Abstract
Background
Along with efficacy, a microbicide’s acceptability will be integral to its impact on the pandemic. Understanding Product Characteristics that users find most acceptable and determining who will use which type of product are key to optimizing use effectiveness.
Objectives
To evaluate psychometrically the Important Microbicide Characteristics (IMC) instrument and examine its relationship to willingness to use microbicides.
Results
Exploratory and confirmatory factor analyses revealed 2 IMC subscales (Cronbach’s coefficient α: Product Characteristics subscale (α = 0.84) and Protective Properties subscale (α = 0.89)). Significant differences on Product Characteristics subscale scores were found for history of douching (P = 0.002) and employment status (P = 0.001). Whether a woman used a method to prevent pregnancy or sexually transmitted disease (STD) in the last 3 months (P < 0.001) and whether she used a condom during the last vaginal sex episode (P < 0.001) were significantly related to her rating of the importance of microbicides being contraceptive. Product Characteristics (r = 0.21) and Protective Properties (r = 0.27) subscale scores and whether a microbicide had contraceptive properties (r = 0.24) were all significantly associated (P < 0.001) with willingness to use microbicides.
Conclusions
Formulation and use characteristics and product function(s) affect willingness to use microbicides and should continue to be addressed in product development. The IMC instrument serves as a template for future studies of candidate microbicides.
Keywords: HIV/sexually transmitted disease protection, instrument development, microbicide acceptability, pregnancy prevention, Product Characteristics, willingness to use microbicides
Given the rise in the proportion of women becoming infected with HIV annually1,2 and their limited ability to require condom use by their sexual partners,3–6 the need for HIV prevention options for women is profound. Topical microbicides, products that could significantly reduce HIV transmission through use that is potentially initiated and controlled by women, are now undergoing clinical efficacy trials. In preparation for US Food and Drug Administration (FDA) approval, behavioral and social scientists have been exploring psychosocial factors hypothesized to be associated with microbicide acceptability and use. It is critical for successful uptake, and thus the impact on rates of HIV transmission, to understand who would use microbicides, why, and under what circumstances. This necessitates understanding the personal, relational, and contextual factors affecting microbicide uptake and use.
Microbicide acceptability research has been conducted hypothetically,7,8 with demonstration or use of surrogate products (ie, over-the-counter spermicides, moisturizers, placebo gels)3,8–17 and in clinical trials.18–23 Such research has elucidated factors associated with acceptability, including product formulation (eg, gel), vehicle attributes (eg, color), timing of insertion in relation to sexual episode, product function (ie, disease and/or pregnancy prevention), side effects (eg, burning), leakage or messiness, dose volume, application process, and how these interplay with sexual pleasure and covert use. Studies show that formulation preferences vary24 and are influenced by such characteristics as, for example, appearance, messiness, affect on sexual pleasure,10,12,15,25–28 cultural norms and practices,13,29–31 and geographic location or climate.15 Ultimately, data suggest that a number of microbicide formulations are needed to maximize global uptake and use.10,32
Many women have expressed preferences for colorless and odorless microbicides20,25,33–36 that do not require waiting between insertion and intercourse12,15,20 and are effective for hours,7,8,20,28 thus minimizing untoward effects on sexual pleasure and the possibility of covert use.37 Microbicides that increase or have a neutral effect on sexual pleasure 10,12,13,15,20,21,28,38 may prove more acceptable than those that do not. The potential for additional lubrication to enhance pleasure and reduce condom irritation and breakage and fears that excessive lubrication might raise concerns among sexual partners about infection, improper hygiene, or infidelity have been noted.12 Studies20,21 have concluded that covert use is feasible and necessary in some circumstances but that successful covert use likely hinges on many factors, including the product, relationship, and context.17,37,39 Identifying and effectively assessing formulation properties that have an impact on use will contribute to our ability to provide users with acceptable products.
Although many candidate microbicides have been found to be safe and well tolerated,23,40–46 women have expressed concern over potential side effects18 and application processes.47 Women may not attempt, or may discontinue, product use or reduce the amount of product applied if the microbicide causes irritation, excessive leakage, or wetness.10,15 In addition, there are mixed opinions regarding product function,8,20 with preferences largely dependent on women’s desires or needs to have children or their use of other contraceptive alternatives.
The Important Microbicide Characteristics (IMC) instrument was developed to ascertain those characteristics of formulation and use that could be rated by degree of importance among potential microbicide users. Although much formative work has been done with respect to these constructs,8,14 there are no psychometrically validated measures that have been used to predict whether particular characteristics have an impact on willingness to use microbicides. We hypothesized that willingness to use a microbicide would vary as a function of the importance that a woman placed on specific Product Characteristics in the context of her own risk.
METHODS
Design and Procedure
A cross-sectional questionnaire was administered using audio computer-assisted self-interview (A-CASI). A detailed description of questionnaire administration procedures is reported elsewhere.48 The definition of a microbicide was presented to participants at screening, at consent, and at key points in the questionnaire when required to respond adequately to subsequent questions (definition provided in Box 1). All procedures were approved by appropriate institutional review boards.
Phoenix Project Questionnaire Content Areas in Order of Administration*
Demographics
Vaginal product use
Preferences for specific microbicide Product Characteristics
Contextual self-efficacy for insertion of vaginal products and negotiation of condom and product use
Perceived norms of friends and significant others regarding participant’s sexual behavior
Sexual partner identification and labeling
Recent STD and HIV risk perception
Relationship context
Relationship quality
Risk communication with partner
Perceived partner norms regarding sexual behavior
Sexual behavior with partner
Perception of STD and HIV risk with partner
Context of participant’s last sexual episode with partner
Willingness to Use Microbicides scale score (by partner, specified event)
Importance of microbicide characteristics (by partner, specified event)
Contextual self-efficacy for microbicide use (by partner, specified event)
Sexual health, STD history, and HIV testing history
Drug use history
Sociotropic cognitions
STD knowledge67
HIV knowledge68
*Before asking participants to respond to items requiring an understanding of microbicides, the following definition was provided (ie, microbicides were defined for participants during screening, at consent, and at key points in the assessment process): microbicides will likely be clear gels, with no color, no taste, and no scent. Women will be able to put microbicides into their vagina before having sex to help reduce the chance of getting HIV. Some microbicides will also reduce the chance of getting other STDs, and still others may prevent pregnancy.
