydroxyapatite coating and bone ongrowth correlated with demographics; (2) if the resorption related to the stem level; and (3) what happens to the implant-bone interface when all hydroxyapatite coating is resorbed? We recovered 13 femoral components from cadaveric specimens 3.3 to 11.2 years after uneventful primary THA. Three cross sections (proximal, medial, distal) of the hydroxyapatite-coated proximal implant sleeve were analyzed by measuring the percentage of residual hydroxyapatite and bone ongrowth on the implant perimeter. Hydroxyapatite resorption was independent of patient age but increased with time in vivo and mostly was gone after 8 years. Bone ongrowth was independent of time in vivo but decreased with aging patients. Only in the most proximal section did less residual hydroxyapatite correlate with less bone ongrowth. Hydroxyapatite resorption, which was more proximal than distal, showed no adverse effects on the implant-bone interface.
Introduction
The use of hydroxyapatite (HA) has been advocated to provide rapid and reliable attachment of bone to metal implants [4, 6, 9, 10, 17, 19, 21, 25, 26]. Augmented bone ongrowth has been documented as early as 3 weeks and as persisting for some years [2–4, 11, 26, 27]. However, as much as 20% to 30% of bone loss at the proximal femur has been reported after THA for the same period as a consequence of proximal stress shielding [8, 21, 30]. It also is expected and observed that the HA coating resorbs with time [1–3, 18, 19, 27, 28], although it is unknown whether this adversely influences the amount of long-term bone ongrowth. Apart from time in vivo and new stress patterns, do other factors influence long-term ongrowth and HA resorption?
Based on short-term bone density studies using dual-energy xray absorptiometry, gender and bone stock are believed to influence ongrowth and resorption [20, 30]. Although age certainly is related to bone stock quality, a direct correlation between age and bone ongrowth onto implants such as uncemented HA-coated hip stems has not yet been proven. In longer-term human histomorphometric retrieval studies [1, 5, 11, 27, 28], the influence of demographics on bone ongrowth and HA resorption could not be studied because of the low number of retrievals or heterogeneity of the described implant designs.
HA coating loss can be caused by several mechanisms, such as osteoclastic resorption during bone remodeling, abrasion, chemical dissolution or delamination. The theory that HA resorption is mainly cell mediated through bone remodeling [2, 19, 28] could be supported if a correlation could be found between HA resorption and stem level as the remodeling differs along the stem. Since the introduction of HA-coated implants, prospectively controlled clinical studies have shown continuing implant fixation [6, 17, 21], but the above questions regarding bone ongrowth and HA resorption at the stem-bone interface cannot be answered.
We therefore addressed three questions: (1) Is the resorption of HA coating and bone ongrowth mainly correlated with time in vivo or with demographics, and if time in vivo is predominant, at which time can we expect all the HA to have completely resorbed? (2) Are HA resorption and/or the amount of bone ongrowth correlated or rather related to the stem level? (3) What happens to the implant-bone interface when all the HA coating is resorbed? Is there still bone ongrowth left to maintain fixation? What levels of bone ongrowth can be associated with clinically stable stem fixation?
Materials and Methods
At autopsy, we performed histomorphometric examinations of metaphyseal surfaces of femoral stems from 13 cadavers by measuring the extent of residual HA coating and the amount of bone ongrowth to find correlations between these histomorphometric parameters and patient demographics (time of implantation, age, height, weight, and clinical score). Values for residual HA and bone ongrowth were measured at three metaphyseal levels, first to correlate the two measures and then to correlate them to the different metaphyseal stem levels. As all stems were mechanically well fixed at the time of retrieval, we assessed the status of HA resorption and the percentage of bone ongrowth in relation to stable stem fixation as expressed in percentage of bone ongrowth.
