Abstract
We have developed an experimental recombinant vesicular stomatitis virus (VSV) vectored plague vaccine expressing a secreted form of Yersinia pestis LcrV protein from the first position of the VSV genome. This vector, given intramuscularly in a single dose, induced high-level antibody titers to LcrV and gave 90–100% protection against pneumonic plague challenge in mice. This single-dose protection was significantly better than that generated by VSV expressing the non–secreted LcrV protein. Increased protection correlated with increased anti-LcrV antibody and a bias toward IgG2a and away from IgG1 isotypes. We also found that depletion of CD4+ cells, but not CD8+ cells, at the time of challenge resulted in reduced vaccine protection, indicating a role for cellular immunity in protection.
Keywords: Yersinia pestis, Vesicular stomatitis virus, CD4+ cells
1. Introduction
Yersinia pestis, a gram-negative bacillus that primarily causes disease of small rodents, can occasionally also infect humans via infected rodent or flea bites, thereby causing bubonic plague [1]. Untreated bubonic plague can progress to a highly contagious pneumonic form of the disease that is associated with high mortality. Humans can also contract pneumonic plague by inhalation of aerosolized infectious droplets following direct contact with an infected individual, thus allowing for the rapid spread of the disease [1, 2]. With antibiotic treatments being only marginally effective [1], the rapidly progressing and often-fatal nature of pneumonic plague poses a danger to public health worldwide. In addition, the possible intentional use of aerosolized Y. pestis or its antibiotic-resistant forms [3, 4] as bioterrorism agents looms as a threat [5].
Vaccination against plague is one approach being pursued to deal with impending infection. However, a safe and effective pneumonic plague vaccine for human is not yet available in the United States [6, 7]. Current vaccine development strategies focus on a few Y. pestis antigens [7], two major candidates being the outer capsule protein (F1) and the low calcium response protein V (LcrV), which induce good protective immune response against challenge with the pathogen [8, 9]. While vaccines based on F1 alone fail to protect against F1− virulent strains [10], LcrV based sub-unit vaccines confer protection against both F1+ and F1− Y. pestis strains [10–18]. LcrV is a 37 kDa protein essential for virulence and critical for production and translocation of the Yersinia outer proteins (Yops) into the eukaryotic cell cytosol [19, 20]. Although the mechanism of protection elicited by LcrV-based vaccines is poorly understood, a number of studies have shown that antibodies to LcrV, generated by either active or passive immunization, can protect from the disease in mouse models [21–26]. Recently, induction of LcrV-specific broad T-cell immunity has also been reported [27, 28], but its role in protection remains to be elucidated.
We had previously reported the development of an experimental pneumonic plague vaccine based on an attenuated recombinant vesicular stomatitis virus (rVSV) vector expressing Yersinia pestis LcrV protein [24]. VSV is a negative-strand RNA virus encoding five structural proteins - nucleocapsid (N), phosphoprotein (P), matrix (M), glycoprotein (G) and RNA dependent RNA polymerase (L). The virus is a natural pathogen of livestock, and human infection is rare and typically asymptomatic [29]. VSV recombinants, that are attenuated for pathogenesis in mice compared to naturally occurring VSV strains [30], can be generated from plasmid DNA [31, 32] and also cause no disease symptoms in non-human primates when given by intranasal (IN), intramuscular (IM) or oral routes [33–35]. Furthermore, VSV can accommodate large insertions of foreign genes whose expression can be controlled based on the site of gene insertion in the VSV genome [36, 37]. Such recombinants induce potent humoral and cellular immune responses in a variety of animal models [29, 38, 39]. Numerous studies have shown that recombinant VSV vectors expressing appropriate foreign antigens are highly effective experimental vaccines [24, 30, 35, 40–50].
In our earlier study, we showed that rVSV vector expressing the Yersinia pestis lcrV gene conferred high-level and long-term protection against intranasal plague challenge in mice, after a single booster immunization [24]. A previous study using a multiple-dose DNA vaccine found that the secreted form of LcrV induced improved antibody responses and better protection of mice from challenge [26]. In the present study, we have incorporated a gene encoding a secreted form of LcrV in the VSV vector (VSV-ssLcrV) and tested its efficacy as a single-dose vaccine against lethal plague challenge. The vector elicits robust antibody responses with increasing immunization dose in mice and confers complete protection against pulmonary Y. pestis challenge. The presence of vaccine induced CD4+ cells at the time of challenge is important for generating complete protection.
