Abstract
Mixed chimerism and donor-specific tolerance are achieved in mice receiving 3 Gy total body irradiation and anti-CD154 monoclonal antibody (mAb) followed by allogeneic bone marrow transplantation (BMT). In this model, recipient CD4 cells are critically important for CD8 tolerance. To evaluate the role of CD4 cells recognizing donor MHC class II directly, we used class II-deficient donor marrow and were not able to achieve chimerism unless recipient CD8 cells were depleted, indicating that directly alloreactive CD4 cells were necessary for CD8 tolerance. To identify the MHC class II+ donor cells promoting this tolerance, we used donor bone marrow (BM) lacking certain cell populations or used positively selected cell populations. Neither donor CD11c+ dendritic cells, B cells, T cells nor donor-derived IL-10 were critical for chimerism induction. Purified donor B cells induced early chimerism and donor-specific cell-mediated lympholysis (CML) tolerance in both strain combinations tested. In contrast, positively selected CD11b+ monocytes/myeloid cells did not induce early chimerism in either strain combination. Donor cell preparations containing B cells were able to induce early deletion of donor-reactive TCR transgenic 2C CD8 T cells, whereas those devoid of B cells had reduced activity. Thus, induction of stable mixed chimerism depends on the expression of MHC class II on the donor marrow, but no requisite donor cell lineage was identified. Donor BM-derived B cells induced early chimerism, donor-specific CML tolerance and deletion of donor-reactive CD8 T cells, whereas CD11b+ cells did not. Thus, BM-derived B cells are potent tolerogenic APCs for alloreactive CD8 cells.
Keywords: B cells, cytotoxic T cells, transplantation, tolerance, MHC class II
Introduction
The induction of donor-specific tolerance is a desirable goal in organ transplantation. Establishment of full donor BM chimerism allows acceptance of donor grafts without immunosuppression (1,2). However, the toxicity of such protocols and the high risk of graft-versus-host disease (GVHD) do not allow clinical application of conventional transplant approaches. We have developed non-myeloablative protocols that achieve mixed lymphohematopoietic chimerism in mice, some relying purely on central tolerance mechanisms (3), while others achieve initial peripheral tolerance using costimulatory blockade with anti-CD154 mAb and CTLA4-Ig (4). Such mice demonstrate donor-specific unresponsiveness in cytotoxicity and proliferation assays and accept donor-type skin grafts permanently, whereas 3rd party grafts are promptly rejected. Some protocols have been translated into non-human primate and patient studies, in which donor-specific tolerance to kidney allografts was successfully established in HLA-identical and haploidentical combinations (5–7).
With the intention of improving protocols to achieve stable mixed chimerism, we have investigated the mechanisms of tolerance induction in murine mixed chimeras. The tolerized CD4 population was previously characterized by our group in CD8-depleted mice with the use of alloreactive TCR transgenic CD4 T cells to track the fate of donor-reactive cells (8,9). Peripheral donor-reactive CD4 T cells were shown to rapidly become anergic, then to undergo clonal deletion within 4–5 weeks following BMT. No skewing of cytokine patterns towards the Th2 phenotype was seen, and no evidence for a role for regulatory T cells was found. Recently we have shown that recipient CD4 T cells are critical for peripheral CD8 tolerance induction (10) in recipients of a non-myeloablative protocol using low dose (3 Gy) total body irradiation (TBI), anti-CD154 mAb and BMT without CD8 depletion (11). CD8 T cells also undergo clonal deletion, which is complete by day 10–14 after BMT. In the early phase prior to deletion, a regulatory CD4 T cell population is required for CD8 cell tolerance, but the regulatory CD4 cell population does not have features of natural Treg, as CD25 depletion and IL-2 neutralization did not interfere with tolerance induction (10).
To evaluate the role of CD4 cells recognizing alloantigen directly on donor APCs for CD8 tolerance, we have now investigated the use of MHC class II-deficient donor BM. The role of defined cell lineages in inducing donor-specific tolerance was addressed by either administering donor BM lacking certain cell populations to evaluate their necessity or by using positively selected BM cell fractions to test for their sufficiency for induction of early peripheral CD8 T cell tolerance. The results demonstrate a strong but not requisite role for donor B cells in this tolerance induction.
Materials and methods
Mice
Female C57BL/6 (B6: H-2b) and B10.A (H-2a) mice were purchased from Frederick Cancer Research Center (Frederick, MD). Female B10.S (H-2s), TCRβ−/− (on C57BL/6 background, H-2b), IL-10−/− (on the C57BL/6 background; H-2b), μMT (on the C57BL/6 background; H-2b) and diphtheria toxin receptor-transgenic (DTR tg, expressing primate DTR under the mouse CD11c promotor; backcrossed 6–7 generations onto the C57Bl/6 background; H-2b) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). I-Aβ−/− (on the C57BL/6 background; H-2b) mice were purchased from Taconic (Germantown, NY). DTR tg as well as 2C TCR tg mice (12) were bred in our animal facility. 2C mice were phenotyped by FACS, and DTR tg mice were genotyped by PCR according to the protocol provided by The Jackson Laboratory. All mice were housed in a specific pathogen-free microisolator environment. Animal experimental protocols were reviewed and approved by the institutional committee at Massachusetts General Hospital.
Conditioning and bone marrow transplantation (BMT)
Eight to twelve week-old mice received 3 Gy of TBI from a 137Cesium irradiator on day –1 with respect to BMT. Hamster anti-mouse CD154 mAb (MR1; 2 mg; produced at National Cell Culture Center, Minneapolis, MN) was administered intraperitoneally (i.p.) on day 0, prior to transplantation with 20–25x106 fully MHC-mismatched bone marrow cells (BMCs) by tail vein injection. CD8 depleting mAb (2.43, 1.44mg), where mentioned, was administered i.p.
Dendritic cell depletion with diphtheria toxin (DT)
Diphtheria toxin was purchased from Sigma-Aldrich (St. Louis, MO) as lyophilized powder and then resuspended with sterile water at a concentration of 1 mg/ml. Depletion of DCs was performed by i.p injection of 100 ng DT in 1 ml PBS 3x/week until day 18. Efficiency of depletion of CD11chigh dendritic cells (DCs) was checked one day after the last DT injection by FACS analysis of collagenase-digested spleen and was approximately 93%.
MACS isolation or depletion of donor cell populations
Donor cells were purified or depleted by magnetic-activated cell sorting (MACS). Donor whole BM was harvested, counted, and incubated according to the manufacturer’s protocol for 15–20 min with either anti-CD11b (Mac-1+ cells), anti-CD19 or anti-CD4&8 magnetic beads in PBS with 0.5% BSA and 2mM EDTA. Cells were washed, resuspended, and sorted with LS (for CD19+ and Mac1+ fractions) or CS columns (for T cell depletion; Miltenyi Biotec) according to the manufacturer’s protocol. Both positive and negative fractions were analyzed for purity by flow cytometry. Mac1+ and CD19+ cells were >97% pure. CD19 and T cell depletion was >99% efficient, but Mac1 depletion was only ~ 60–80% efficient.
Preparation of B6 mice with a traceable population of transgenic CD8 cells specific for the Ld MHC class I antigen (2C/B6 chimeras)
Mice were prepared as previously described (10). Briefly, 5 million BMC from 2C-TCR transgenic B6 mice in which a transgenic T cell receptor recognizing Ld is expressed on >99% of CD8+ T cells, were transplanted into naïve B6 mice treated with 3 Gy TBI on the same day. The resulting mice were chimeric for the 2C TCR and are referred to as 2C/B6 mice. After 8 weeks, the percentage of 2C cells among CD8+ T cells in the peripheral blood was analyzed by flow cytometry using the clonotypic antibody 1B2. 2C cells ranged from 3.8% to 31.4% of peripheral blood CD8 cells. These mice then received the conditioning protocol described above and were transplanted with BMCs from B10.A (Ld positive) or B10.S (Ld negative) donor mice to assess deletion of donor-specific CD8 cells at the time of multilineage chimerism studies.
Flow cytometric analysis of multilineage chimerism in white blood cells
Donor chimerism after allogeneic BMT
Three-color flow cytometric analysis was used to determine multilineage chimerism. Donor-derived cells were identified by fluorescein isothiocyanate-conjugated anti-MHC class I antibodies (anti-H-2Dd mAb 34-2-12 for H-2a, anti-H-2Db mAb KH95 for H-2b). The cells were counterstained with phycoerythrin (PE)- or allophycocyanin-conjugated anti-CD4, -CD8, -B220 (Becton-Dickinson [BD]/PharMingen, San Diego, CA), and -Mac1 (Caltag, San Francisco, CA) mAbs. Negative control mAbs included HOPC1-FITC (prepared in our laboratory) and rat anti-mouse IgG2a-PE or -APC (Becton-Dickinson/Pharmingen). Propidiumiodine stain was used to exclude dead cells from analysis. Chimerism was analyzed among CD4 and CD8 T cells, B cells and granulocytes at 2, 6 and 10 weeks post BMT. A lineage was defined as chimeric when ≥5% donor MHC class I positive cells were found within the respective cell lineage.