Thirteen patients from a prospectively followed series of more than 750 consecutive patients receiving primary ABG®-I (Stryker, Caen, France) prostheses provided written consent for retrieval of the prostheses postmortem. All 13 patients (10 women, three men; age at the time of the surgery, 58–86 years) had uneventful THAs and died from causes unrelated to their hip disease (Table 1). The time from implantation (stem in vivo) ranged from 3.3 to 11.2 years. The femoral stems were made of titanium alloy (Ti6Al4V) with the proximal third HA-coated by plasma spray onto a macrorelief surface. The titanium substrate had a roughness of 3 to 4 μm Ra. The coating had a HA content greater than 90% and porosity less than 10%. Crystallinity was 100% before coating and greater than 75% thereafter. The grain size was 20 to 50 μm, and the strength of the tensile bond was 62 to 65 MPa. The thickness of the HA layer was 60 ± 15 μm.
Table 1.
Patient demographics
| Patient number | Gender | Age at THA (years) | Diagnosis | Time from implantation (years) | Weight (kg) | Height (m) | Merle d’Aubigné-Postel score | Harris hip score | Cause of death | Alignment stem |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 63 | Osteoarthritis | 3.3 | 85 | 1.39 | 18 | 100 | Cardiac arrest | Neutral |
| 2 | Female | 86 | Osteoarthritis | 4.5 | 65 | 1.62 | 18 | 100 | Cardiac arrest | Neutral |
| 3 | Male | 65 | Osteoarthritis | 5.2 | 65 | 1.69 | 18 | 100 | Cerebral hemorrhage | Neutral |
| 4 | Female | 81 | Osteoarthritis | 5.4 | 52 | 1.60 | 18 | 97 | Cardiac arrest | 4° valgus |
| 5 | Male | 58 | Osteoarthritis | 5.7 | 50 | 1.52 | 15 | 90 | Cardiac arrest | Neutral |
| 6 | Male | 72 | Osteoarthritis | 6.1 | 65 | 1.66 | 18 | 100 | Cerebral hemorrhage | 4° varus |
| 7 | Female | 66 | Osteoarthritis | 6.2 | 75 | 1.63 | 18 | 100 | Aortic aneurysm | 2° varus |
| 8 | Female | 86 | Osteoarthritis | 6.6 | 65 | 1.60 | 18 | 95 | Pancreatitis | Neutral |
| 9 | Female | 65 | Fracture | 8.0 | 60 | 1.71 | 18 | 100 | Cardiac arrest | Neutral |
| 10 | Female | 73 | Rheumatoid arthritis | 8.3 | 50 | 1.55 | 18 | 100 | Lung carcinoma | Neutral |
| 11 | Female | 60 | Rheumatoid arthritis | 8.11 | 57 | 1.60 | 15 | 76 | Foramen magnum trapping | Neutral |
| 12 | Female | 69 | Osteoarthritis | 10.5 | 103 | 1.64 | 13 | 73 | Cardiac failure | Neutral |
| 13 | Female | 66 | Osteoarthritis | 11.2 | 70 | 1.68 | 14 | 75 | Subdural hematoma | 3° varus |
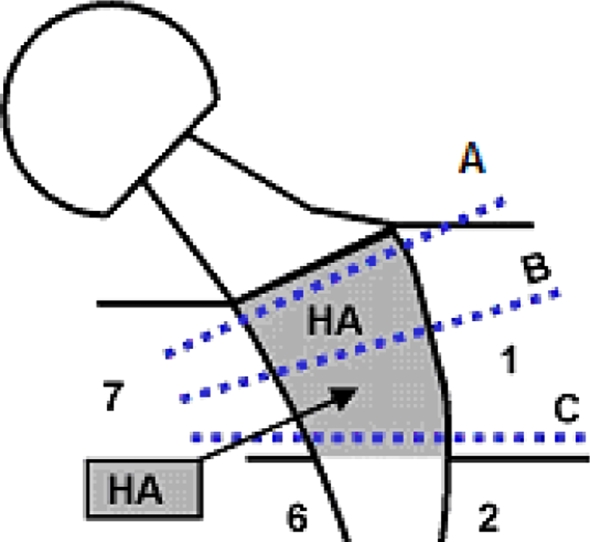
The prostheses and surrounding bone were collected postmortem, immersed in buffered formalin for 7 days, and then immersed in 70% ethanol for 24 hours. Three cross sections were cut from the metaphyseal femur proximal to a line separating proximal Gruen Zones 1 and 7 (regions with HA coating) from the distal stem (Fig. 1). The three metaphyseal sections were proximal (A), medial (B), and distal (C). Each segment was embedded in a polymethylmethacrylate resin and a representative section (approximately 20 μm thick) was cut from each segment using a microcutting and grinding technique adapted from a technique described by Donath and Breuner [7]. The sections underwent paragon staining (a combination of basic fuchsin and toluidine blue) for qualitative histology and quantitative histomorphometry.