Participant Recruitment and Enrollment
Women from 4 states in the northeastern United States participated. Eligibility criteria included the following: 18 to 55 years old; black/African American (black), Latina/Hispanic (Latina), or white; vaginal sex with at least 1 male sex partner in the last 12 months; HIV-negative or of unknown HIV status; and not pregnant. Nonproportional quota sampling was used to recruit subgroups sufficient to address the overall research questions. Details of recruitment procedures, including the effectiveness of nonproportional quota sampling procedures, can be found elsewhere.49 Participants were reimbursed for time spent to complete the questionnaire and for travel or child/elder care expenses, where appropriate.
Measures
The questionnaire consisted of an eligibility screener and items assessing a full compliment of sexual risk variables and contexts (see Box 1). Items were generated by means of analyses of qualitative acceptability studies conducted among low- and high-risk women using surrogate products in formative studies or actual experimental products in early clinical trials. Items and framing of items were further developed and ordered after cognitive interviews and expert reviews of draft items. After a partner identification section, 1 partner was randomly selected and the remainder of the questionnaire asked the participant to focus on the selected partner to set a specific context for subsequent questions. This resulted in a questionnaire that capitalized on contextual flow, leading logically from one item or construct to the next, with transitions as necessary. The final item order for the IMC instrument is denoted by item numbers in Table 1.
TABLE 1.
Component and Factor Pattern Item Loadings in Subsample 1 (n = 260)
| Product Characteristics |
Protective Properties |
|||
|---|---|---|---|---|
| Variable | PCA | MLFA | PCA | MLFA |
| 1. How important would it have been to you that the microbicide would not have been noticeable to you? | 0.68 | 0.58 | ||
| 2. How important would it have been to you that the microbicide would not have been noticeable to [partner]? | 0.69 | 0.60 | ||
| 3. How important would it have been to you that the microbicide would have made sex feel better? | 0.49 | 0.43 | ||
| 4. How important would it have been to you that the microbicide would have been a [formulation preference]? | 0.69 | 0.59 | ||
| 5. How important would it have been to you that the microbicide would have been clear, that is, have no color? | 0.81 | 0.83 | ||
| 6. How important would it have been to you that the microbicide would have had no scent or odor? | 0.74 | 0.77 | ||
| 7. How important would it have been to you that the microbicide would have had no side effects? | 0.45 | 0.47 | ||
| 12/13. How important would it have been to you that the microbicide could have been inserted with an applicator/without an applicator (participant’s preference)? | 0.63 | 0.53 | ||
| 14. How important would it have been to you that the microbicidec would have allowed you to have sex immediately after applying the product? | 0.60 | 0.52 | ||
| 9. How important would it have been to you that the microbicidewould have protected you against STDs other than HIV? | 0.88 | 0.83 | ||
| 10. How important would it have been to you that the microbicide would have protected you against HIV? | 0.88 | 0.85 | ||
| 11. How important would it have been to you that the microbicide would have protected [partner] from disease too? | 0.85 | 0.76 | ||
PCA indicates Principal Components Analysis; MLFA, Maximum Likelihood Factor Analysis.
Sample Characteristics
The final sample (N = 531) included 166 (31.3%) Latinas, 193 (36.3%) black women, and 172 (32.4%) white women. One hundred fifty-five women (29.2%) reported 1 male vaginal sex partner in the last 12 months, whereas 376 (70.8%) reported 2 or more. The mean age of the sample was 33.8 years (SD = 9.6). Black women were significantly older (Mean [M] = 35.4 years, SD = 9.5) than white (M = 33.0 years, SD = 10.5) or Latina (M = 32.8 years, SD = 8.4) women [F(2,528) = 4.235; P = 0.015]. Twelve percent were married. More than half reported a high school education or less (54%), were not employed (55%), and reported an annual household income of less than $15,000 (52%).
MEASURE DEVELOPMENT
Overview
Importance of Microbicide Characteristics (IMC) Instrument
An investigation of the underlying constructs within the 14-item IMC instrument was undertaken. The complete sample (N = 531) was divided randomly into 2 subsamples. Subsample 1 (n = 265) was used for exploratory dimensional analysis (EDA) and initial reliability analyses. Subsample 2 (n = 266) was used for confirmatory factor analysis (CFA) and further instrument refinement. A final CFA was done using the complete sample to obtain more stable estimates of item loadings, model fit, and reliability.
Exploratory Dimensional Analyses
Procedure and Results
EDAs were conducted on 260 complete sets of responses to the 14 items. A principal components analysis (PCA) and a maximum likelihood factor analysis (MLFA) were conducted to examine a 2-dimensional solution based on a convergence of results of the Scree Test,50 the minimum average partial (MAP) procedure,51 and the parallel analysis (PA) procedure,52,53 which helped to determine the underlying dimensional structure. The varimax-rotated patterns of item loadings for the MLFA and PCA indicated similar results and supported a 2-dimensional solution. A final PCA and MLFA were conducted after removal of 2 items with low (<0.4) loadings, and these respective patterns are presented in Table 1. This 2-factor solution accounted for 54% of the variance, with 9 items representing a “Product Characteristics” factor and 3 items representing a “Protective Properties” factor. The reliability of the 9-item Product Characteristics subscale, as measured by the Cronbach coefficient (α) statistic,54 was 0.85, and the reliability of the 3-item Protective Properties subscale was 0.87.