2. Materials and methods
2.1. Construction of plasmids and virus recovery
The plasmid pVSV-ssLcrV1 used to derive a VSV recombinant expressing a secreted form of low calcium response protein V (LcrV) of Yersinia pestis was generated as follows. The ssLcrV coding region, containing a signal sequence (from the human plasminogen activator protein) upstream of the LcrV open reading frame (ORF), was amplified by PCR using the pBS-LcrV plasmid [24] as a template. The signal sequence was added upstream of the LcrV ORF in the forward primer. The PCR was performed using Vent DNA polymerase (New England Biolabs), forward primer 5′-GATCGATC GTCGACAACATGGATGCAATGAA GAGAGGGCTCTGCTGTGTGCTGCTGCTG TGTGGAGCAGTCTTCGT TTCGATTAGAGCCTACGAACAAAACCC -3′ [sequence encoding signal (underlined) followed by 2nd codon of LcrV] and reverse primer 5′-CGA TCCCCCCGGGCTAGCTCATTTACCAGACGTGTCATCTA GCAG -3′. The forward and the reverse primers respectively introduced Sal I and Nhe I restriction enzyme sites (in bold) upstream and downstream of the ssLcrV coding sequence. The PCR product was digested with Sal I and Nhe I, purified and ligated to Xho I-Nhe I digested pVSV1XN [51] to generate pVSV-ssLcrV1. Recombinant VSV expressing secreted LcrV from the first position in the VSV genome was recovered from these plasmids as described previously [31]. Briefly, Baby Hamster Kidney (BHK-21) cells were grown to 70% confluency and infected with vTF7-3 [52], a vaccinia virus recombinant expressing T7 RNA polymerase, at a multiplicity of infection (MOI) of 10 for 1 hr followed by transfection with pVSV-ssLcrV1 along with the support plasmids pBS-N, pBS-P and pBS-L. At 48 hrs post transfection, cell supernatants were passaged onto fresh BHK-21 cells. Medium containing the virus was collected after 48 hrs. Virus stocks were prepared from individual plaques grown in BHK-21 cells and were stored at − 70°C
2.2. Indirect Immunofluorescence
BHK-21 cells seeded on glass cover slips were infected with VSV-ssLcrV1, VSV-LcrV1 or recombinant wild-type (rwt)-VSV. At 3 hrs post infection, cells were washed twice with phosphate buffered saline (PBS) and fixed with 3% Para formaldehyde at room temperature. Following this, cells were washed with PBS containing 10mM glycine (PBS-glycine) and permeabilized with 1% Triton-x-100 in PBS-glycine. Cells were then incubated with an equal mixture of 1:200 diluted anti-LcrV monoclonal antibodies (MAbs) - Va13 and Va48 (Santa Cruz Biotechnology, CA), followed by incubation with goat anti-mouse Alexa Flour 594 IgG (Molecular probes, Eugene, OR) diluted 1:500. The cells were then washed twice with PBS-glycine, mounted on slides and imaged using a Nikon Eclipse 80i fluorescence microscope equipped with a Nikon Plan Apochromat 60X oil objective and a Photometrics CoolSnap camera.
2.3. Metabolic labeling and SDS-PAGE of cells infected with recombinant viruses
BHK-21 cells were infected with recombinant viruses at an MOI of 100. After 4 hrs, cells were washed with methionine-free Dulbecco’s modified Eagle’s medium (DMEM) followed by labeling with 100 μCi of [35S]-methionine for 2 hrs. Cell supernatants were then collected and infected cells were washed with PBS and lysed with detergent lysis buffer (1% nonidet P-40, 0.4% deoxycholate, 50mM Tris-HCl, pH8.0, 62.5 mM EDTA) on ice for 5 min to prepare lysates. Both lysates and supernatants were clarified at 16,000 g for 2 min at 4°C and then analyzed by 10% SDS-PAGE. Protein bands were visualized by autoradiography.
2.4. Vaccination of mice
Six to eight week-old Balb/C female mice were obtained from either Charles River Laboratories or Taconic Laboratories and then rested for a minimum of 1 week before inoculation. Animals were housed under BSL-2 conditions in microisolator cages. Viruses were diluted in serum free DMEM. Mice were vaccinated intramuscularly (IM) into the left hind leg muscle with doses of virus as indicated in the text in a total volume of 50μL. Immunizations performed at Yale University, and immunizations and challenges carried out at the Public Health Research Institute (PHRI), Newark, NJ, were approved by the respective Institutional Animal Care and Use Committees.