2C deletion analysis
2C CD8 T cells were identified by staining with the unconjugated 1B2 clonotypic mAb (13) and anti-mouse IgG1-APC as secondary Ab and counterstained with CD8-PE. Relative deletion was quantified by dividing the % of 2C CD8 T cells in the spleen on day 7 by the % of 2C CD8 T cells present in peripheral blood before B10.A BMT.
Cell mediated lympholysis (CML) assay
CML assay was performed as previously described(11). Spleen cells from control and experimental mice were suspended in CML medium consisting of RPMI 1640 (Mediatech, Herdon, VA) with 10% fetal calf serum (Sigma, St. Louis, MO), 0.09 mM nonessential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate, 100U/mL penicillin, 10 ug streptomycin, 0.05 mM 2-mercaptoethanol, and 0.01 M HEPES buffer. Responder and stimulator cells (8x105 each; stimulators 30 Gy irradiated) were plated in triplicates in a 96-well round-bottomed plate. Cells were incubated for 5 days at 37°C in 6% CO2. A series of twofold dilutions was then prepared, allowing for cytolytic readout at a total of 5 different responder-to-target ratios. Target cells were prepared with a 2 day incubation with concanavalin-A stimulation (2 μg/mL) followed by labeling with 51Cr (1 μCi/mL). Eight thousand target cells were added to each well and incubated for 4 hours at 37°C in 6% CO2. Plates were harvested by using the Titerek supernatant collection system (Skatron, Inc., Sterling, VA) and 51Cr release was quantified with an automated gamma counter. Percent specific lysis (PSL) was calculated as [(experimental release − spontaneous release)/(total release − spontaneous release)] x 100%.
Skin grafting
Mice were shaved and anesthetized with ketamine/xylazine. Full thickness tail skin (0.5–1.0 cm2) from donor and irrelevant 3rd party mice were grafted on either side of the back of recipient mice. Grafts were checked for viability on a daily base starting from day 7 after skin transplantation for about 3 weeks, and then followed 2x/week and later 1x/week until day 100. A graft was considered rejected, when <10% of the graft remained viable.
Results
CD4 T cell recognition of donor MHC class II is required for CD8 tolerance induction
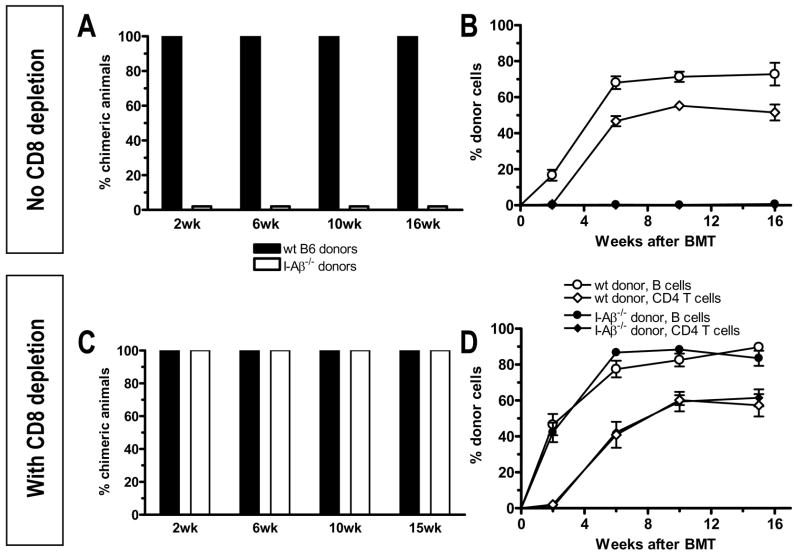
To investigate the role of directly alloreactive CD4 T cells for CD8 tolerance, we transplanted either wild-type B6 or MHC class II-deficient I-Aβ−/− (H-2b) marrow to fully MHC-mismatched B10.S (H-2s) recipients. All animals achieved durable multilineage chimerism following wild-type B6 BMT. In contrast, I-Aβ−/− BM failed to engraft (Figure 1A, B). Depletion of CD8 T cells (Figure 1C, D), however, permitted stable multilineage chimerism in 100% of animals receiving either MHC class II-positive or -negative BM with similar incidence and levels of chimerism in all lineages between the groups. Thus, directly alloreactive recipient CD4 T cells are required to tolerize CD8 T cells in this model.
Figure 1. Requirement for direct CD4 alloreactivity for CD8 tolerance.
(A) B10.S mice received a wild-type B6 or I-Aβ−/− BM graft after conditioning with TBI and anti-CD154. The incidence of mixed chimerism for the B cell lineage at indicated time points is shown. Chimerism was defined as ≥5% donor MHC class I positive cells among B220+ B cells. One representative experiment out of two is shown (n = 7 animals/group per experiment). (B) The percentage of donor chimerism ±SEM over time is shown for B cells and CD4 T cells in peripheral blood of both groups shown in panel A. (C) Mice were treated as described for panel A. In addition, they received one dose of anti-CD8 mAb. CD8 T cells were <0.5% of lymphocytes in white blood cells by 2 weeks after BMT. The incidence of chimerism for the B cell lineage is shown at indicated time points. One representative experiment out of two is shown (n = 6–7 animals/group per experiment). (D) The percentage of donor chimerism ±SEM over time is shown for B cells and CD4 T cells in peripheral blood of the groups shown in panel C. In all animals, B cell chimerism is similar to chimerism of myeloid cell lineages, and CD4 T cell chimerism is similar to CD8 chimerism (unless CD8s were depleted).
Donor BM-derived CD11c+ dendritic cells are not required for induction of stable mixed chimerism
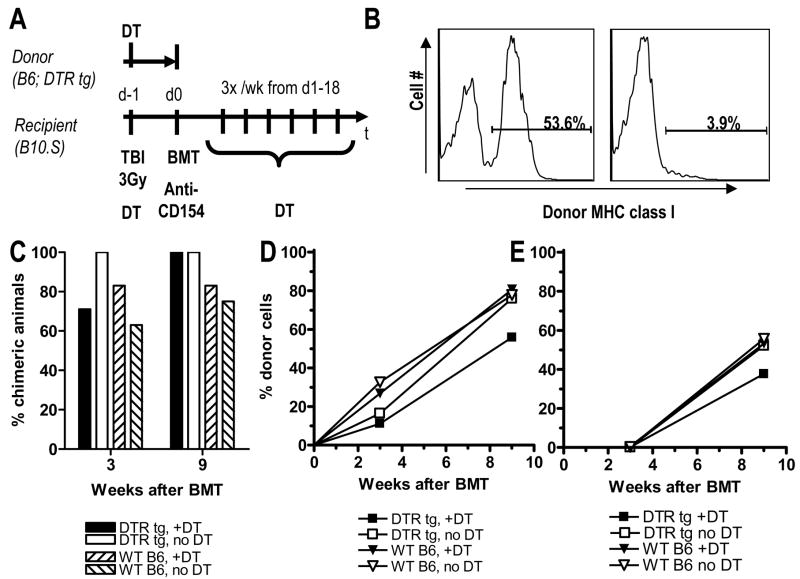
In an attempt to identify the tolerogenic MHC class II+ donor APC, we used diphtheria toxin receptor transgenic (DTR tg) donors expressing primate DTR under the mouse CD11c promoter. Treatment of DTR tg mice with DT depletes 90–95% of CD11c+ DCs from the spleen, lasting for 48–72 hours (14). Figure 2A shows the experimental design. DTR tg or wt B6 donors were either treated or not treated with one dose of DT on day -1. On day 0, BMT with 20x106 BMCs from all 4 groups was performed. B10.S recipients of BM from DT-treated DTR tg or wt B6 donors were also treated with DT, starting on day -1 and then continued 3x/wk until day 18. One day after the last DT injection, one mouse in each group with comparable levels of peripheral chimerism was euthanized for FACS assessment of depletion of donor-derived CD11c+ DCs in collagenase-digested spleen. As shown in Figure 2B, gated CD11c+ splenocytes demonstrated a 93% reduction in donor-derived DCs in the DT-treated compared to the untreated recipient of DTR tg BM.
Figure 2. Donor CD11c+ DCs are not necessary for induction of chimerism.
(A) Experimental design: B10.S mice received conditioning with 3Gy TBI and anti-CD154. In addition, they were or were not treated with diphtheria toxin (DT). On day 0 they received 20x106 BMCs from diphtheria toxin receptor-transgenic (DTR tg) or wild-type B6 mice, which were either treated or not treated with DT on the day before BM harvest. Panel (B) shows the percentage of donor-derived cells among gated CD11c+ splenocytes one day after the last DT injection for two individual mice receiving DTR tg BM either without (left panel) or with DT treatment (right panel). Both of these mice had similar levels of multilineage chimerism (around 15% for B cells and 30% for granulocytes). (C) Incidence of donor chimerism in the B cell lineage, which is representative also for the granulocyte lineage, is shown 3 and 9 weeks after BMT for mice of the 4 groups mentioned above (n = 7–8/group; one of 2 similar experiments is shown). (D, E) Levels of donor chimerism in the 4 groups mentioned above is shown for B cells (D) and CD4 T cells (E).