Fig. 1.
Three cross sections, proximal (A), medial (B), and distal (C), were cut from the metaphyseal femur proximal to a line separating proximal Gruen Zones 1 and 7 (regions with HA coating) from the distal stem.
A Polyvar microscope (Reichert-Jung, Vienna, Austria) was used for qualitative analysis; quantitative analysis was performed on an Axioskop® microscope (Carl Zeiss, Jena, Germany) equipped with a color image analyzing system (SAMBA technology; Alcatel, Paris, France). The pathologists (MT, AA) were blinded to all clinical information, except for patient identification and cause of death. They successively identified regions of implant, bone, and lacunae, including all soft tissues. The methods used were described previously [28]. For each section, the total implant perimeter and the percentage of implant perimeter covered by bone and/or by residual HA coating were measured. Bone-implant contact was defined as direct ongrowth of bone to the HA coating or to the titanium surface after HA resorption and represented the amount of osseointegration. The lengths of bone or HA contact were divided by the length of the implant interface to provide a parameter illustrating the percentage of the implant covered by bone or HA. Means and standard deviations were calculated for each section. The percentages of bone ongrowth and residual HA coating were compared among the three section levels (A, B, and C) and correlated to the total time in vivo and patient demographics (gender, age, diagnosis, time in vivo, weight, height, clinical score, and stem position).
The histomorphometric parameters for the femoral components of Cases 1, 3, 4, 5, and 7 were reported previously [28], but because of the small data sample, correlations between the histomorphometric and demographic data were not reported and the study focused on histologic findings instead. In another histomorphometric study [27], nearly total resorption was observed after more than 6 years’ implantation. Therefore, we used a 6-year cutoff after implantation to compare amounts of HA coating resorption between two groups with time of implantation before and after this threshold (Group I consisting of Cases 1–6 and Group II consisting of Cases 7–13).
To answer our first question, we used linear regression to correlate HA resorption and bone ongrowth with time in vivo and patient demographic factors such as age. In addition, based on the threshold value of 6 years in vivo, we compared the two groups above and below this threshold using the unpaired Student’s t test. To answer our second question, we used linear regression to correlate HA resorption with bone ongrowth and to correlate these two parameters with the three metaphyseal levels. The paired Student’s t test was used to compare the differences between these three levels. To answer our third question, we used only descriptive statistics. The assumptions regarding linearity (correlation testing) and normality (comparisons) were confirmed using the runs test (linearity) and Kolmogorov-Smirnov test (normality). Trends and evidence levels (p values) also were validated using nonparametric test alternatives.