2.5. ELISA for total serum antibody to LcrV
Serum samples were heat inactivated at 56°C for 30 min. An enzyme linked immunosorbent assay (ELISA) to determine the total serum antibody specific to LcrV was performed as described by Palin et al. [24], with minor modifications. Briefly, 100 ng of purified GST-LcrV protein [from Northeast Biodefense Center (NBC) Protein Expression Core, Wadsworth Center, NY] in 100 μL capture buffer (20 mM Tris-HCl, pH 8.5 and 0.1 M NaCl) was added to each well of a 96-well plate (Corning) for 16 hrs at 4°C. Plates were blocked with 200 μL 10% bovine serum in PBS for 30 min. 2-fold serially diluted serum samples (in duplicate) were added to the plates and incubated for 1.5 hrs at room temperature. Goat-anti-mouse secondary antibody conjugated to horseradish peroxidase (HRP) was added at a dilution of 1:10,000 (100 μL/well) and incubated for further 1.5 hrs. Immunopure ABTS tablet (Pierce), dissolved in 10 mL substrate buffer (0.1 M acetate, 0.05% Tween 20, pH 4.2) to which 5 μL 30% H202 was added, was used as the substrate solution (100 μL/well) and plates were read at 415 nm on a Biorad microplate reader at 20 min following addition of substrate. Titers were calculated as the reciprocal of the maximum serum dilution giving an OD415 of 0.2, after subtracting the non-specific binding in serum of VSV-eGFP1, a recombinant VSV expressing enhanced Green Fluorescence Protein (eGFP) from the first position [51], infected animals.
2.6. Isotype determination of LcrV specific antibodies
ELISA assays to determine the levels of IgG1 and IgG2a bound specifically to LcrV were performed as described above (section 2.5), except using HRP-conjugated goat anti-mouse IgG1 (used at 1:5,000 dilution) or IgG2a (used at 1:10,000 dilution). To calculate the amounts of IgG1 or IgG2a bound to LcrV, we also generated standard curves using a range of concentrations of mouse IgG1 or IgG2a (Sigma) bound directly to the ELISA plate and detected with HRP-conjugated goat anti-mouse IgG1 or or IgG2a (Sigma).
2.7. Depletion of CD4+and/or CD8+cells
To determine the extent of CD4+ and/or CD8+ cell depletion by MAbs, female Balb/C mice, obtained from Charles River laboratories were rested for 1 week before administration of 500 μg of rat anti-mouse MAbs specific for mouse CD4 (clone GK1.5) and/or mouse CD8 (clone 2.43) by the intraperitoneal (IP) route. Control animals were either injected with 500 μg of the isotype control Rat IgG2b (clone LTF-2) or left untreated (naïve). All MAbs were purchased from Bio Express Cell Culture Services, West Lebanon, NH. Splenocytes were isolated from these animals at three days post MAb injection and stained with a phycoerythrin (PE)- conjugated rat anti-mouse CD4 antibody (Pharmingen) and an allophycocyanin (APC)- conjugated rat anti-mouse CD8 antibody (Pharmingen). CD4+ and CD8+ cell depletion from the splenocytes were assessed by a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed by using FlowJo analysis software (Tree Star, Inc., San Carlos, CA).
In the depletion-challenge study, VSV-ssLcrV1 vaccinated mice were injected (IP) with 1 mg (500 μg/day/mouse) of isotype control, or anti-mCD4 and/or anti-mCD8 MAbs, as indicated, one day prior to and on the day of challenge.
2.8. Y. pestis challenge
All challenge work with Y. pestis was performed in a biosafety cabinet in a BSL3-equipped animal facility at the PHRI, NJ. Mice vaccinated IM were challenged with 10 LD50 (10,000 cfu) of Y. pestis (C092 strain) by the IN route in a volume of 20 μL/nostril, following anesthetization with Ketamine and Xylazine (40–80 mg/kg and 5 mg/kg of body weight, respectively). Liquid inocula (20 μl) were delivered to the nares of the mice 5.0 μl at a time using a pipettman with an aerosol barrier tip. Mice were challenged at the indicated times post-inoculation and were observed daily for up to 14 days. Any animal exhibiting extreme lethargy or distress during this period was euthanized.