Figure 2C shows the incidence of multilineage mixed chimerism 3 and 9 weeks after BMT in animals receiving DTR tg BM with or without DT treatment. Control animals receiving wt B6 BM with or without DT treatment are also shown. Although the incidence of chimerism was slightly lower in recipients of wt compared to tg donor bone marrow, no difference was observed between DT-treated and untreated recipients of DTR tg (or wt) bone marrow in the incidence (Figure 2C) or levels (Figure 2D, E) of donor chimerism. Therefore, donor BM-derived CD11c+ DCs are not necessary for induction of stable mixed chimerism in this model.
Donor BM-derived B cells are not required for induction of chimerism and tolerance
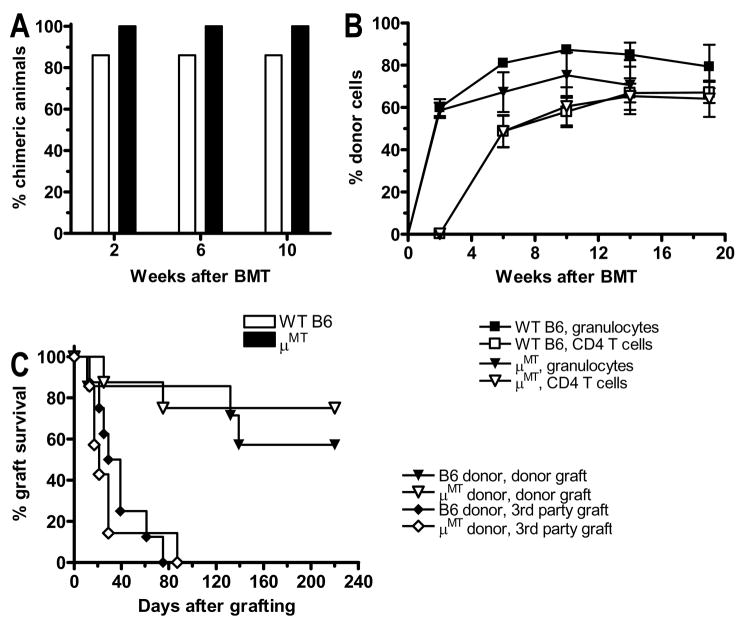
B cells represent the major MHC class II+ cell population in the bone marrow. To determine whether or not B cells are essential for tolerance induction with our regimen, we performed BMT using either wt B6 or B cell-deficient μMT mice as donors in two different fully MHC-mismatched strain combinations, B6→B10.S and B6→B10.A. No difference in the incidence (Figure 3A) or levels (Figure 3B) of donor chimerism was seen when marrow from wild-type versus μMT B6 donors was given to B10.S recipients. These mice were skin-grafted with donor type B6 and 3rd party B10.A skin. Long-term acceptance of donor-type skin grafts was achieved in 60–70% of animals in both groups, whereas 3rd party grafts were promptly rejected, demonstrating donor-specific tolerance (Figure 3C).
Figure 3. Donor B cells are not required for induction of stable chimerism and tolerance.
(A) B10.S mice received conditioning as in Methods followed by transplantation with BMCs from wt B6 or B cell-deficient μMT mice. Incidence of donor chimerism in the granulocyte lineage is shown (n = 7–8/group; one representative experiment of 2 is shown). (B) Levels of chimerism (mean ± SEM) in granulocytes and CD4 T cells are shown for groups in panel A. (C) The groups in panel A were grafted with donor B6 or 3rd party B10.RIII skin 2 weeks after BMT, and graft survival is shown over 100 days.
Donor BM-derived T cells are not required for chimerism or tolerance induction using BMT with anti-CD154
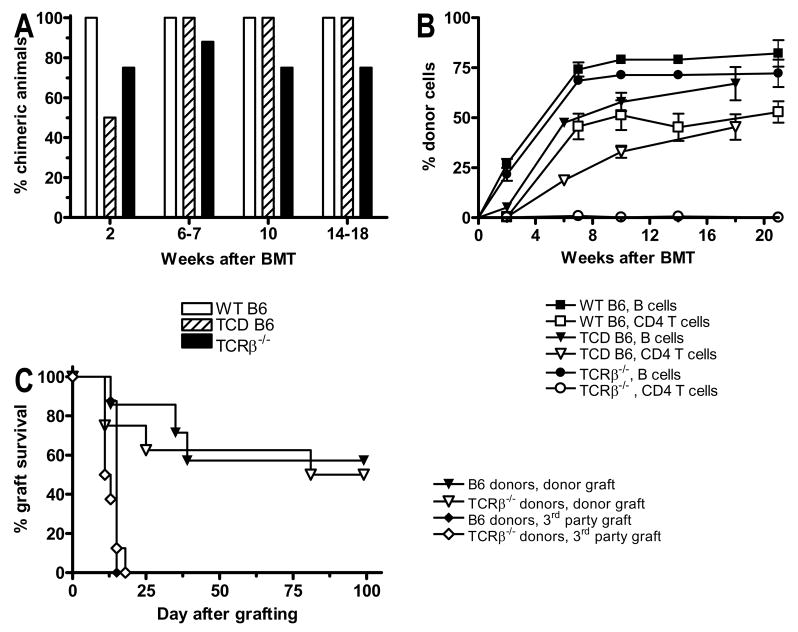
Donor T cells have been shown to promote engraftment of allogeneic model in humans and in a variety of mouse models. We therefore evaluated the role of donor BM-derived T cells for induction of stable mixed chimerism using the fully MHC-mismatched B6 (H-2b)→B10.S (H-2s) strain combination. Recipient mice were conditioned with 3Gy TBI and anti-CD154 before receiving 20x106 BMCs that were either unfractionated or ex vivo depleted of CD4+ and CD8+ T cells. No difference in the incidence of long-term stable chimerism was observed (Figure 4A), although the levels of chimerism were lower in the T cell-depleted group in the first 10 weeks after BMT (Figure 4B). Since new donor T cells are generated in the recipient thymus of chimeric mice within a few weeks and may be of importance for the maintenance of chimerism and tolerance, we next used TCRβ−/− mice, which lack αβT cells, as BM donors in the same strain combination. Long-term stable mixed chimerism was observed in about 80% of animals receiving TCRβ−/− BM and 100% of those receiving wild-type B6 BM (Figure 4A). Levels of B cell and myeloid chimerism were comparable to that in the group receiving wild-type B6 BM (Figure 4B and data not shown). This experiment was repeated in a different strain combination (B6→B10.A) with similar results, although an overall lower incidence of chimerism (60–70%) was seen in both the experimental and the control group (data not shown). To test for donor-specific tolerance, mice receiving either wt B6 or TCRβ−/− donor cells were grafted with tail skin from either wt B6 or 3rd party B10.RIII (H-2r) mice. Third party skin was promptly rejected in both groups, whereas long-term (>100 days) acceptance of donor-derived skin grafts was observed in 60% of BMT recipients, with no difference between those receiving wild-type or TCRβ−/− marrow (Figure 4C). Thus, donor BM-derived T cells are not necessary for induction of long-term stable mixed chimerism and skin graft tolerance in this model.
Figure 4. Donor T cells are not necessary for induction of chimerism and tolerance.
B10.S mice received the conditioning regimen described under Methods followed by allogeneic BMT. (A) Incidence of donor chimerism in the B cell lineage, which is similar to chimerism in the granulocyte lineage (not shown), is shown for mice that were transplanted with either whole BMCs from wt B6 mice (open bars), with wt B6 BMCs depleted ex vivo of CD4 and CD8 T cells (hatched bars), or with whole BMCs from TCRβ−/− mice (closed bars; n = 7–8 for each group). (B) Levels of donor chimerism (mean ± SEM) are shown for the B cell and CD4 T cell lineages in the three groups shown in panel A. (C) The groups transplanted with whole BMCs from wild-type B6 or TCRβ−/− mice were grafted with donor B6 or 3rd party B10.RIII skin 12 weeks after BMT, and graft survival was followed over 100 days (n=7–8/group).