Results
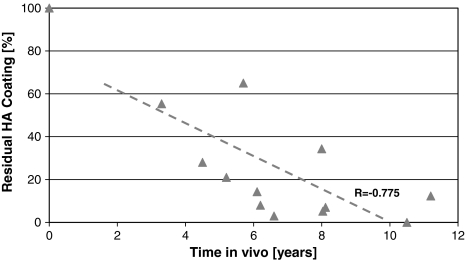
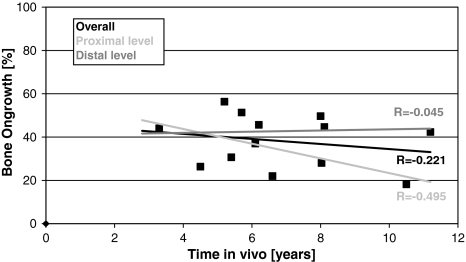
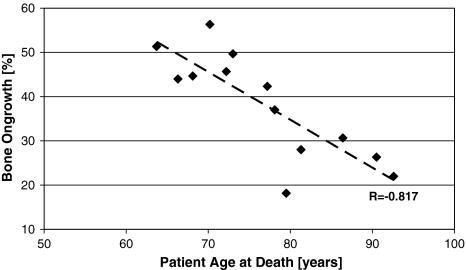
HA resorption (total of three stem levels) increased (r = −0.775; p = 0.002) with time in vivo as measured by the residual HA (Fig. 2). Less residual HA and thus increased resorption with time in vivo was observed for each section (A: r = −0.709, p = 0.007; B: r = −0.779, p = 0.002; and C: r = −0.756, p = 0.003). Also, the average residual HA level was higher (p = 0.02) when time in vivo was less than 6 years (36.7% ± 22.2% [n = 6]) than when it was more than 6 years (10.1% ± 10.0% [n = 7]). Beyond 8 years, the HA was almost gone (Fig. 2). Overall bone ongrowth ranged between 18% and 56% and was independent (r = 0.221; p = 0.46) of the time in vivo (Fig. 3). At the proximal level (A), bone ongrowth decreased somewhat (r = −0.495; p = 0.085) with time in vivo beyond 3 years, while the bone ongrowth remained flat at the medial level (B) (r = 0.044; p = 0.89) and distal level (C) (r = 0.045; p = 0.90) (Fig. 3). Bone ongrowth correlated (r = −0.817; p = 0.0007) with patient age, with younger patients having higher bone ongrowth than older patients (Fig. 4). HA resorption however did not correlate (r = −0.396; p = 0.20) with patient age. Patient height, weight, and body mass index had no influence on either residual HA (p = 0.13–0.86) or bone ongrowth (p = 0.51–0.93).
Fig. 2.
A graph shows the correlation between residual HA coating and time in vivo (r = −0.775; p = 0.002).
Fig. 3.
A graph shows the correlation between bone ongrowth and time in vivo overall (r = −0.221; p = 0.46) and for the proximal (r = −0.495; p = 0.085) and distal (r = −0.045; p = 0.88) levels.
Fig. 4.
A graph shows the correlation between patient age and overall bone ongrowth (r = −0.817; p = 0.0007).
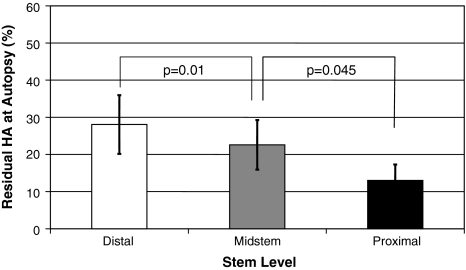
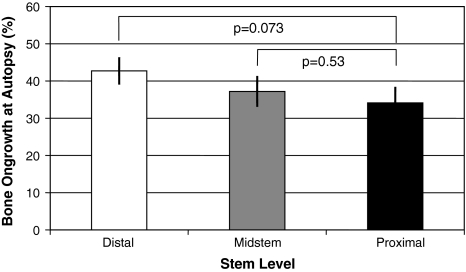
Bone ongrowth and HA resorption correlated (r = 0.716; p = 0.009) only in the most proximal zone, with lower bone ongrowth associated with lower levels of residual HA. We observed no correlation for the medial (r = 0.265; p = 0.41) or distal (r = 0.200; p = 0.53) stem level or for overall values (r = 0.519; p = 0.08). HA resorption was highest (p = 0.045) most proximally, with less residual HA in the proximal level (13.0% ± 14.9%) than in the medial (22.6% ± 23.1%) and distal (28.1% ± 27.3%) levels (Fig. 5). Bone ongrowth was not correlated (p = 0.07–0.53) with the metaphyseal stem levels (A: 34.1% ± 15.6%; B: 37.2% ± 14.9%; C: 42.7% ± 13.2%) (Fig. 6).
Fig. 5.
A comparison between residual HA values measured at the different stem levels shows HA resorption was highest (p = 0.045) in the most proximal section. Data are expressed as means with standard errors of the mean.
Fig. 6.