3. Results
3.1. Construction and characterization of a recombinant VSV expressing a secreted form of the Yersinia pestis LcrV protein
To generate a recombinant VSV that expressed a secreted form of the Y. pestis LcrV protein, a DNA sequence encoding signal sequence (ss) for secretion was added upstream of the LcrV ORF and inserted into the plasmid vector pVSV1XN [51]. This allowed the recovery of a virus, VSV-ssLcrV1 (Fig. 1A), expressing a secreted form of LcrV from the first position of the VSV genome, the position that maximizes mRNA and protein expression. VSV transcription occurs sequentially starting from the 3′-end, with transcription being attenuated by about 30% at each subsequent gene junction [53]. Appropriate VSV-specific transcription start, stop, and polyadenylation signals flanking the ssLcrV-coding region were incorporated as indicated (Fig. 1A). The recovered virus grew to titers nearly equivalent to wild-type VSV and had a normal plaque size.
Figure 1.
Recombinant VSV expressing the secreted form of Yernisia pestis lcrV gene (A) Schematic representation of the recombinant VSV with ssLcrV inserted upstream of the N gene (VSV-ssLcrV1) used in this study, showing the gene order in 3′ to 5′ direction on the negative-strand genome. (B) Indirect immunofluorescence microscopy of BHK-21 cells infected with the indicated viruses. Cells were fixed at 3 hrs post infection, permeabilized and stained using anti-LcrV monoclonal antibodies (MAbs), followed by an AlexaFlour-594 secondary antibody. The differential interference contrast (DIC) images are shown in the left column, the fluorescence images are shown in the middle column and the merged images are shown in the right column.
To verify expression of the secreted LcrV protein and to examine its localization in cells, we infected cells with VSV-ssLcrV1, VSV-LcrV1, or recombinant wild-type (rwt)-VSV and then performed indirect immunofluorescence microscopy using two monoclonal antibodies (MAbs) to LcrV protein. As shown in Fig. 1B, the protein encoded by VSV-ssLcrV1 was present in a reticular network throughout the cytoplasm, the pattern expected for a secreted protein in the endoplasmic reticulum. In contrast, the LcrV protein encoded by VSV-LcrV1 showed a more diffuse cytoplasmic localization. Control cells infected with rwt-VSV showed no staining for LcrV protein. Differential interference contrast (DIC) images are included to show the presence of cells in all fields.
The expression of the secreted LcrV protein was also verified by metabolically labeling VSV-ssLcrV1 infected BHK-21 cells with [35S]-methionine, followed by analysis of lysates and supernatants from the infected cells by SDS-PAGE and autoradiography (Fig. 2). Because VSV shuts off host protein synthesis, the cell lysates from rVSV- infected cells show mainly the viral encoded proteins L, G, N, P and M (Fig. 2). High-level expression of a protein with the expected mobility of LcrV (~37kDa) was identified both in cell lysates and in supernatants from cells infected with VSV-ssLcrV1 (Fig. 2, lanes 3 and 7), confirming the secretion of the ssLcrV protein. Control cells infected with VSV-LcrV1 [24], expressed the protein only in the cell lysates and not in the medium (Fig. 2, lanes 4 and 8). The LcrV band was absent in lysates and supernatants from mock infected cells or cells infected with rwt-VSV (Fig. 2, lanes 1, 2, 5 and 6).
Figure 2.
Metabolic labeling of proteins expressed from recombinant VSV vectors. BHK-21 cells, were infected with the indicated viruses or mock infected and then metabolically labeled with [35S]-methionine. Cell lysates and cell supernatants were fractionated by 10% SDS-PAGE and the gel image collected on a phosphorimager. Positions of VSV encoded proteins are indicated on the left side of the gel image.
3.2. Intramuscular vaccination with VSV-ssLcrV1 elicits improved antibody response to LcrV with increasing immunizing dose
Because our earlier work showed that, a single intranasal (IN) immunization (106 pfu) with VSV-LcrV1 generated low-level and variable protection (10%) to Y. pestis challenge [24], we generated the vector expressing secreted LcrV (VSV-ssLcrV1) and evaluated the antibody response generated by this vector. A single IN vaccination with a 106 pfu of VSV-ssLcrV1 generated a modestly improved protection against intranasal Y. pestis challenge when compared to VSV-LcrV1 (not shown). To determine if better protection could be obtained using a higher immunizing dose, we initiated a dose response study. Previous studies with VSV vectors have shown little effect of virus dose when given intranasally [37], but substantial effects of dose when given by the intramuscular (IM) route (S. Kapadia and J.K. Rose, unpublished results). We therefore tested the effect of virus dose on the antibody response to VSV-ssLcrV1 given IM.