Donor-derived IL-10 is not required for induction of CD8 and CD4 tolerance
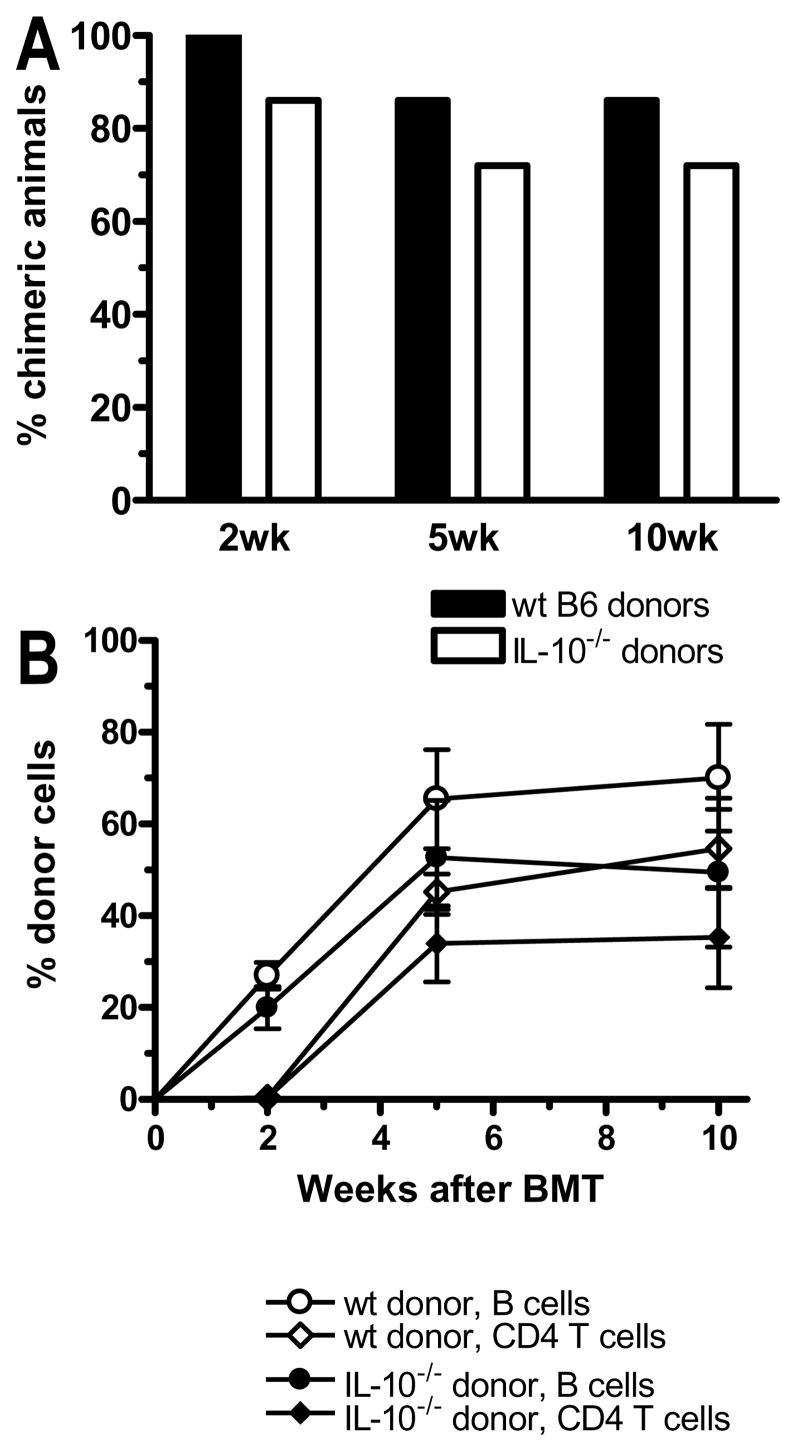
Since the cytokine IL-10 can be produced by multiple cell types, including T cells, B cells and dendritic cells and has been implicated in many tolerance models, we considered the possibility that IL-10 produced by any of these donor cell populations might play a role in tolerance induction in our model. We therefore compared the outcome of allogeneic BMT with our conditioning regimen using either wt or IL-10−/− BM donors. No difference was found between the ability of wt or IL-10−/− BM to induce multilineage chimerism (Figure 5), indicating that donor-derived IL-10 is not required for tolerance induced by allo-BMT and anti-CD154.
Figure 5. Donor-derived IL-10 is not required for induction of chimerism.
B10.S mice received the conditioning regimen described under Methods followed by allogeneic BMT. (A) Incidence of donor chimerism in the B cell lineage, which is similar to chimerism in the granulocyte lineage (not shown), is shown for mice that were transplanted with either T cell-depleted BMCs from wt B6 mice (open bars) or from IL-10 KO mice (closed bars; n = 7–8/group). (B) Levels of donor chimerism (mean ± SEM) are shown for the B cell and CD4 T cell lineages in the two groups shown in panel A.
Early donor-specific CML tolerance is achieved by BMT with 3Gy TBI and anti-CD154
To further analyze the ability of various cell types to induce tolerance in our model, we transplanted fractionated BMC populations. Since donor stem cells are not transplanted to all groups with this approach, long-term chimerism cannot be used as a read-out of tolerance. We therefore used short-term measures of tolerance in these animals, including the level of early (d7) splenic chimerism in the granulocyte and B cell lineage. Mice transplanted with our regimen were compared to a group of mice receiving the same regimen without anti-CD154. Figure 6A shows that chimerism is undetectable by d7 after BMT in the group not receiving anti-CD154, indicating that rejection occurs in the absence of anti-CD154, consistent with our previous results (15). We recently demonstrated in chimeras prepared with our regimen that donor-specific unresponsiveness of cytotoxic T cells (CTL) is induced by 2 weeks after BMT (11), concomitant with deletion of donor-reactive 2C CD8 T cells (10). To evaluate tolerance at an earlier time point, we analyzed CTL responses at day 8 after BMT in the spleens of mice treated with 3Gy TBI and anti-CD154 followed by allogeneic BMT. Unresponsiveness to donor targets with measurable responses to 3rd party antigens was observed (Figure 6B), indicating that donor-specific unresponsiveness was established by day 8. We therefore used three parameters – early chimerism, donor-specific CML responses and deletion of donor-reactive CD8 T cells – as measures of tolerance induced by different marrow fractions. This analysis was performed in two different strain combinations: B6→B10.S and B10.A→B6 (deletion of Ld-reactive 2C cells could only be tested in the latter).
Figure 6. Tolerance can be assessed by early chimerism and cytotoxicity assays 7–8 days after BMT.
(A) B6 mice received either conditioning with TBI and anti-CD154 mAb or with TBI only without anti-CD154. They were then transplanted with BMCs from fully MHC-mismatched B10.A mice. The percentage of donor B cells and granulocytes in the spleen is shown on day 7 after BMT. (B) B6 mice received conditioning with TBI and anti-CD154 and were then transplanted with BMCs from fully MHC-mismatched B10.A mice. Cytotoxic activity of whole splenocytes on day 8 after BMT was measured in a 51Cr release assay against donor B10.A (closed symbols) or 3rd party B10.RIII targets (open symbols) after a 5 day restimulation culture with the respective stimulators (mean ± SEM, 4 mice/group). One representative experiment out of three is shown.