Confirmatory Factor Analyses
Procedure and Results
A CFA using the LISREL 8.7 structural equation modeling (SEM) program55 was conducted on 263 complete sets of subsample 2 participant responses to the 12 retained items from the EDA. The initial model examined a specified 2-dimensional solution with 9 item parameters freed to load on a Product Characteristics factor, 3 item parameters freed to load on a Protective Properties factor, a correlation allowed to be freely estimated between the latent constructs, and each item error variance allowed to be freely estimated. This model was then revised to eliminate 1 additional item that had a low (<0.4) item loading in the CFA on the Product Characteristics factor and to allow 2 item error covariances to be freely estimated. Although the overall revised model had a significant minimum fit function χ2 statistic [χ2(41) = 79.1, P < 0.001], several alternative fit indices indicated good fit. These included the Comparative Fit Index56 (CFI = 0.98), the Non-Normed or Tucker-Lewis Index57 (TLI = 0.97), and the standardized root mean square residual58 (SRMR = 0.048). In general, because values range above 0.95 for the CFI and TLI, in combination with SRMR values of <0.06, model fit is typically considered very good.59 Reliability (α) for the 2 scales in this subsample was also high (0.84 in the 8-item Product Characteristics subscale and 0.91 in the 3-item Protective Properties subscale).
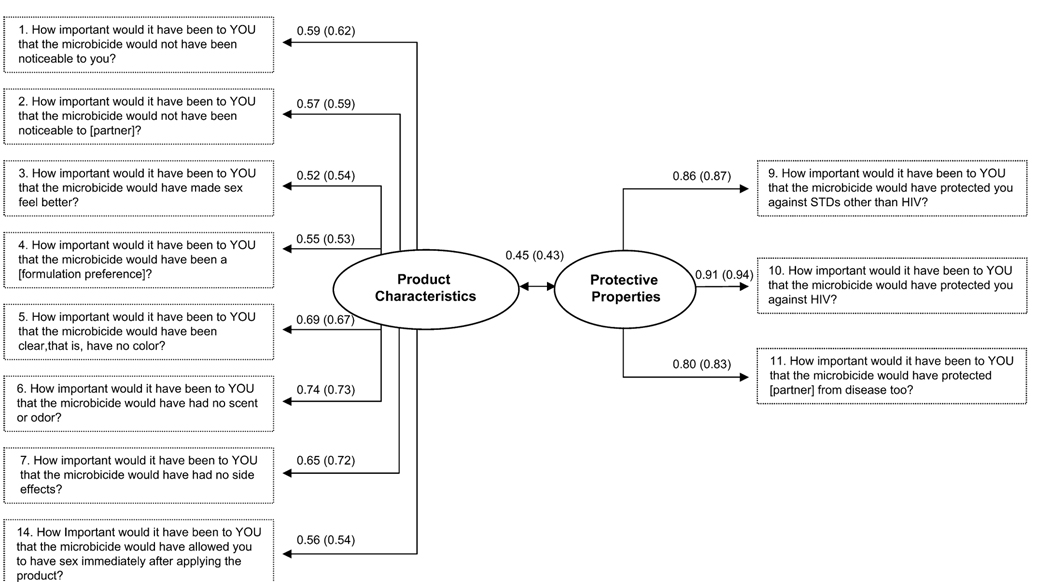
A final CFA examined the revised model using the responses of the complete sample (N = 523). The overall model based on the minimum fit function x2 statistic was significant [χ2(41) = 134.7; P < 0.001], but the alternative indices indicated very good model fit (CFI = 0.98, TLI = 0.97, SRMR = 0.044). Reliability within the whole sample was high in the 8-item Product Characteristics subscale (α = 0.84) and in the 3-item Protective Properties subscale (α = 0.89). Figure 1 presents for comparison the item loadings and correlation between the latent constructs (0.45) from the complete sample and those from subsample 2.
FIGURE 1.
Two-factor model of IMC instrument with item loadings and the correlation between factors for the CFA on the complete sample (N = 523) and CFA coefficient values for subsample 2 (n = 263) in parentheses.
Subgroup Reliability Analyses
Reliability analyses were also conducted within each racial/ethnic group on the 2 subscales, and internal consistency was found to be generally high. For Latinas (n = 165), the reliability was 0.87 for the Product Characteristics subscale and 0.85 for the Protective Properties subscale; for African Americans (n = 188), the reliability was 0.84 for the Product Characteristics subscale and 0.91 for the Protective Properties subscale; and for whites, the reliability was 0.80 for the Product Characteristics subscale and 0.89 for the Protective Properties subscale.
RESULTS
Validity Analyses
Overview
Concurrent validity analyses were conducted on the 2 subscales. Demographic and theoretically relevant variables were examined for their relationship to the 8-item Product Characteristics subscale and the 3-item Protective Properties subscale. Variables such as sociodemographics,60 substance use,61 history of sexually transmitted diseases (STDs),62–64 incarceration history,65 race,66 and douching67 have all been associated with HIV risk. Variables analyzed in the validity analyses were chosen based on these established or hypothesized associations with HIV risk. Decisions as to what variables to include in each analysis were made as a function of their relevance to the subscale constructs being captured. For instance, with respect to the Product Characteristics subscale, variables were chosen that investigators hypothesized to be related to the particular vehicle-associated characteristics of the product. For example, a history of use of other vaginal products was postulated to affect current microbicide formulation preferences. Likewise, variables such as HIV testing history were not included, because formulation characteristics are likely unaffected by an individual’s testing history. With respect to the Protective Properties subscale, variables were chosen that investigators hypothesized to be related to the efficacy of the product as illustrated by the items of that subscale. A complete list of variables examined during validity analyses is presented in Table 2.