In an initial study, five groups of mice (five mice per group) were immunized IM with escalating doses (105, 106, 107, 108, or 109 pfu) of VSV-ssLcrV1. A group of three mice received the control virus VSV-eGFP1 by the same route. Blood was collected from these mice at 28 and 53 days post immunization (dpi) and anti-LcrV titers in pooled sera were determined using ELISA. As shown in Fig. 3, each escalating IM dose of VSV-ssLcrV1 ranging from 105 – 109 pfu elicited increasing anti-LcrV titers that improved with time. The increase was more prominent in the two higher dose groups (108 and 109 pfu), with over two-fold increase in anti-LcrV titers in the 109 pfu group, relative to the 108 pfu group.
Figure 3.
Dose-response of anti-LcrV serum titers in VSV-ssLcrV1 immunized mice. Mice in five different groups were immunized intramuscularly (IM) on day 0 with 105, 106, 107, 108 and 109 pfu of VSV-ssLcrV1. Blood was collected, by retro-orbital bleeding, on days 28 and 53 post inoculation. Serum samples were pooled and total IgG titers, specific to LcrV, were determined at the indicated days using ELISA.
In order to determine if the higher doses of VSV-ssLcrV1 might be potentially pathogenic in mice, we also weighed the mice daily for two weeks following VSV immunizations (not shown). The amount and kinetics of weight loss in mice are a sensitive measure of viral pathogenesis [30]. While weight loss was negligible in mice immunized with 105 and 106 pfu, similar to that receiving the control virus VSV-eGFP1, mice in 107 and 108 pfu dose groups showed a transient weight loss (up to 5% by day1 post immunization) and began to recover by day 2. Mice in the 109 pfu dose group showed the greatest weight loss (up to 10% by day 2), but these and all other mice recovered to their pre-immunization body weights by day four.
3.3. A single dose of VSV-ssLcrV1 confers high-level protection against Y. pestis challenge
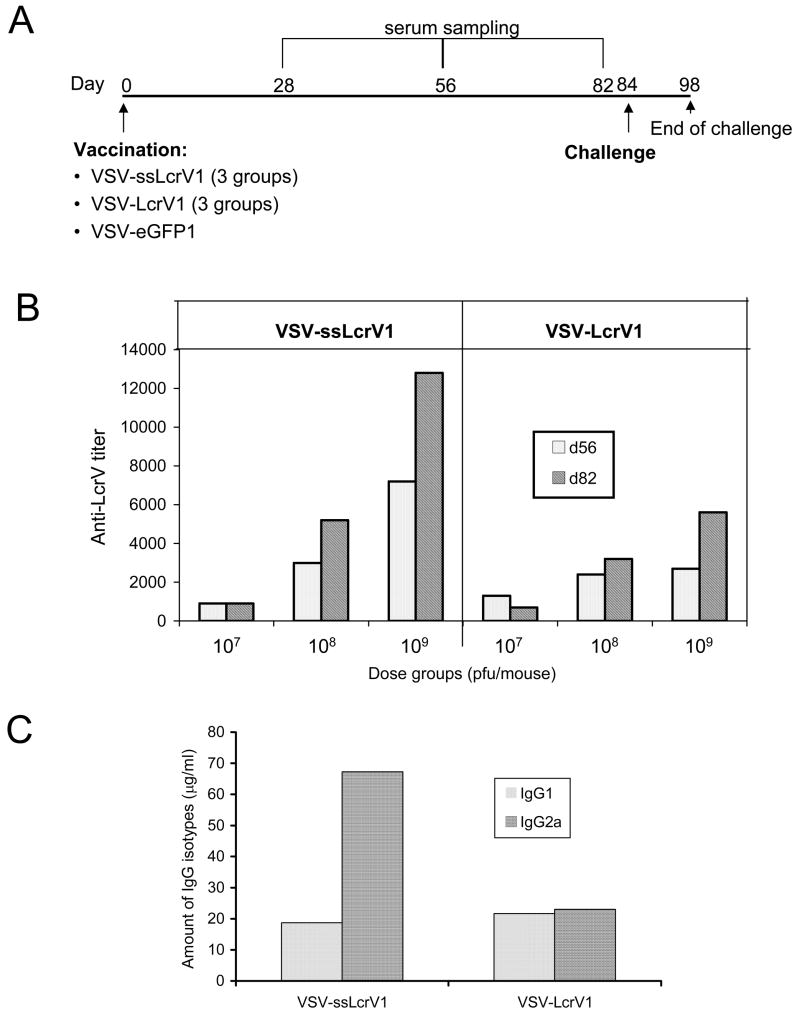
Because the dose response study showed that we could achieve very high anti-LcrV titers with a single high dose of VSV-ssLcrV1, we compared antibody responses and degree of protection from Y. pestis challenge conferred by increasing doses of VSV-ssLcrV1 and VSV-LcrV1, the vector that we had used earlier [24]. The immunization timeline for this challenge study is represented in Fig. 4A. Three groups of eight mice each were vaccinated (IM) with VSV-ssLcrV1, at doses of 107, 108 and 109 pfu. In parallel, three additional groups received the same doses of VSV-LcrV1. Eight control mice were immunized (IM) with VSV-eGFP1. Blood was collected at indicated time-points (Fig. 4A) and anti-LcrV titers were determined using pooled sera from each immunization group. Increasing doses of either virus led to increased antibody titers. In addition, the titers induced by VSV-ssLcrV1 were generally higher than those induced by VSV-LcrV1 (Fig. 4B).