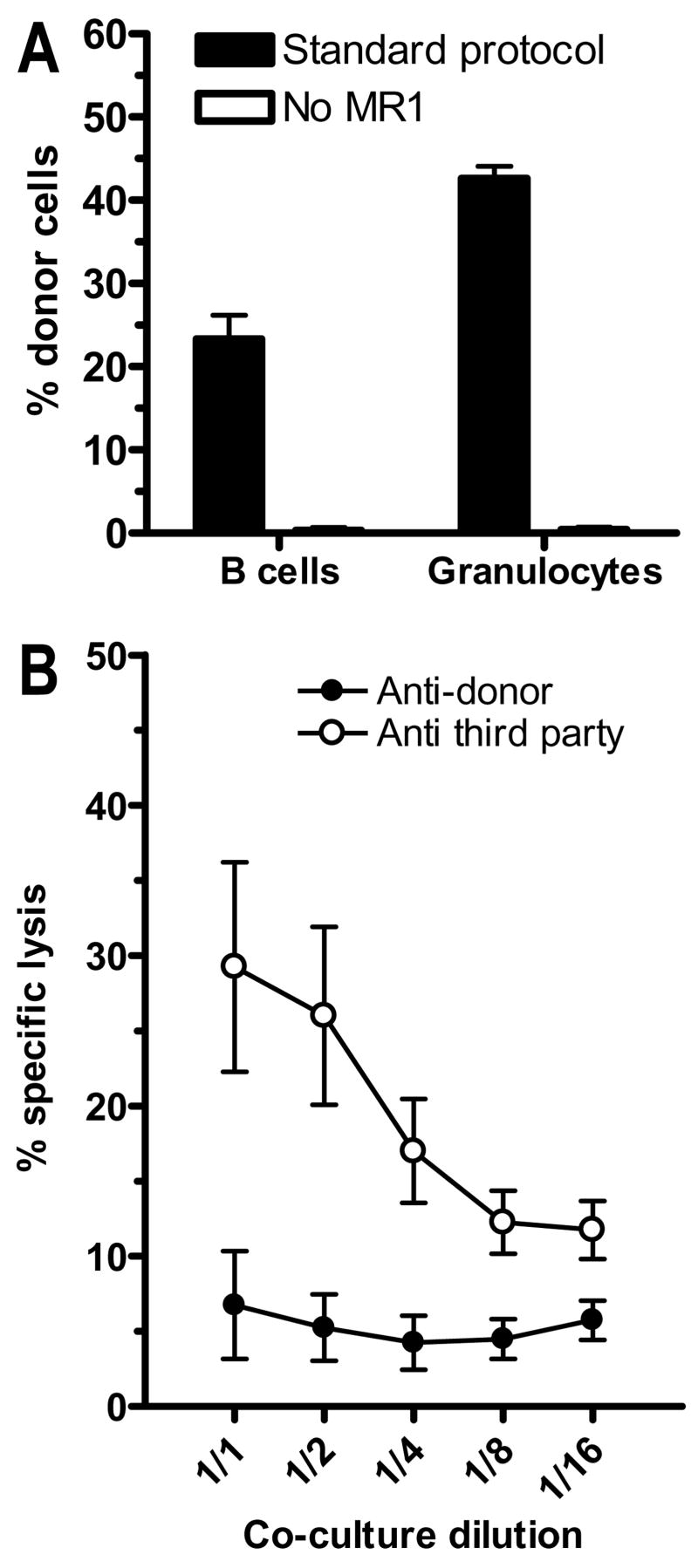
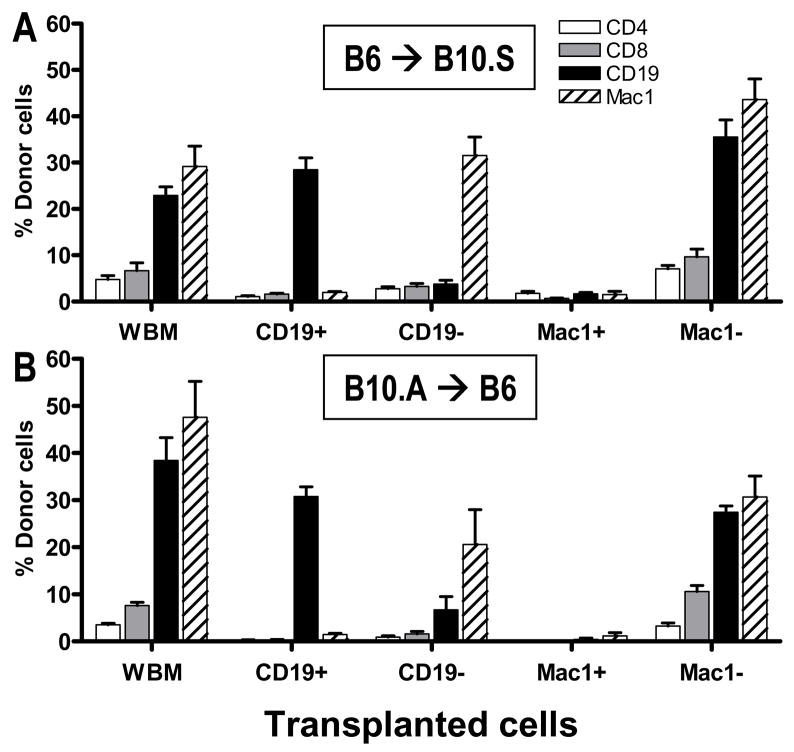
B cells but not CD11b+ cells are sufficient to achieve early chimerism
Mice received 3Gy TBI with anti-CD154 followed by 25x106 total BMCs (WBM), isolated B cells (CD19+), B cell-depleted BMCs (CD19−), isolated CD11b+ cells (Mac1+) or CD11b-depleted BMCs (Mac1−). In both strain combinations, positively isolated CD19+, but not Mac1+ cells led to substantial chimerism on Day 7 after transplant (Figure 7A, B). The Mac1-depleted fraction led to chimerism in every combination. However, the Mac1 depletion was only about 70% efficient, whereas all the other fractions were highly purified. Analysis of mice receiving the CD19-depleted fractions revealed that B cells were not required for early chimerism induction in either strain combination. This result corresponds to the results obtained with μMT donor mice (Figure 3).
Figure 7. Donor B cells but not Mac1+ cells promote early chimerism.
Recipient mice were conditioned as in Methods, then transplanted with 25x106 whole BMCs (WBM), MACS-selected CD19+ or Mac1+ cells or MACS depleted CD19− or Mac1− cells from a fully allogeneic donor strain. Percentage of donor B cells and granulocytes in spleen on day 7 after BMT is shown. Pooled data from 2–3 experiments per strain combination are shown. (A) Recipient mice were 10.S, donor mice were B6 (n=6/group from 2 experiments). (B) Recipient mice were B6 and donor mice were B10.A (n=10–12/group from 3 experiments).
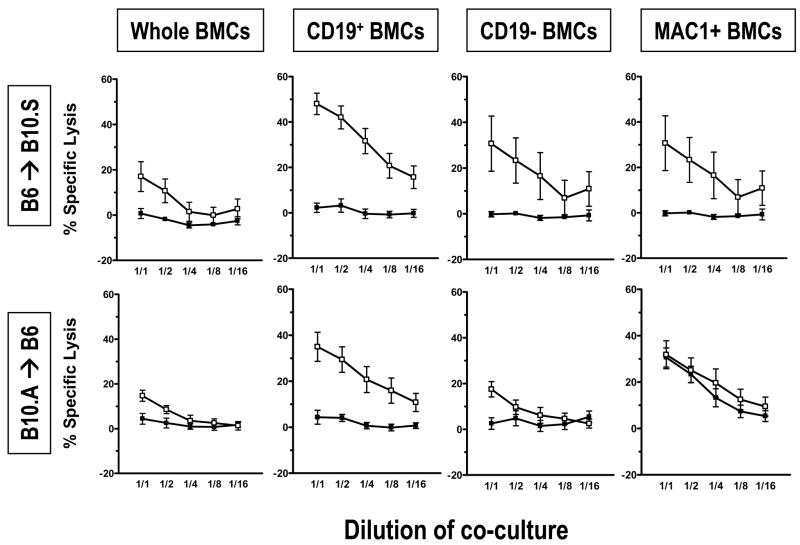
Donor BM-derived B cells induce early donor-specific CTL unresponsiveness
Figure 8 shows the results of Day 7 CML assays performed using spleens from mice transplanted with the marrow cell fractions described above, the chimerism of which is shown in Figure 7. Recipients of Mac1− marrow are not presented because of the impurity of the inoculum. All groups that achieved donor chimerism showed unresponsiveness to the donor in CML assays. In recipients of Mac1+ cells, which did not induce chimerism in either strain combination, anti-donor CTL reactivity could only be detected in the B10.A→B6, but not in B6→B10.S strain combination. In the groups receiving CD19− cells, no anti-donor CTL response could be detected in either strain combination. The groups receiving WBM, CD19− or Mac1− cells (data not shown) showed low responses to 3rd party targets, suggesting the possible existence of a CD19− Mac1− non-specifically suppressive cell population in the donor marrow. Most strikingly, isolated B cells induced donor-specific unresponsiveness in all animals, which retained strong 3rd party responses, indicating that donor BM-derived B cells, although not absolutely required, are sufficient to reliably induce early chimerism and donor-specific CML tolerance.
Figure 8. Donor B cells induce donor-specific unresponsiveness in CML assay seven days after BMT.
Recipient B10.S or B6 mice were conditioned as in Methods, then transplanted with 25x106 whole BMCs (WBM), MACS-selected CD19+ or Mac1+ cells or MACS-depleted CD19− from fully MHC-mismatched B6 or B10.A donor mice as indicated. Cytotoxic activity in whole splenocytes on day 7 after BMT was measured in a 51Cr release assay against donor B6 (closed symbols) or 3rd party B10.RIII targets (open symbols) after a 5 day restimulation culture with the respective stimulators. Each line indicates mean ± SEM of % specific lysis for pooled mice from 2–3 different experiments. Upper row: recipient mice were B10.S, donor mice were B6 (n=6/group from 2 experiments); lower row; recipient mice were B6 and donor mice were B10.A (n=8–12/group from 3 experiments).