A comparison between bone ongrowth levels measured at the different stem levels shows bone ongrowth was not correlated (p = 0.07–0.53) with the metaphyseal stem levels. Data are expressed as means with standard errors of the mean.
All stems were well fixed at retrieval and the ranges of residual HA and bone ongrowth were 0% to 55% and 18% to 56%, respectively (Table 2). The three lowest levels of residual HA still showing stable stem fixation were 0%, 3%, and 5%, respectively, whereas the three lowest levels of bone ongrowth that secured stem fixation were 18%, 22%, and 26%, respectively (Table 2).
Table 2.
Percentages of bone ongrowth and residual hydroxyapatite coating
| Patient number | Bone ongrowth (%) | Residual hydroxyapatite (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1A–7A | 1B–7B | 1C–7C | Mean | 1A–7A | 1B–7B | 1C–7C | Mean | |
| 1 | 51 | 40 | 39 | 44 | 15 | 68 | 83 | 55 |
| 2 | 34 | 18 | 27 | 26 | 2 | 3 | 4 | 3 |
| 3 | 48 | 54 | 67 | 56 | 12 | 20 | 31 | 21 |
| 4 | 25 | 25 | 42 | 31 | NA | NA | NA | NA |
| 5 | 61 | 45 | 48 | 51 | 50 | 68 | 77 | 65 |
| 6 | 37 | 35 | 37 | 37 | 11 | 18 | 14 | 14 |
| 7 | 36 | 52 | 49 | 46 | 5 | 7 | 11 | 8 |
| 8 | 33 | 12 | 21 | 22 | 3 | 2 | 8 | 3 |
| 9 | 40 | 55 | 54 | 50 | 31 | 31 | 41 | 34 |
| 10 | 6 | 40 | 36 | 28 | 0 | 10 | 5.6 | 5 |
| 11 | 24 | 54 | 56 | 45 | 2 | 10 | 11 | 8 |
| 12 | 9 | 19 | 27 | 18 | 0 | 0 | 0 | 0 |
| 13 | 40 | 35 | 52 | 42 | 6 | 11 | 20 | 12 |
A = proximal; B = medial; C = distal; NA = not available.
Discussion
HA coating accelerates early bone ongrowth whereas proximal stress shielding-induced periprosthetic bone resorption seemingly plays a contrary role during the local bone remodeling process. One relates to initial repair primarily in cancellous bone while the other relates to long-term adaptation, which radiographically seems to occur in the proximal cancellous areas and in the cortical areas [6, 15–17, 20, 25, 30]. However, these opposing processes of bone gain and loss do not work at the same time, but whether they persist and to what degree are unknown. Although short-term retrieval studies report the HA coating after 2 to 4 years implantation is partly broken down through osteoclastic resorption [2, 3, 5, 10, 11, 18, 19, 27, 28], none of these studies reported whether patient demographics might influence residual HA coating or bone ongrowth. Therefore, we asked three questions: (1) Is the resorption of HA coating and bone ongrowth mainly correlated with time in vivo or with demographics, and when time in vivo is predominant, at which point can we expect all the HA to have completely resorbed? (2) Are HA resorption and/or the amount of bone ongrowth correlated with each other or rather related to the metaphyseal stem level? (3) What happens to the implant-bone interface when all the HA coating is resorbed, how much bone ongrowth is left, and which minimum levels can be associated with clinically stable fixation?
Our study has some limitations. First, the quality of the initial bone stock and exact parameters of patient activity level were not known and these two factors have a major influence on general bone remodeling [18, 20, 24]. Second, we did not study interobserver variability although the two pathologists who performed the measurements were from a dedicated professional laboratory specializing in histology and histomorphometry; their interpretations were checked by two other staff members from that laboratory. Third, with only three patients showing nonneutral stem alignment, we could not examine the influence of varus or valgus positioning on the histomorphometric bone remodeling parameters. Positioning might well influence long-term bone remodeling and we could not account for these influences. However, we studied retrievals from patients having only one femoral stem design. The harvesting, preparation, and analysis were the same for all specimens and the pathologists were blinded to all clinical data, except for cause of death.