Figure 4.
Comparison of humoral immune responses of mice to VSV-ssLcrV1 and VSV-LcrV1 vaccinations. (A) Timeline for dose response vaccination-challenge study. Mice were vaccinated IM with 107, 108 and 109 pfu doses of rVSVs encoding either ssLcrV or LcrV and challenged IN with Y. pestis (CO92 strain). VSV-eGFP1 immunization (107 pfu) was used as control. (B) Anti-LcrV titer profile at 56 and 82 days post immunization, in mice immunized with 107, 108 and 109 pfu doses of either VSV-ssLcrV1 (left panel) or VSV-LcrV1 (right panel). (C) Amount of anti-LcrV specific IgG1 and IgG2a isotypes in VSV-ssLcrV1 (left) of VSV-LcrV1 (right) vaccinated mice in the 109 pfu dose group. Serum antibodies that bound LcrV were quantitated using secondary IgG1- and IgG2-specific antibodies. Concentrations of mouse IgG1 or IgG2a were then calculated from standard curves run in parallel using a range of concentrations of purified mouse IgG1 or IgG2a.
Analysis of IgG subclass binding LcrV protein (109 pfu dose) showed that VSV-ssLcrV1 vaccination induced an LcrV-specific IgG2a response which was about 3-fold higher than the IgG1 response (Fig. 4C). In contrast, the VSV-LcrV1 vaccination induced similar levels of LcrV-specific IgG1 and IgG2a (Fig. 4C).
To measure the efficacy of these single dose recombinant VSV vaccines, animals from these immunization groups were subjected to pulmonary challenge (IN) with 10 LD50 Y. pestis (CO92 strain) at about 3 months post vaccination. In the VSV-ssLcrV1 groups, seven out of eight animals in the 109 pfu group survived the challenge (Fig. 5A). Four out of eight animals survived in the 108 pfu group, while only one out of eight mice survived in the 107 pfu group (Fig 5A). All mice in the VSV-eGPF1 control group died by day 5 post-challenge. The higher level of protection in the 109 pfu group was statistically highly significant [p = 0.001, Fisher Exact Test (when compared to control vaccinated animals)]. In comparison, in both 109 and 108 pfu groups of the VSV-LcrV1 set, only three of eight animals survived the challenge, while none survived in the 107 pfu group (Fig. 5B).
Figure 5.
Protective efficacy of single-dose VSV-ssLcrV or VSV-LcrV vaccine in a plague challenge assay. (A) Survival profile of VSV-ssLcrV1 vaccinated mice following lethal plague challenge. Mice were immunized (IM) with VSV-ssLcrV1 at three different doses - 107 pfu (open triangle), 108 pfu (open diamond) or 109 pfu (open square). The control mice received VSV-eGFP1 (open circle). All animals were challenged at 3 months post vaccination with 10LD50 Y. pestis (CO92) and observed for 14 days. (B) Similar to A, except that animals were vaccinated with three different doses of VSV-LcrV1 - 107 pfu (open triangle), 108 pfu (open diamond) or 109 pfu (open square).