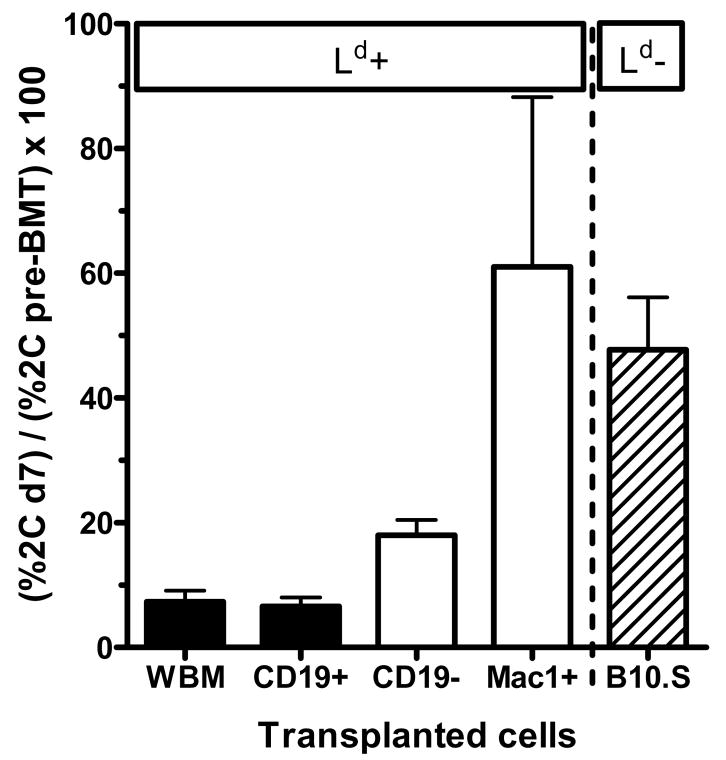
Donor BM-derived B cells induce early deletion of donor-reactive CD8 T cells
We recently described a model system to analyze deletion of donor-reactive CD8 T cells by using 2C/B6 syngeneic chimeric mice (10). B6 mice are irradiated with 3Gy TBI and then transplanted with syngeneic BMCs from TCR-transgenic syngeneic mice expressing the alloreactive 2C TCR on CD8 T cells. This TCR recognizes the Ld molecule with high affinity (12). The resulting 2C/B6 chimeras express the transgenic receptor on 5–30% of their peripheral CD8 T cells, allowing analysis of deletion of a donor-specific CD8 T cell population while retaining a polyclonal response. Eight weeks after syngeneic 2C BMT, these mice were then treated with 3Gy TBI and anti-CD154 followed by BMT from donors either expressing (B10.A) or not expressing (B10.S) the Ld molecule. Our previous studies with this system demonstrated deletion of donor-reactive CD8 T cells in recipients of this protocol as early as d7 post-BMT (10), consistent with results using a different transgenic TCR in a different model (16). We now analyzed the capacity of isolated BM cell fractions to induce deletion of donor-reactive 2C CD8 T cells (Figure 9). Mice that were transplanted with cell fractions containing B cells (WBM, CD19+) showed profound deletion of 2C cells by d7, whereas recipients of fractions not containing B cells showed less (CD19−) or no (Mac1+) 2C deletion compared to the non-specific control (B10.S). Thus, donor BM-derived B cells are able to induce profound, early deletional CD8 T cell tolerance after BMT with anti-CD154.
Figure 9. B cells induce early deletional CD8 T cell tolerance.
Recipient B6 mice expressing a transgenic donor-specific alloreactive TCR on a fraction of recipient CD8 T cells were generated (“2C/B6 chimeras”, for details see Methods section). Eight weeks later, these 2C/B6 chimeras received conditioning with TBI and anti-CD154 and were then transplanted with 25x106 whole BMCs (WBM), MACS-selected CD19+ or Mac1+ cells or MACS-depleted CD19− cells from fully MHC-mismatched ligand-bearing B10.A donor mice (Ld positive; black columns indicating B cell-containing and white columns indicating B cell-depleted grafts) or whole BMCs from non-ligand bearing B10.S donor mice (Ld negative; hatched columns). The percentage of 2C+ cells among splenic CD8 T cells was determined by FACS on day 7 after BMT and then normalized to the percentage of 2C TCR expression among peripheral blood CD8 T cells 1 week before BMT. Mean + SEM are shown for 5 animals/group.
Discussion
We have investigated the role of donor MHC class II expression and individual marrow cell populations in the induction of stable mixed chimerism and tolerance using a protocol consisting of 3 Gy TBI on day -1 and anti-CD154 mAb on day 0 followed by fully MHC-mismatched allogeneic BMT (11). We demonstrate here that MHC class II expression on donor BM-derived APCs is critical for CD8 cell tolerance. Furthermore, we found that a transplant consisting of purified B cells is sufficient to induce early donor-specific CD8 tolerance, as measured by donor chimerism, donor-specific unresponsiveness in CML assays and deletion of donor-reactive CD8 T cells (2C/B6 model) by 7 days after BMT. Analysis of chimerism and tolerance in experiments using B cell-deficient BM (either ex vivo depleted or gene-targeted) suggests that B cells are sufficient, but not required for rapid deletional tolerance of alloreactive peripheral CD8 cells. We also demonstrate that donor BM-derived CD11c+ DCs and donor-derived T cells are not necessary to establish durable mixed chimerism, and that CD11b+ cells are incapable of inducing even initial chimerism or tolerance.
A number of studies have investigated the role of B cells for T cell tolerance induction to self and foreign antigens. Naïve (but not memory) T and B cell tolerance to a soluble protein antigen can be induced with small resting B cells as APCs (17–19). In a cardiac allograft model, donor B cell pretreatment combined with depletion of recipient B cells by anti-IgD monoclonal antibody prolonged graft survival times indefinitely (20). Injection of syngeneic B cells coated with ovalbumin (OVA) was shown to induce OVA-specific deletional CD8 T cell tolerance (21). This was recently confirmed using retrovirally transduced B cells to express the specific antigen, which led to peripheral, but not intrathymic CD8 tolerance (22). Thus, the ability of donor B cells to rapidly induce donor-specific CD8 unresponsiveness in our model is consistent with previous findings. The kinetics of deletion in our 2C/B6 model (10) are also comparable to those in the OVA model. However, in contrast to a recent study in the heart transplant model (23), early tolerance of CD8 cells in BMT recipients was critically dependent on CD4 cell recognition of donor class II MHC molecules, whereas in the heart allograft model donor MHC class II was dispensable. Taken together, the present data suggest that presentation of both class I and class II MHC by donor B cells to recipient CD8 and CD4 cells, respectively, may facilitate the CD4-CD8 interactions required for CD8 tolerance. One study suggested that the type of B cell activation may influence their ability to tolerize CD8 T cells, as B cells activated with LPS, but not those activated with anti-CD40/anti-Ig, were tolerogenic, with surface TGF-β expession and IL-10 production as possible mediators (24). While blockade of CD154-CD40 interactions undoubtedly plays a critical role in rendering donor B cells tolerogenic, the molecular mechanism by which B cells promote CD8 cell tolerance has not been identified in our studies. We tested the role of donor IL-10 by using IL-10 KO donor BM and found no difference in the ability to induce tolerance compared to wild-type donors in our model. In a preliminary experiment, we were also unable to detect a role for TGF-β in tolerance induction (C. Gibbons and M. Sykes, unpublished data).
Dendritic cells, initially described as the most potent inducers of adaptive immune responses, have recently attracted attention as mediators of tolerance to self and foreign antigens (25). “Tolerogenic DCs” have an immature phenotype, with low expression of MHC class II and costimulatory molecules such as CD80 and CD86 (26). These cells efficiently endocytose antigen and can induce T cell anergy or T regulatory cells in vivo, depending on the experimental conditions. However, there is no prior information on the requirement for donor-derived DCs in the context of allogeneic BMT. A recent in vitro study showed that immature allogeneic DCs induced specific T cell tolerance to a subsequent challenge with immunogenic DCs. Furthermore, recipient DCs loaded with donor apoptotic cells were able to “cross-tolerize” the direct alloantigen presentation pathway (27). In some cases DCs may promote T cell tolerance by producing indoleamine-2, 3-dioxygenase (IDO) (28), but this pathway did not appear to be involved in a mixed chimerism model related to the one examined here (29). In the context of allo-BMT, Kaufman et al described a facilitating cell population (FC) in the donor marrow, which is CD8+ CD3+TCR- and promotes engraftment of hematopoietic stem cells without the capacity to induce GVHD (30). The same group later reported induction of T regulatory cells by FCs (31) and found that this population included 65% CD11c+ cells resembling precursor plasmacytoid DCs (32). However, the FC population is a very small fraction of bone marrow whose practical utility may be limited. Our study suggests that with a minimal conditioning regimen for fully mismatched allo-BMT, donor-derived CD11c+ DCs are not necessary for induction of multilineage mixed chimerism. Moreover, since at least some of these cells should be included in the Mac1+ marrow population, our data indicate that they are not effective at inducing early peripheral CD8 cell tolerance. Nevertheless, it is possible that FC may promote the partial 2C deletion and initial chimerism seen in recipients of CD19-negative bone marrow cells.
Macrophages have been implicated in tolerance induction to a foreign protein antigen (33), and peritoneal macrpohages were reported to facilitate engraftment of allogeneic hematopoietic stem cells in rats (34). Monocytes can differentiate into DCs and therefore potentially exert similar functions as described for DCs (35). In vitro studies revealed that blockade of the B7-CD28 costimulatory pathway leads to an alternative type of macrophage activation which mediates suppression of T cell responses (36). Here we show that a BM-derived highly purified CD11b+ (Mac1+) population was not able to induce early chimerism. One simple explanation would be that these cells, which may be short-lived, were not present in sufficient numbers by 7 days after BMT. Alternatively, the Mac1+ bone marrow fraction, which contains myeloid cells at various stages of differentiation, may not contain sufficient mature monocytes to exert tolerogenic effects (37). In addition, strong early anti-donor CML responses were seen in at least one strain combination, suggesting active rejection of these cells. While our data suggest that donor monocytes/macrophages are insufficient to induce early chimerism and tolerance, they do not indicate whether or not this population is necessary, since MACS depletion of CD11b+ cells, which represent about 40% of total BMCs, was only about 70% efficient.