TABLE 2.
Variables Examined in Concurrent Validity Analyses for the Product Characteristics Subscale, the Protective Properties Subscale, and the Importance for Pregnancy Protection Item
| Variable | Product Characteristics Subscale | Protective Properties Subscale | Pregnancy Protection Item |
|---|---|---|---|
| Willingness to Use Microbicides* | † | † | † |
| Employment status | † | ns | ns |
| Education | ‡ | ns | ns |
| Household income | ‡ | ns | ns |
| Age | ns | ns | ns |
| Race/ethnicity | ns | ns | ns |
| Ever incarcerated | ns | ns | ns |
| No. sexual partners in last 12 months | ns | ns | ns |
| History of sexual lubricant use | ns | — | ns |
| History of vaginal medication use | ns | — | ns |
| No. times moved in the last year | ns | — | ns |
| HIV testing history | — | ns | ns |
| History of STDs | — | ns | ns |
| Frequency of condom use | ns | ‡ | ‡ |
| History of douching | † | — | ns |
| History of spermicide use | ‡ | ns | ns |
| Condom use during last vaginal sex | ns | ns | † |
| Under influence of drug other than alcohol last vaginal sex | — | ns | ‡ |
| Pressured/forced to have sex last time | ns | ‡ | ns |
| Traded sex last time | ‡ | ‡ | ns |
| Length of relationship with sexual partner | ‡ | ns | ns |
| Used method to prevent pregnancy or STD in last 3 months | — | ns | † |
| Partner type (main, casual, other) | ns | ns | ‡ |
| Medical reason prevented pregnancy | — | ns | ‡ |
| Participant: concurrent sexual partnerships | ns | ns | ns |
| Perception of partner’s behaviors that put her at risk | ns | ns | ns |
| Perception of risk of contracting HIV from partner | — | ns | ns |
| Perception of risk of contracting STD from partner | — | ns | ns |
| Wanted to have sex last time with partner | ns | ns | ns |
| Participant under influence of alcohol last vaginal sex | — | ns | ns |
| Partner under influence of alcohol or other drug last vaginal sex | — | ns | ns |
Willingness to Use Microbicide scale score (see note in Table 3 for further details).
Significant after Bonferroni adjustment.
Nominally significant.
ns indicates nonsignificant; —, not used in analysis.
Each subscale item used a 5-point Likert scale ranging from 1 (not at all important) to 5 (extremely important). The Product Characteristics subscale (M = 30.3, SD = 6.7) had a potential score range of 8 to 40, whereas the Protective Properties subscale (M = 13.7, SD = 2.4) had a potential score range of 3 to 15. Participant scores were represented across the full range on both scales.
Concurrent Validity
Results
Of the 22 variables examined with respect to the Product Characteristics subscale, 14 were not statistically significant (P > 0.05), 5 were nominally significant (P < 0.05) but not significant after applying a Bonferroni adjustment (P ≤ 0.0022), and 3 were significant (P ≤ 0.0022): history of douching (Welch/Brown-Forsythe robust test of equality of means [F(1, 273) = 9.92; P = 0.002]), employment status (Welch/Brown-Forsythe robust test of equality of means [F(1, 510) = 11.27; P = 0.001]), and willingness to use microbicides (Pearson correlation coefficient [r(483) = 0.21; P < 0.001]). Means, SDs, effect sizes, and any post hoc test differences are presented in Table 3A.
TABLE 3.
Means, SDs, Effect Sizes, and Post Hoc Differences for Bonferroni-Adjusted Significant Differences for Product Characteristics, Protective Properties, and Importance for Pregnancy Protection
| A. PC subscale: significant after Bonferroni adjustment | |||||
|---|---|---|---|---|---|
| Variable | N | Mean | Mean | P | Ω2 |
| History of douching | |||||
| No history | 132 | 31.7 | 5.7 | 0.002 | 0.013 |
| One or more times | 391 | 29.8 | 7.0 | ||
| Employment status | |||||
| Not employed | 280 | 29.4 | 7.0 | 0.001 | 0.020 |
| Full- or part-time | 233 | 31.4 | 6.2 | ||
| Willingness to Use Microbicides*(correlation with PC subscale) | |||||
| 485 | r = 0.21 | <0.001 | (r2 = 0.042) | ||
| B. PP subscale: significant after Bonferroni adjustment | |||||
| Willingness to Use Microbicides*(correlation with PP subscale) | |||||
| 485 | r = 0.27 | <0.001 | (r2 = 0.072) | ||
| C. Importance that product protects against pregnancy: significant after Bonferroni adjustment | |||||
| Method to prevent pregnancy or STD in past 3 months | |||||
| No | 217 | 3.6 | 1.5 | <0.001 | 0.033 |
| Yes | 302 | 4.1 | 1.2 | ||
| Condom use during last vaginal sex | |||||
| No | 282 | 3.7 | 1.5 | <0.001 | 0.037 |
| Yes | 215 | 4.2 | 1.2 | ||
| Willingness to Use Microbicides*(correlation with importance that product protects against pregnancy) | |||||
| 485 | r = 0.24 | <0.001 | (r2 = 0.060) | ||
Ω2 is a population-adjusted measure of systematic variance accounted for.
r2 is a commonly used measure of variance used with the Pearson correlation statistic.
PC indicates Product Characteristic; PP, Protective Property.
From Morrow KM, Fava JL, Rosen RK, et al. Willingness to use microbicides varies by race/ethnicity, experience with prevention products, and partner type. Health Psychol. (In press).