The difference in survival between the VSV-ssLcrV1 vaccinated and VSV-LcrV1 vaccinated animals (109 dose) approached statistical significance in this first experiment (p=0.059). In a second experiment (10 animals per group) using the same dose of each vector and the same challenge, we obtained 100% protection in the VSV-ssLcrV1 vaccinated animals and only 50% protection in the VSV-LcrV1 vaccinated animals. This difference was highly significant (p = 0.016).
3.4. Survival did not fully correlate with antibody titer
Earlier passive transfer studies have shown that antibody to LcrV is sufficient to provide protection from disease in the mouse model [21, 23]. Although in our studies, increased antibody titers to LcrV generally yielded higher protection, analysis of antibody responses in individual mice often did not correlate well with protection. Comparison of mouse survival profiles to their anti-LcrV titers in the 108 pfu dose groups, revealed that there was a large variation in the titers among individual mice and that some mice with low titers survived the Y. pestis challenge (Fig. 6).
Figure 6.
Correlation of pre-challenge anti-LcrV titers and mouse survival. Mice were vaccinated with 108 pfu of either VSV-ssLcrV1 or VSV-LcrV1 and challenged at day 84 post vaccination with 10 LD50 (10,000 cfu) of Y. pestis (CO92 strain). Anti-LcrV serum titers in individual mice at day 82 post vaccination are plotted for those mice that survived challenge (alive, filled triangle) or succumbed to challenge (dead, open inverted triangle). The horizontal bars represent the geometric means for the two groups.
3.5. Role of CD4+T-cells in protection elicited by VSV-ssLcrV1 vaccination
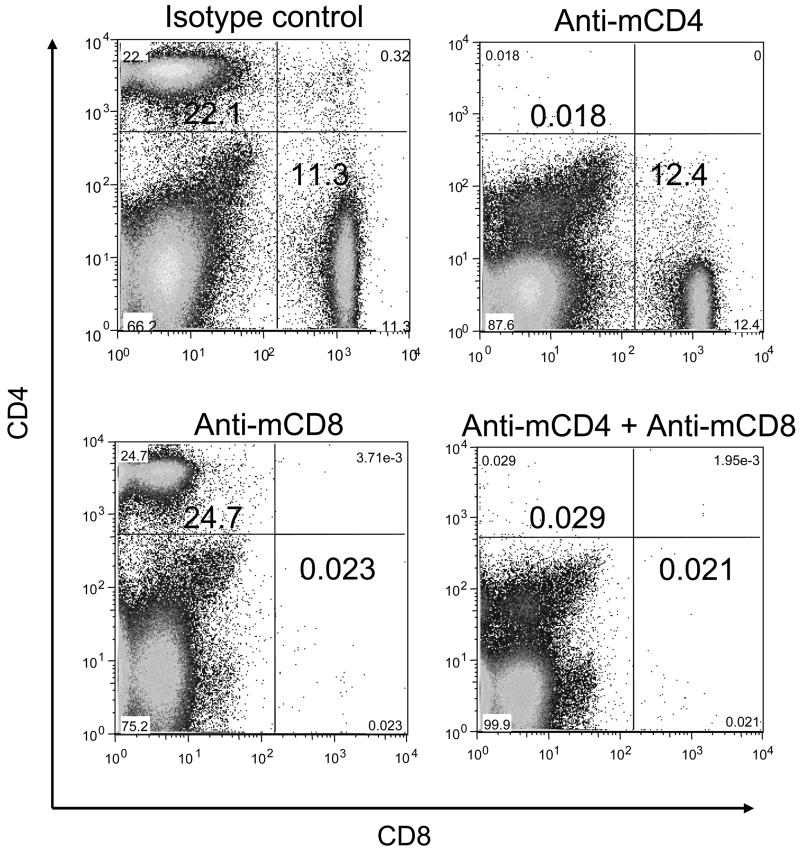
Because LcrV antibody titers did not correlate completely with protection (Fig. 6), we thought that cellular immune responses might contribute to protection against pulmonary plague infection induced by VSV vectors. To examine a potential role of CD4+ and CD8+ cells in VSV-ssLcrV1 vaccinated mice at the time of challenge we depleted these cells singly or together just before challenge and then determined the extent of protection. A control experiment was performed to evaluate the extent of depletion using mouse specific MAbs. Figure 7 shows representative FACS plots of splenocytes, obtained from mice injected intraperitonealy (IP) with anti-mCD4, anti-CD8 or isotype control MAbs and, stained with appropriately conjugated rat anti-mouse CD4 or CD8 antibodies. Relative to an isotype control MAb treated mouse, anti-mCD4 and/or anti-mCD8 MAb treatment resulted in a nearly complete, specific depletion of CD4+ and/or CD8+ cells in mice, confirming the efficiency of the depletion (Fig. 7).