GVHD induced by donor T cells is a major obstacle to the use of BMT to induce donor-specific tolerance in the HLA-matched and especially the HLA-mismatched transplant setting (38). Unfortunately, depleting T cells from the donor graft to avoid GVHD reduces the efficiency of engraftment (39,40). In the context of allograft tolerance, it is therefore important to know whether T cells are an important component of the donor BM for tolerance induction. Furthermore, recent findings suggest that activated T cells may express MHC class II MHC (41), so that T cells could theoretically be the source of donor class II promoting tolerance in our model. However, the results of our studies using either ex vivo T cell depletion or gene-targeted T cell-deficient donor mice demonstrate that donor T cells are not necessary to induce or maintain chimerism or skin graft tolerance with our costimulatory blockade-based regimen. Umemura et al have obtained similar results using CD4−, CD8− and CD4/8 double-deficient mice as BM donors in a protocol involving treatment with anti-lymphocyte serum followed by allo-BMT and rapamycin (42). In contrast, mice receiving sublethal TBI followed by T cell-depleted BMT achieved chimerism that was not accompanied by skin graft tolerance, whereas T cell-replete BMT assured skin graft tolerance (43). Another study showed that central T cell tolerance in lethally irradiated recipients can be achieved by antigen-expressing T cells in the presence of isolated class I MHC disparity (44), but this study did not address the necessity of donor T cells, which have been shown in other models to be dispensible for central tolerance induction (B. Nikolic and M. Sykes, unpublished data). Our results are consistent with those in a mixed chimerism model using busulfan and anti-CD154 conditioning, in which T cell-depleted marrow was effective in inducing tolerance (45). Taken together, these findings indicate that donor T cells are not necessary for induction or maintenance of stable mixed hematopoietic chimerism or central or peripheral tolerance in regimens where the host-vs-graft response is adequately suppressed or tolerized.
Taken together, our study demonstrates a potent effect of donor-derived B cells for induction of early CD4 and CD8 T cell tolerance in a fully allogeneic BMT model using a minimal anti-CD154-based conditioning regimen. Donor B cells were sufficient, but not required in both strain combinations tested to induce peripheral T cell tolerance. In the same model, donor-derived T cells are dispensable. Together with the finding that MHC class II expression on donor BM is critical for CD8 tolerance, the results of this study suggest that MHC class II presented by any hematopoietic cell can play this tolerance-promoting role and that no particular cell type is absolutely required.
One possible explanation for the ability of multiple sources of donor class II MHC to promote tolerance is that exosomes, which can be produced by B cells (46,47) and dendritic cells (48), present MHC class II in a manner that is tolerogenic to T cells. Circulating donor class II-bearing exosomes have been implicated in an oral tolerance model (49), and exogenous donor MHC-bearing exosome administration promoted graft survival in a rat heart transplantation model (50). Indeed, exosome production is induced by B cells upon interactions with CD4 T cells recognizing them (51). However, a role for soluble exosomes would not readily explain the role for CD4 cells in tolerizing CD8 cells in our model. We think that direct killing of donor-reactive CD8 cells by recipient CD4 cells is also an unlikely explanation for this requirement, as our published studies show that the early partial deletion of peripheral donor-reactive CD8 cells is similar in control and CD4-depleted mice, and that it is only after marrow rejection that these cells appear to expand in the CD4-depleted group (10). Thus, we favor a model in which CD4-CD8 interactions involve APC populations that are conditioned by CD4 cells to be tolerogenic for CD8 cells, culminating in CD8 cell deletion. It is possible that pickup and presentation of intact donor MHC molecules by recipient APCs promotes tolerance induction in our model and that donor APC populations are not involved, consistent with the observed requirement for recipient but not donor B7 molecules in achieving tolerance (Kurtz et al, manuscript submitted). Such pickup may occur through the production of membrane exosomes (52). Further studies in progress should help to address these possibilities.
These findings have important implications for an understanding of tolerance mechanisms and for the clinical use of hematopoietic cell transplantation to induce organ allograft tolerance, for which non-toxic regimens are required. Combined transplantation of bone marrow and kidney can induce donor-specific tolerance and avoid long-term immunosuppression in monkeys and humans (5,53). Donor kidneys have survived without immunosuppression for over ten years in monkeys and eight years in patients (6,7,54). It is interesting to note that the monkeys and some of the patients lost initial mixed hematopoietic chimerism over weeks to months, but retained the kidney nonetheless; therefore, only transient donor chimerism seems to be necessary to achieve donor-specific tolerance when kidney transplantation and BMT are performed simultaneously. Thus, in light of our findings it would be interesting to consider a solid organ transplant combined with costimulatory blockade and BMT vigorously depleted of donor T cells, but enriched with a tolerogenic, non-self-renewing cell population such as B cells to minimize the risk of GVHD.
Acknowledgments
We thank Drs. Nicolas Degauque and Emanuel Zorn for critical review of the manuscript, Christopher Borges for assistance with IL-10−/− experiments, Mr. Orlando Moreno for outstanding animal husbandry and technical assistance, and Ms. Kelly Walsh for expert assistance with the manuscript.
Nonstandard abbreviations
- BMT
bone marrow transplant
- BM
bone marrow
- CML
cell-mediated lympholysis
- GVHD
graft-versus-host disease
- TBI
total body irradiation
- DTR tg
diphtheria toxin receptor-transgenic
- BMCs
bone marrow cells
- DT
diphtheria toxin
- DCs
dendritic cells
- CTL
cytotoxic T cell
- FC
facilitating cell population
Footnotes
This work was supported by NIH grant RO1HL49915. Thomas Fehr was supported by a research fellowship of the Swiss Foundation for Medical and Biological Grants (with support of Novartis Switzerland) and a research fellowship of the Walter and Gertrud Siegenthaler Foundation (Medical Faculty, University of Zurich, Switzerland). Fabienne Haspot was supported by a research fellowship of the FRM (Fondation pour la Recherche Medicale, France) and a research fellowship of the American Society of Blood and Marrow Transplantation.