We observed HA coating was increasingly resorbed with time from implantation and was nearly completed at 8 years. No other demographic factor correlated with resorption. Bauer et al. [2, 3] and Hardy et al. [11] did not report a correlation between the extent of residual HA and duration in vivo, but their series were too small (three to seven cases) and the followup was too short (25 months) to document substantial HA resorption. However, longer followup [1, 27, 28] or animal experiments [18] indicate a tendency toward total HA coating resorption with increased time of implantation.
The only demographic factor that influenced the amount of bone ongrowth was age, with younger patients having higher bone ongrowth percentages than older patients. This may relate to greater initial bone stock in younger people but also can be explained by the fact that in older patients the resorptive component of the remodeling process is more prominent, especially in patients with senile osteoporosis. Our data do not support those of Linder et al. [14] showing inferior bone ingrowth in patients with osteopenic rheumatoid arthritis as compared with patients with osteoarthritis. Søballe et al. [23] also did not observe less bone fixation of HA-coated implants in osteopenic versus normal bone after 4 weeks’ implantation, but with titanium porous implants bony fixation was less in osteopenic bone. Also, Shih et al. [22] observed impaired bone ingrowth of porous cobalt/chromium plugs in areas with cancellous bone in ovariectomized dogs compared with female controls. Therefore, it seems percentages of long-term bone ongrowth are affected mainly by the individual bone stock quality, which is intimately related to the age of the patient. The observation that HA resorption is independent of age supports this conclusion but also indicates older age is not a contraindication for the use of HA-coated implants.
Only at the most proximal stem level did the lower bone ongrowth correlate with lower levels of residual HA. Both values decreased from distal to proximal, but the effect and evidence were stronger for HA resorption. Thus, the extent and metaphyseal location of the HA resorption strongly resembled the new postoperative proximal stress shielding patterns [12, 13, 29]. These patterns can be qualitatively predicted for hip stems in general, showing highest stress shielding most proximally and a rapid reduction in stress shielding going more distally. In other words, when we presume a relationship between the amount and location of resorption of the HA and the known pattern of progressing load transfer from proximal to distal, our histomorphometric results suggest the bone remodeling process (as dictated by the local stress shielding patterns, which are described in finite-element studies [12, 13, 29]) is a major regulating factor in HA resorption. Bauer et al. [2] also reported a general increase of bone apposition from proximal to distal (in the coated portion of the stem). Coathup et al. [5] compared bone remodeling around one femoral stem design with three different proximal coatings in 21 postmortem cases and observed a larger amount of bone ingrowth into the surface of the plain porous implants at the most distal metaphyseal level compared with the two proximal levels. However, bone ongrowth in the porous HA-coated regions was distributed more evenly without large differences. As they also noted more ingrowth and ongrowth of bone to the porous HA-coated surface, it is thought the HA counteracts the bone resorptive action of the local stress shielding, at least during the early postoperative years [5].
As bone ongrowth was independent of implantation time in vivo and independent of HA resorption on the medial and distal parts of the coated stem, we presume the long-term implant fixation is not disturbed by ongoing HA coating resorption. This means, during the remodeling process, part of the resorbed HA coating layer will be replaced by bone. This has been observed in numerous experimental and retrieval studies [1, 18, 27, 28]. In a dog study using weightbearing implants, Overgaard et al. [18] reported completely resorbed HA coating was replaced by 36% ± 6.0% (range, 26%–42%) bone in direct contact with the implant surface, suggesting the implant was firmly fixed despite loss of the ceramic coating. Aebli et al. [1] in a single human retrieval study, noted complete HA coating resorption after 9.5 years of good function, with a 34% average bone-implant contact at the originally coated part. We also observed retrieved specimens with little HA coating (0%–8%) remaining but with bone ongrowth percentages between 18% and 45%. These data indicate lack of correlation between HA coating resorption and amount of bone ongrowth; suggesting, after an initial burst of accelerated bone ongrowth with all its positive effects [4, 6, 21], HA has no additional beneficial or negative role during the middle to long term for implant fixation or for the bone remodeling process.