Of the 27 variables examined with respect to the Protective Properties subscale, 23 were not statistically significant (P > 0.05), 3 were nominally significant (P < 0.05) but not significant after applying a Bonferroni adjustment (P ≤ 0.0018), and 1 was significant (P ≤ 0.0018): willingness to use microbicides (Pearson correlation coefficient [r(483) = 0.27; P < 0.001]). The effect size is presented in Table 3B.
Importance of Contraceptive Function
One other theoretically important dependent variable was examined: the importance of microbicides protecting against pregnancy. This variable was removed as a potential subscale item based on the previously described psychometric analyses, but because of its theoretic importance, it was analyzed using the same 523 participants utilized in the previous psychometric and validity analyses. It had a mean of 3.9 (SD = 1.4) and a median value of 4 (range from 1 [not at all important] to 5 [extremely important]). Of the 31 variables examined with respect to this item, 24 were not statistically significant (P > 0.05), 4 were nominally significant (P < 0.05) but not significant after applying a Bonferroni adjustment (P ≤ 0.0016), and 3 were significant (P ≤ 0.0016): used method to prevent pregnancy or STD in last 3 months (Welch/Brown-Forsythe robust test of equality of means [F(1, 405) = 17.60; P < 0.001]), condom use during last vaginal sex episode (Welch/Brown-Forsythe robust test of equality of means [F(1, 494) = 21.14; P < 0.001]), and willingness to use microbicides (Pearson correlation coefficient [r(485) = 0.24; P < 0.001]). Means, SDs, and effect sizes are presented in Table 3C.
DISCUSSION
Current microbicide candidate Product Characteristics, its ability to protect the user (or her partner) from disease, and its contraceptive ability were all significantly associated with a woman’s willingness to use microbicides. While Product Characteristics are only a single aspect of acceptability influencing willingness to use microbicides, they are an important aspect. These properties as well as use parameters and product function affect whether a microbicide is tried and likely used correctly and consistently by those who need it.
Results suggest that, in this US-based sample, Product Characteristics rated as important vary as a function of history of douching and employment status. In addition, several factors, including socioeconomic factors, were nominally significant (see Table 2). Women who reported lower levels of socioeconomic indices (ie, low income/underemployed, lower education level) appear less concerned about Product Characteristics than higher socioeconomic status participants. This finding has support in marketing research. Studies have found that those in lower income groups rated sensory appeal (ie, smell, appearance, texture, taste) as less important in food choice than those with higher incomes68 and that families with lower incomes were more sensitive to price and less loyal to specific product brands than those with higher incomes.69 Race/ethnicity was not significantly associated with vehicle-related characteristics. This leads one to consider whether race/ethnicity (in US populations) or access to resources is better considered in targeting potential microbicide users. These data suggest that access to resources may have a limiting effect on microbicide use or, minimally, that those with limited resources would be less discriminating of specific characteristics as long as the product was effective.
Analyses show that women who have a history of douching rate the presented Product Characteristics as less important than those without a history of douching. It is unclear why a woman with a history of douching would find the characteristics represented in the subscale less important unless she would see douching as a mechanism for cleansing her body of any untoward characteristic. If this is the case, education regarding the hazards of douching with respect to microbicide use and HIV transmission is important, especially if a specific microbicide would need to reside in the vagina for some time after coitus. This relationship should be explored in greater detail in future microbicide acceptability research because it may be better understood through examination of a multivariate relationship between product function and participant characteristics.
Not surprisingly, in terms of effect size, whether a product protects a woman (or her partner) against HIV and other STDs is most strongly associated with her willingness to use the product. Women who had used some method to prevent pregnancy or STDs in the last 3 months and women who used condoms at their last sexual episode rated a microbicide’s ability to prevent pregnancy higher in importance than those who did not. It may be that women currently using contraception would prefer to use microbicides for disease and pregnancy prevention. Whether as an alternative to their usual strategies or in addition to them remains an empirical question.
While reliability and concurrent validity analyses suggest that the IMC instrument can be a useful tool for microbicide research, these results represent early efforts to measure and understand a woman’s decision-making process for using reproductive health products. Future research should allow for further refinement of the instrument and generalizability studies. In addition, head-to-head studies of Product Characteristics should be conducted (eg, conjoint analyses) to discern relative importance and preference for certain formulations, devices, use parameters, and efficacy profiles that exist at that point in time. With a more in-depth understanding of microbicide preferences and needs, future generations of microbicidal products can be developed that suit those needs. Additionally, behavioral interventions can then be created to help women decide which microbicide may best suit her sexual context and how to use that product correctly and consistently in her own life.
Admittedly, each new candidate microbicide is unique in its properties, use parameters, and functions. Consequently, it is crucial to measure product-specific dimensions and users’ perceptions of and preferences for each new formulation or device. The current study presents an instrument format and conceptual base for measuring microbicide preferences specific to formulation and function. The Product Characteristics and Protective Properties subscales can be tailored to each new candidate microbicide, and thus used as a template for future scales measuring the importance of formulation/device, application process, and Protective Properties. For example, with respect to a cervical barrier plus microbicide gel combination product, item 4 could be changed to: “How important would it have been to you that the microbicide would have been inserted with a cervical barrier like a diaphragm?” Additionally, as daily use products, coitally independent products, and oral formulations progress in the microbicide development pipeline, items measuring the acceptability of these characteristics would need to be added. Instruments could then be validated using standard psychometric analyses, as done here, to confirm factor structure, reliability, and other instrument characteristics. A parallel example is the measurement of “decisional balance” for health behaviors. Decisional balance refers to the measurement of perceived pros and cons of adopting, modifying, or stopping a particular behavior (eg, STD and pregnancy prevention,70,71 adherence to antiretroviral therapy, 72 others73). These measures share the same format and conceptual basis, yet the questions are tailored to the specific health behavior being studied. Similarly, the IMC instrument could provide the format and conceptual basis for each new microbicide product, with items tailored to product-specific characteristics.