References
- 1.Sayegh MH, Fine NA, Smith JL, Rennke HG, Milford EL, Tilney NL. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann Intern Med. 1991;114:954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- 2.Fehr T, Sykes M. Tolerance induction in clinical transplantation. Transpl Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Sharabi Y, Sachs DH. Mixed chimerism and permanent specific transplantation tolerance induced by a nonlethal preparative regimen. J Exp Med. 1989;169:493–502. doi: 10.1084/jem.169.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, Zhao G, Sykes M. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Cosimi AB, Colvin RB, Powelson J, Eason J, Kozlowski T, Sykes M, Monroy R, Tanaka M, Sachs DH. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation. 1995;59:256–262. [PubMed] [Google Scholar]
- 6.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, Preffer F, Tolkoff-Rubin N, Dey BR, Saidman SL, Kraus A, Bonnefoix T, McAfee S, Power K, Kattleman K, Colvin RB, Sachs DH, Cosimi AB, Sykes M. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, Fishman JA, Dey B, Ko DS, Hertl M, Goes NB, Wong W, Williams WW, Jr, Colvin RB, Sykes M, Sachs DH. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtz J, Shaffer J, Lie A, Anosova N, Benichou G, Sykes M. Mechanisms of early peripheral CD4 T-cell tolerance induction by anti-CD154 monoclonal antibody and allogeneic bone marrow transplantation: evidence for anergy and deletion but not regulatory cells. Blood. 2004;103:4336–4343. doi: 10.1182/blood-2003-08-2642. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz J, Lie A, Griffith M, Eysaman S, Shaffer J, Anosova N, Turka L, Benichou G, Sykes M. Lack of Role for CsA-Sensitive or Fas Pathways in the Tolerization of CD4 T Cells Via BMT and Anti-CD40L. Am J Transplant. 2003;3:804–816. doi: 10.1034/j.1600-6143.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 10.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4(+)CD25(−)T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8(+) T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi Y, Ito H, Kurtz J, Wekerle T, Ho L, Sykes M. Earlier Low-Dose TBI or DST Overcomes CD8+ T-Cell-Mediated Alloresistance to Allogeneic Marrow in Recipients of Anti-CD40L. Am J Transplant. 2004;4:31–40. doi: 10.1046/j.1600-6135.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 12.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 13.Kranz DM, Tonegawa S, Eisen HN. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung S, Unutmaz D, Wong P, Sano G, De los SK, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wekerle T, Sayegh MH, Ito H, Hill J, Chandraker A, Pearson DA, Swenson KG, Zhao G, Sykes M. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- 16.Seung E, Mordes JP, Rossini AA, Greiner DL. Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning. J Clin Invest. 2003;112:795–808. doi: 10.1172/JCI18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eynon EE, Parker DC. Parameters of tolerance induction by antigen targeted to B lymphocytes. J Immunol. 1993;151:2958–2964. [PubMed] [Google Scholar]
- 19.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 20.Niimi M, Hara M, Witzke O, Morris PJ, Wood KJ. Donor resting B cells induce indefinite prolongation of fully allogeneic cardiac grafts when delivered with anti-immunoglobulin-D monoclonal antibody: evidence for tolerogenicity of donor resting B cells in vivo. Transplantation. 1998;66:1786–1792. doi: 10.1097/00007890-199812270-00037. [DOI] [PubMed] [Google Scholar]
- 21.Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188:1977–1983. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner-Klein M, Dresch C, Marconi P, Brocker T. Transcriptional targeting of B cells for induction of peripheral CD8 T cell tolerance. J Immunol. 2007;178:7738–7746. doi: 10.4049/jimmunol.178.12.7738. [DOI] [PubMed] [Google Scholar]
- 23.Kishimoto K, Yuan X, Auchincloss H, Jr, Sharpe AH, Mandelbrot DA, Sayegh MH. Mechanism of action of donor-specific transfusion in inducing tolerance: role of donor MHC molecules, donor co-stimulatory molecules, and indirect antigen presentation. J Am Soc Nephrol. 2004;15:2423–2428. doi: 10.1097/01.ASN.0000137883.20961.2D. [DOI] [PubMed] [Google Scholar]
- 24.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 25.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 26.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 27.Nouri-Shirazi M, Guinet E. Direct and indirect cross-tolerance of alloreactive T cells by dendritic cells retained in the immature stage. Transplantation. 2002;74:1035–1044. doi: 10.1097/00007890-200210150-00024. [DOI] [PubMed] [Google Scholar]
- 28.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff C, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2, 3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 29.Pree I, Bigenzahn S, Fuchs D, Koporc Z, Nierlich P, Winkler C, Brandacher G, Sykes M, Muehlbacher F, Langer F, Wekerle T. CTLA4Ig promotes the induction of hematopoietic chimerism and tolerance independently of Indoleamine-2, 3-dioxygenase. Transplantation. 2007;83:663–667. doi: 10.1097/01.tp.0000255594.23445.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaufman CL, Colson YL, Wren SM, Watkins S, Simmons RL, Ildstad ST. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood. 1994;84:2436–2446. [PubMed] [Google Scholar]
- 31.Colson YL, Christopher K, Glickman J, Taylor KN, Wright R, Perkins DL. Absence of clinical GVHD and the in vivo induction of regulatory T cells after transplantation of facilitating cells. Blood. 2004;104:3829–3835. doi: 10.1182/blood-2004-01-0393. [DOI] [PubMed] [Google Scholar]
- 32.Fugier-Vivier IJ, Rezzoug F, Huang Y, Graul-Layman AJ, Schanie CL, Xu H, Chilton PM, Ildstad ST. Plasmacytoid precursor dendritic cells facilitate allogeneic hematopoietic stem cell engraftment. J Exp Med. 2005;201:373–383. doi: 10.1084/jem.20041399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukic ML, Leskowitz S. Tolerance induction with bovine gamma globulin in mouse radiation chimaeras depends on macrophages. Nature. 1974;252:605–607. doi: 10.1038/252605a0. [DOI] [PubMed] [Google Scholar]
- 34.Fandrich F, Zhou X, Schlemminger M, Lin X, Dresske B. Future strategies for tolerance induction: a comparative study between hematopoietic stem cells and macrophages. Hum Immunol. 2002;63:805–812. doi: 10.1016/s0198-8859(02)00444-5. [DOI] [PubMed] [Google Scholar]
- 35.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, Stanley ER, Randolph GJ, Merad M. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tzachanis D, Berezovskaya A, Nadler LM, Boussiotis VA. Blockade of B7/CD28 in mixed lymphocyte reaction cultures results in the generation of alternatively activated macrophages, which suppress T-cell responses. Blood. 2002;99:1465–1473. doi: 10.1182/blood.v99.4.1465. [DOI] [PubMed] [Google Scholar]
- 37.Lord BI, Woolford LB, Molineux G. Kinetics of neutrophil production in normal and neutropenic animals during the response to filgrastim (r-metHu G-CSF) or filgrastim SD/01 (PEG-r-metHu G-CSF) Clin Cancer Res. 2001;7:2085–2090. [PubMed] [Google Scholar]
- 38.Blazar BR, Murphy WJ. Bone marrow transplantation and approaches to avoid graft-versus-host disease (GVHD) Philos Trans R Soc Lond B Biol Sci. 2005;360:1747–1767. doi: 10.1098/rstb.2005.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Barrett TA, Bluestone JA, Vallera DA. Lethal murine graft-versus-host disease induced by donor gamma/delta expressing T cells with specificity for host nonclassical major histocompatibility complex class Ib antigens. Blood. 1996;87:827–837. [PubMed] [Google Scholar]
- 40.Martin PJ. Donor CD8 cells prevent allogeneic marrow graft rejection in mice: potential implications for marrow transplantation in humans. J Exp Med. 1993;178:703–712. doi: 10.1084/jem.178.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeGuern C. Regulation of T-cell functions by MHC class II self-presentation. Trends Immunol. 2003;24:633–638. doi: 10.1016/j.it.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Umemura A, Monaco AP, Maki T. Donor T cells are not required for induction of allograft tolerance in mice treated with antilymphocyte serum, rapamycin, and donor bone marrow cells. Transplantation. 2000;70:1005–1009. doi: 10.1097/00007890-200010150-00003. [DOI] [PubMed] [Google Scholar]
- 43.Umemura A, Morita H, Li XC, Tahan S, Monaco AP, Maki T. Dissociation of hemopoietic chimerism and allograft tolerance after allogeneic bone marrow transplantation. J Immunol. 2001;167:3043–3048. doi: 10.4049/jimmunol.167.6.3043. [DOI] [PubMed] [Google Scholar]
- 44.Tian C, Bagley J, Forman D, Iacomini J. Induction of central tolerance by mature T cells. J Immunol. 2004;173:7217–7222. doi: 10.4049/jimmunol.173.12.7217. [DOI] [PubMed] [Google Scholar]
- 45.Adams AB, Durham MM, Kean L, Shirasugi N, Ha J, Williams MA, Rees PA, Cheung MC, Mittelstaedt S, Bingaman AW, Archer DR, Pearson TC, Waller EK, Larsen CP. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J Immunol. 2001;167:1103–1111. doi: 10.4049/jimmunol.167.2.1103. [DOI] [PubMed] [Google Scholar]
- 46.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arita S, Baba E, Shibata Y, Niiro H, Shimoda S, Isobe T, Kusaba H, Nakano S, Harada M. B cell activation regulates exosomal HLA production. Eur J Immunol. 2008;38:1423–1434. doi: 10.1002/eji.200737694. [DOI] [PubMed] [Google Scholar]
- 48.Montecalvo A, Shufesky WJ, Stolz DB, Sullivan MG, Wang Z, Divito SJ, Papworth GD, Watkins SC, Robbins PD, Larregina AT, Morelli AE. Exosomes As a Short-Range Mechanism to Spread Alloantigen between Dendritic Cells during T Cell Allorecognition. J Immunol. 2008;180:3081–3090. doi: 10.4049/jimmunol.180.5.3081. [DOI] [PubMed] [Google Scholar]
- 49.Ostman S, Taube M, Telemo E. Tolerosome-induced oral tolerance is MHC dependent. Immunology. 2005;116:464–476. doi: 10.1111/j.1365-2567.2005.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC. Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transplant. 2006;6:1541–1550. doi: 10.1111/j.1600-6143.2006.01344.x. [DOI] [PubMed] [Google Scholar]
- 51.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Segura E, Guerin C, Hogg N, Amigorena S, Thery C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179:1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 53.Buhler LH, Spitzer TR, Sykes M, Sachs DH, Delmonico FL, Tolkoff-Rubin N, Saidman SL, Sackstein R, McAfee S, Dey B, Colby C, Cosimi AB. Induction of kidney allograft tolerance after transient lymphohematopoietic chimerism in patients with multiple myeloma and end-stage renal disease. Transplantation. 2002;74:1405–1409. doi: 10.1097/00007890-200211270-00011. [DOI] [PubMed] [Google Scholar]
- 54.Kawai T, Poncelet A, Sachs DH, Mauiyyedi S, Boskovic S, Wee SL, Ko DS, Bartholomew A, Kimikawa M, Hong HZ, Abrahamian G, Colvin RB, Cosimi AB. Long-term outcome and alloantibody production in a non-myeloablative regimen for induction of renal allograft tolerance. Transplantation. 1999;68:1767–1775. doi: 10.1097/00007890-199912150-00022. [DOI] [PubMed] [Google Scholar]