We found the HA coating layer was slowly resorbed and was almost completely resorbed after 8 years of implantation. Age or other patient factors such as gender, height, and weight had no apparent influence on this process. The amount of bone ongrowth to the stem observed postmortem, however, was related to the age of the patient at time of death but not to the time of implantation. The extent and metaphyseal location of the HA coating resorption reflected the ongoing proximal stress shielding patterns after THA. These findings suggest the amount of HA coating resorption has no influence on the amount of bone ongrowth and therefore will not disturb long-term fixation of the implant.
Acknowledgments
We thank Michel Therin and Antoine Alves from Biomatech (Chasse-sur-Rhone, France) for histomorphometric work on the cadaver specimens and Drs. J. P. Boutrand and R. Eloy for supervising the study performed at Biomatech.
Footnotes
All the histomorphometry was performed at Biomatech (Chasse-sur-Rhone, France) and funded by Stryker® (Caen, France).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aebli N, Krebs J, Schwenke D, Stich H, Schwawalder P, Theis JC. Degradation of hydroxyapatite coating on a well-functioning femoral component. J Bone Joint Surg Br. 2003;85:499–503. [DOI] [PubMed]
- 2.Bauer TW, Geesink RC, Zimmerman R, McMahon JT. Hydroxyapatite-coated femoral stems: histological analysis of components retrieved at autopsy. J Bone Joint Surg Am. 1991;73:1439–1452. [PubMed]
- 3.Bauer TW, Stulberg BN, Ming J, Geesink RG. Uncemented acetabular components: histologic analysis of retrieved hydroxyapatite-coated and porous implants. J Arthroplasty. 1993;8:167–177. [DOI] [PubMed]
- 4.Chambers B, St Clair SF, Froimson MI. Hydroxyapatite-coated tapered cementless femoral components en total hip arthroplasty. J Arthroplasty. 2007;22:71–74. [DOI] [PubMed]
- 5.Coathup MJ, Blunn GW, Flynn N, Williams C, Thomas NP. A comparison of bone remodelling around hydroxyapatite-coated, porous-coated and grit-blasted hip replacements retrieved at post-mortem. J Bone Joint Surg Br. 2001;83:118–123. [DOI] [PubMed]
- 6.D’Antonio JA, Capello WN, Jaffe WL. Hydroxyapatite coated hip implants: multicenter three-year clinical and roentgenographic results. Clin Orthop Relat Res. 1992;285:102–109. [PubMed]
- 7.Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues: the Sage-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–326. [DOI] [PubMed]
- 8.Engh CA, Bobyn JD. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin Orthop Relat Res. 1988;231:7–28. [PubMed]
- 9.Geesink RG, de Groot K, Klein CP. Chemical implant fixation using hydroxyl-apatite coatings: the development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clin Orthop Relat Res. 1987;225:147–170. [PubMed]
- 10.Geesink RG, de Groot K, Klein CP. Bonding of bone to apatite-coated implants. J Bone Joint Surg Br. 1988;70:17–22. [DOI] [PubMed]
- 11.Hardy DC, Frayssinet P, Guilhem A, Lafontaine MA, Delince PE. Bonding of hydroxyapatite-coated femoral prostheses: histopathology of specimens from four cases. J Bone Joint Surg Br. 1991;73:732–740. [DOI] [PubMed]
- 12.Huiskes R. The various stress patterns of press-fit, ingrown, and cemented femoral stems. Clin Orthop Relat Res. 1990;261:27–38. [PubMed]
- 13.Huiskes R, Weinans H, Grootenboer HJ, Dalstra M, Fudala B, Slooff TJ. Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech. 1987;20:1135–1150. [DOI] [PubMed]