While microbicide use will be contextualized by relationship, sexual norms, socioeconomic status, and other domains, responding to any measure within a larger assessment instrument is also, by necessity, contextualized by all the questions that precede it. It is important for future research to capitalize on the relational and sexual contexts that likely shape responses. When the IMC is tailored to a specific new product, items are modified or new items are created, or the current version is used in new cultures and contexts, sociocultural norms with respect to sexuality, natalism, and gender equity, at a minimum, should be considered. The 3 items not retained in the current analyses may hold greater significance in other contexts and should be retained until psychometric analyses can be conducted to ascertain their importance to new and different samples of potential microbicide users. In particular, item 8 (“How important would it have been to you that the microbicide would have protected you against pregnancy?”) may hold significant weight among women who have limited access to contraception or in pronatal environments. Likewise, a product’s duration of effectiveness and the type of application process (the 2 other items not retained) may affect overall product confidence, comfort, and self-efficacy with product use, and thus may have more or less relevance for certain populations.
While a strength of the current study is that the sample was derived from several urban northeastern US sites, results may not generalize to women in more rural areas or internationally. This sample contains women from lower socioeconomic strata and with limited formal education. It is imperative for researchers in other sociogeographic arenas to use the instrument with a clear understanding that language and culture need to be accounted for and the instrument reevaluated. Further, it is important to consider procedural issues so that needs for privacy and ability to negotiate methods such as A-CASI are considered.
ACKNOWLEDGMENTS
The authors thank the following people for their contributions: Hilda Castillo, Allison Cohn, Michelle Gomez, Alyssa Israel, Luz Lopez, Angela Martinez, Mayra Morales, C. Teal Pedlow, and Andronike Tsamas, research staff; Liz Salomon and Larry Shulman, site coordinators; and Susan Cu-Uvin, Kenneth H. Mayer, and Patricia Symonds, coinvestigators. They also thank the women who participated in the study and all the community-based organizations that collaborated to facilitate recruitment efforts. Aspects of qualitative item development and further details of scale psychometric analyses can be obtained from K. M. Morrow.
Funded by National Institutes of Mental Health grant R01MH64455.
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2004. vol. 16. Atlanta, GA: US Department of Heath and Human Services, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 2.Joint United Nations Program on HIV/AIDS. [Accessed August 30, 2006];Report on the Global AIDS Epidemic. Available at: http://www.unaids.org/en/HIV_data/2006GlobalReport/default.asp.
- 3.Beksinska ME, Rees VH, McIntyre JA, et al. Acceptability of the female condom in different groups of women in South Africa—a multicentred study to inform the national female condom introductory strategy. S Afr Med J. 2001;91:672–678. [PubMed] [Google Scholar]
- 4.Smith LA. Partner influence on noncondom use: gender and ethnic differences. J Sex Res. 2003;40:346–350. doi: 10.1080/00224490209552200. [DOI] [PubMed] [Google Scholar]
- 5.Wingood GM, DiClemente RJ. The influence of psychosocial factors, alcohol, drug use on African-American women’s high-risk sexual behavior. Am J Prev Med. 1998;15:54–59. doi: 10.1016/s0749-3797(98)00027-0. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Geneva, Switzerland: World Health Organization; The Female Condom: A review. 1997
- 7.Darroch JE, Frost JJ. Women’s interest in vaginal microbicides. Fam Plann Perspect. 1999;31:16–23. [PubMed] [Google Scholar]
- 8.Holt BY, Morwitz VG, Ngo L, et al. Microbicide preference among young women in California. J Womens Health. 2006;15:281–294. doi: 10.1089/jwh.2006.15.281. [DOI] [PubMed] [Google Scholar]
- 9.Francis-Chizororo M, Natshalaga NR. The female condom: acceptability and perception among rural women in Zimbabwe. Afr J Reprod Health. 2003;7:101–116. [PubMed] [Google Scholar]
- 10.Hammett TM, Mason TH, Joanis CL, et al. Acceptability of formulations and application methods for vaginal microbicides among drug-involved women: results of product trials in three cities. Sex Transm Dis. 2000;27:119–126. doi: 10.1097/00007435-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Macaluso M, Demand M, Artz L, et al. Female condom use among women at high risk of sexually transmitted disease. Fam Plann Perspect. 2000;32:138–144. [PubMed] [Google Scholar]
- 12.Mason TH, Foster SE, Finlinson HA, et al. Perspectives related to the potential use of vaginal microbicides among drug-involved women: focus groups in three cities in the United States and Puerto Rico. AIDS Behav. 2003;7:339–351. doi: 10.1023/b:aibe.0000004726.61630.96. [DOI] [PubMed] [Google Scholar]
- 13.Pool R, Whitworth JA, Green G, et al. An acceptability study of female-controlled methods of protection against HIV and STDs in south-western Uganda. Int J STD AIDS. 2000;11:162–167. doi: 10.1258/0956462001915606. [DOI] [PubMed] [Google Scholar]