- 14.Linder L, Carlsson A, Marsal L, Bjursten LM, Brånemark PI. Clinical aspects of osseointegration in joint replacement: a histological study of titanium implants. J Bone Joint Surg Br. 1988;70:550–555. [DOI] [PubMed]
- 15.Maloney WJ, Jasty M, Burke DW, O’Connor DO, Zalenski EB, Bragdon C, Harris WH. Biomechanical and histologic investigation of cemented total hip arthroplasties: a study of autopsy-retrieved femurs after in vivo cycling. Clin Orthop Relat Res. 1989;249:129–140. [PubMed]
- 16.McCarthy CK, Steinberg GG, Agren M, Leahey D, Wyman E, Baran DT. Quantifying bone loss from the proximal femur after total hip arthroplasty. J Bone Joint Surg Br. 1991;73:774–778. [DOI] [PubMed]
- 17.Oosterbos CJ, Rahmy AI, Tonino AJ, Witpeerd W. High survival rate of hydroxyapatite-coated hip prostheses: 100 consecutive hips followed for 10 years. Acta Orthop Scand. 2004;75:127–133. [DOI] [PubMed]
- 18.Overgaard S, Lind M, Josephsen K, Maunsbach AB, Bünger C, Søballe K. Resorption of hydroxyapatite and fluorapatite ceramic coatings on weight-bearing implants: a quantitative and morphological study in dogs. J Biomed Mater Res. 1998;39:141–152. [DOI] [PubMed]
- 19.Overgaard S, Lind M, Rahbek O, Bünger C, Søballe K. Improved fixation of porous-coated versus grit-blasted surface texture of hydroxyapatite-coated implants in dogs. Acta Orthop Scand. 1997;68:337–343. [DOI] [PubMed]
- 20.Rahmy AI, Gosens T, Blake GM, Tonino A, Fogelman I. Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating: a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporos Int. 2004;15:281–289. [DOI] [PubMed]
- 21.Rasquinha VJ, Ranawat CS, Mauriello AJ Jr. Hydroxyapatite: catalyst or conjuror? J Arthroplasty. 2002;17(4 suppl 1):113–117. [DOI] [PubMed]
- 22.Shih LY, Shih HN, Chen TH. The effect of sex and estrogen therapy on bone ingrowth into porous coated implants. J Orthop Res. 2003;21:1033–1040. [DOI] [PubMed]
- 23.Søballe K, Hansen ES, Brockstedt-Rasmussen H, Hjortdal VE, Juhl GI, Pedersen CM, Hvid I, Bünger C. Fixation of titanium and hydroxyapatite-coated implants in arthritic osteopenic bone. J Arthroplasty. 1991;6:307–316. [DOI] [PubMed]
- 24.Sychterz CJ, Engh CA. The influence of clinical factors on periprosthetic bone remodeling. Clin Orthop Relat Res. 1996;322:285–292. [DOI] [PubMed]
- 25.Tanzer M, Kantor S, Rosenthall L, Bobyn JD. Femoral remodeling after porous-coated total hip arthroplasty with and without hydroxyapatite-tricalcium phosphate coating: a prospective randomized trial. J Arthroplasty. 2001;16:552–558. [DOI] [PubMed]
- 26.Tisdel CL, Goldberg VM, Parr JA, Bensusan JS, Staikoff LS, Stevenson S. The influence of a hydroxyapatite and tricalcium-phosphate coating on bone growth into titanium fiber-metal implants. J Bone Joint Surg Am. 1994;76:159–171. [DOI] [PubMed]
- 27.Tonino A, Oosterbos C, Rahmy A, Thèrin M, Doyle C. Hydroxyapatite-coated acetabular components: histological and histomorphometric analysis of six cups retrieved at autopsy between three and seven years after successful implantation. J Bone Joint Surg Am. 2001;83:817–825. [PubMed]
- 28.Tonino AJ, Therin M, Doyle C. Hydroxyapatite-coated femoral stems: histology and histomorphometry around five components retrieved at post mortem. J Bone Joint Surg Br. 1999;81:148–154. [DOI] [PubMed]
- 29.van Rietbergen B, Huiskes R. Load transfer and stress shielding of the hydroxyapatite-ABG hip: a study of stem length and proximal fixation. J Arthroplasty. 2001;16:55–63. [DOI] [PubMed]
- 30.Venesmaa PK, Kroger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy x-ray absorptiometry: a 3-year follow-up study. J Bone Miner Res. 2001;16:1056–1061. [DOI] [PubMed]