- 14.Weeks MR, Mosack KE, Abbott M, et al. Microbicide acceptability among high-risk urban U.S. women: experiences and perceptions of sexually transmitted HIV prevention. Sex Transm Dis. 2004;31:682–690. doi: 10.1097/01.olq.0000143113.04524.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coggins C, Elias CJ, Atisook R, et al. Women’s preferences regarding the formulation of over-the-counter vaginal spermicides. AIDS. 1998;12:1389–1391. doi: 10.1097/00002030-199811000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Coetzee N, Blanchard K, Ellertson C, et al. Acceptability and feasibility of Micralax applicators and of methyl cellulose gel placebo for large-scale clinical trials of vaginal microbicides. AIDS. 2001;15:1837–1842. doi: 10.1097/00002030-200109280-00013. [DOI] [PubMed] [Google Scholar]
- 17.Koo HP, Woodsong C, Dalberth BT, et al. Context of acceptability of topical microbicides: sexual relationships. J Soc Issues. 2005;61:67–93. doi: 10.1111/j.0022-4537.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bentley ME, Fullem AM, Tolley EE, et al. Acceptability of a microbicide among women and their partners in a 4-country phase I trial. Am J Public Health. 2004;94:1159–1164. doi: 10.2105/ajph.94.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentley ME, Morrow KM, Fullem A, et al. Acceptability of a novel vaginal microbicide during a safety trial among low-risk women. Fam Plann Perspect. 2000;32:184–188. [PubMed] [Google Scholar]
- 20.Morrow KM, Rosen R, Richter L, et al. The acceptability of an investigational vaginal microbicide, PRO 2000 Gel, among women in a phase I clinical trial. J Womens Health. 2003;12:655–666. doi: 10.1089/154099903322404302. [DOI] [PubMed] [Google Scholar]
- 21.Rosen R, Morrow K, Carballo-Diéguez A, et al. The Acceptability of a Novel Vaginal Microbicide, PMPA Gel, Among Female Users: HPTN 050. Presented at the Microbicides 2004 Conference; March, 2004; London, England. [Google Scholar]
- 22.Vandebosch A, Goetghebeur E, Ramjee G, et al. Acceptability of COL-1492, a vaginal gel, among sex workers in one Asian and three African cities. Sex Transm Infect. 2004;80:241–243. doi: 10.1136/sti.2003.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sadr WM, Mayer KH, Maslankowski L, et al. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. AIDS. 2006;20:1109–1116. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- 24.Harrison PF. Acceptability research: outcomes and future directions. The Microbicide Quarterly. 2006;4:10–12. [Google Scholar]
- 25.Hardy E, Jimenez AL, de Padua KS, et al. Women’s preferences for vaginal antimicrobial contraceptives. III. Choice of a formulation, applicator, and packaging. Contraception. 1998;58:245–249. doi: 10.1016/s0010-7824(98)00104-8. [DOI] [PubMed] [Google Scholar]
- 26.Hira SK, Spruyt AB, Feldblum PJ, et al. Spermicide acceptability among patients at a sexually transmitted disease clinic in Zambia. Am J Public Health. 1995;85:1098–1103. doi: 10.2105/ajph.85.8_pt_1.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raymond EG, Chen PL, Condon S, et al. Acceptability of five nonoxynol-9 spermicides. Contraception. 2005;71:438–442. doi: 10.1016/j.contraception.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammett TM, Norton GD, Mason TH, et al. Drug-involved women as potential users of vaginal microbicides for HIV and STD prevention: a three-city survey. J Womens Health Gend Based Med. 2000;9:1071–1080. doi: 10.1089/152460900445983. [DOI] [PubMed] [Google Scholar]
- 29.Mwanza B, Siziya S, Chisembele M, et al. Dry sex and the acceptability of microbicides gel [abstract PC50]. Presented at: Microbicides 2006; Cape Town. 2006. [Google Scholar]
- 30.Mwanza B, Siziya S, Chisembele M, et al. Vaginal washing: a threat in evaluating microbicide effectiveness [abstract PC51]. Presented at: Microbicides 2006; Cape Town. 2006. [Google Scholar]
- 31.Ray S, Gumbo N, Mbizvo M. Local voices: what some Harare men say about preparation for sex. Reprod Health Matters. 1996;4:34–45. [Google Scholar]
- 32.Severy LJ, Newcomer S. Critical issues in contraceptive and STI acceptability research. J Soc Issues. 2005;61:45–65. [Google Scholar]
- 33.Ezechi O, Onwujekwe D, Odunukwe N, et al. Acceptability of vaginal microbicide among Nigerian serodiscordant couples with female negative partner [abstract PC13]. Presented at: Microbicides 2006; Cape Town. 2006. [Google Scholar]
- 34.Morrow K, Srikrishnan AK, Rosen R, et al. Acceptability of vaginal microbicide use in Chennai, India: a qualitative study among low- and high-risk men [abstract PC45]. Presented at: Microbicides 2006; Cape Town. 2006. [Google Scholar]
- 35.Oladele D, Onwuatuelo I, Adeiga A, et al. Acceptability of Savvy (C31G) gel in phase III randomized clinical trial in Lagos Nigeria [abstract PC57]. Presented at: Microbicides 2006; Cape Town. 2006. [Google Scholar]
- 36.Simkhada P, Simkhada B, Bhatta P. Opinions, preferences and barriers for the use vaginal microbicides by female sex workers (FSWs) in Nepal. Presented at: Microbicides 2006; Cape Town. 2006. [Google Scholar]
- 37.Woodsong C. Covert use of topical microbicides: implications for acceptability and use. Int Fam Plan Perspect. 2004;30:94–98. doi: 10.1363/3009404. [DOI] [PubMed] [Google Scholar]
- 38.Severy LJ, Spieler J. New methods of family planning: implications for intimate behavior. J Sex Res. 2000;37:258–265. [Google Scholar]
- 39.Biddlecom AE, Fapohunda BM. Covert contraceptive use: prevalence, motivations, and consequences. Stud Fam Plann. 1998;29:360–372. [PubMed] [Google Scholar]
- 40.Mauck CK, Weiner DH, Ballagh SA, et al. Single and multiple exposure tolerance study of polystyrene sulfonate gel: a phase I safety and colposcopy study. Contraception. 2004;70:77–83. doi: 10.1016/j.contraception.2004.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer KH, Karim SA, Kelly C, et al. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. AIDS. 2003;17:321–329. doi: 10.1097/00002030-200302140-00005. [DOI] [PubMed] [Google Scholar]
- 42.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20:543–551. doi: 10.1097/01.aids.0000210608.70762.c3. [DOI] [PubMed] [Google Scholar]
- 43.Coggins C, Blanchard K, Alvarez F, et al. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex Transm Infect. 2000;76:480–483. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias CJ, Coggins C, Alvarez F, et al. Colpscopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception. 1997;56:387–389. doi: 10.1016/s0010-7824(97)00176-5. [DOI] [PubMed] [Google Scholar]
- 45.Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. AIDS. 2000;14:85–88. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz JL, Mauck C, Lai JJ, et al. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind phase I safety study. Contraception. 2006;74:133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Vail JG, Cohen JA, Kelly KL. Improving topical microbicide applicators for use in resource-poor settings. Am J Public Health. 2004;94:1089–1092. doi: 10.2105/ajph.94.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrow KM, Fava JL, Rosen RK, et al. Willingness to use vaginal microbicides varies by race/ethnicity and partner type. Health Psychol. doi: 10.1037/0278-6133.26.6.777. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrow KM, Vargas S, Rosen RK, et al. The utility of non-proportional quota sampling for recruiting at-risk women for microbicide research. AIDS Behav. doi: 10.1007/s10461-007-9213-z. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cattell RB. The Scree Test for the number of factors. Multivariate Behav Res. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- 51.Velicer WF. Determining the number of components from the matrix of partial correlations. Psychometrika. 1976;41:321–327. [Google Scholar]
- 52.Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- 53.O’Connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer’s MAP test. Behav Res Methods Instrum Comput. 2000;32:396–402. doi: 10.3758/bf03200807. [DOI] [PubMed] [Google Scholar]
- 54.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 55.LISREL 8.7 forWindows [computer program] Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- 56.Bentler PM. Comparative fit indexes in structural equation models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 57.Tucker LR, Lewis C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika. 1973;38:1–10. [Google Scholar]
- 58.Bentler PM. EQS: A Structural Equations Program. Los Angeles, CA: BMDP Statistical Software; 1989. [Google Scholar]
- 59.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 60.Zierler S, Krieger N. Reframing women’s risk: social inequalities and HIV infection. Annu Rev Public Health. 1997;18:401–436. doi: 10.1146/annurev.publhealth.18.1.401. [DOI] [PubMed] [Google Scholar]
- 61.Sikkema KJ, Heckman TG, Kelly JA, et al. HIV risk behaviors among women living in low-income, inner-city housing developments. Am J Public Health. 1996;86:1123–1128. doi: 10.2105/ajph.86.8_pt_1.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimley DM, Annang L, Lewis I, et al. Sexually transmitted infections among urban shelter clients. Sex Transm Dis. 2006;33:666–669. doi: 10.1097/01.olq.0000223285.18331.4d. [DOI] [PubMed] [Google Scholar]
- 63.Chan DJ. Factors affecting sexual transmission of HIV-1: current evidence and implications for prevention. Current HIV Research. 2005;3:223–241. doi: 10.2174/1570162054368075. [DOI] [PubMed] [Google Scholar]
- 64.Risbud A. Human immunodeficiency virus (HIV) and sexually transmitted diseases (STDs) Indian J Med Res. 2005;121:369–376. [PubMed] [Google Scholar]
- 65.Bond L, Semaan S. At risk for HIV infection: incarcerated women in a county jail in Philadelphia. Women Health. 1996;24:27–45. doi: 10.1300/j013v24n04_02. [DOI] [PubMed] [Google Scholar]
- 66.Hallfors DD, Iritani BJ, Miller WC, et al. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97:125–132. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Annang L, Grimley DM, Hook EW., 3rd Vaginal douche practices among black women at risk: exploring douching prevalence, reasons for douching, and sexually transmitted disease infection. Sex Transm Dis. 2006;33:215–219. doi: 10.1097/01.olq.0000205046.11916.c5. [DOI] [PubMed] [Google Scholar]
- 68.Steptoe A, Pollard TM, Wardle J. Development of a measure of the motives underlying the selection of food: the food choice questionnaire. Appetite. 1995;25:267–284. doi: 10.1006/appe.1995.0061. [DOI] [PubMed] [Google Scholar]
- 69.Andrews RL, Currim IS. Identifying segments with identical choice behaviors across product categories: an intercategory logit mixture model. Int J Res Mark. 2002;19:65–79. [Google Scholar]
- 70.Galavotti C, Cabral RJ, Lansky A, et al. Validation of measures of condom and other contraceptive use among women at high risk for HIV infection and unintended pregnancy. Health Psychol. 1995;14:570–578. doi: 10.1037//0278-6133.14.6.570. [DOI] [PubMed] [Google Scholar]
- 71.Grimley DM, Riley GE, Bellis JM, et al. Assessing the stages of change and decision-making for contraceptive use for the prevention of pregnancy, sexually transmitted diseases, and acquired immunodeficiency syndrome. Health Educ Q. 1993;20:455–470. doi: 10.1177/109019819302000407. [DOI] [PubMed] [Google Scholar]
- 72.Highstein GR, Willey C, Mundy LM. Development of stage of readiness and decisional balance instruments: tools to enhance clinical decision-making for adherence to antiretroviral therapy. AIDS Behav. 2006;10:563–573. doi: 10.1007/s10461-005-9043-9. [DOI] [PubMed] [Google Scholar]
- 73.Prochaska JO, Velicer WF, Rossi JS, et al. Stages of change and decisional balance for 12 problem behaviors. Health Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]