Figure 7.
Depletion of CD4+ and CD8+ T-cells in mice using mouse specific MAbs. Representative FACS plots are shown for splenocytes obtained from mice injected intraperitonealy (IP) with anti-mouse isotype control (top left), anti-mCD4 (top right), anti-mCD8 (bottom left) or combined anti-mCD4 + anti-mCD8 (bottom right) MAbs and stained with approrpiately conjugated rat anti-mouse CD4 and CD8 antibodies on day 3 post injection.
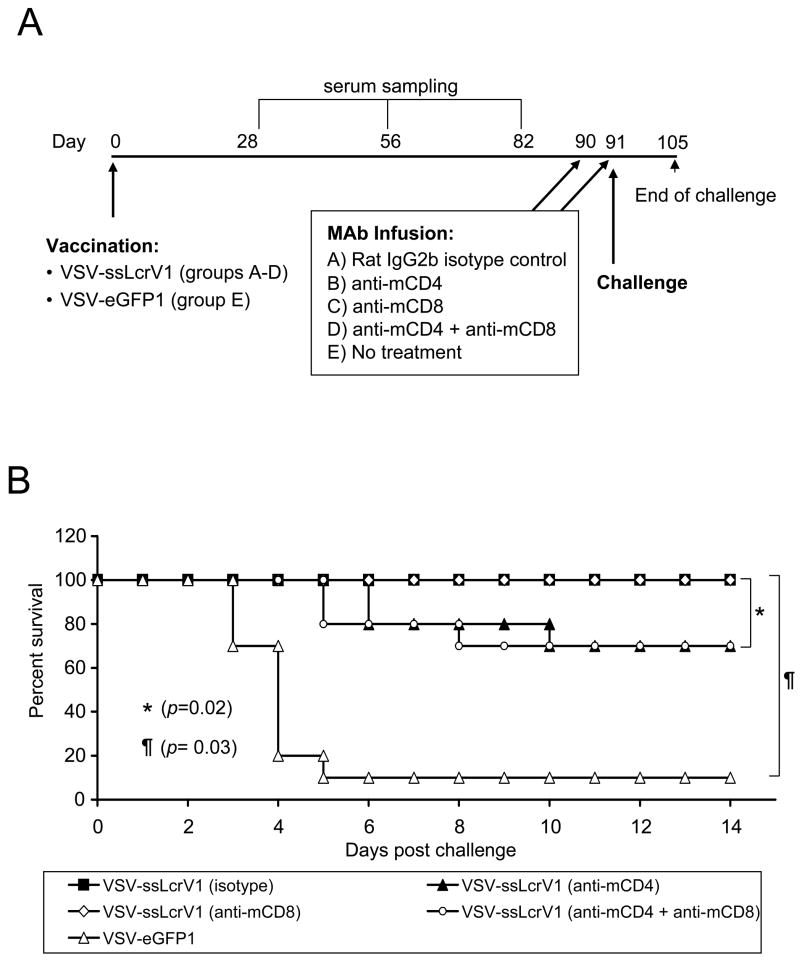
Figure 8A gives the plan and timeline for the depletion/challenge study. Four groups of ten mice each (groups A-D) were given 109 pfu of VSV-ssLcrV1 by the IM route, while ten control mice received similar doses of VSV-eGFP1. The CD4+ and/or CD8+ cells were depleted in some of these immunization groups with injection (IP) of MAbs specific for mouse CD4 and/or mouse CD8 as indicated (Fig. 8A), followed by pulmonary Y. pestis challenge approximately 3 months post vaccination. Complete protection [p = 0.03 (Fisher Exact Test), as compared to VSV-eGFP1 vaccinated mice] was obtained in the VSV-ssLcrV1 vaccinated animals, which received the isotype control MAb (group A) or the anti-mCD8 MAb (group C), as shown in Fig. 8B. In contrast, two groups of vaccinated mice that received the anti-mCD4 MAb, with or without depletion of CD8+ cells (groups B and D), elicited significantly reduced [p=0.02 (Fisher Exact Test)] levels of protection (70%). Note that greater statistical significance was achieved in the latter case because there were more animals involved in this calculation. Surprisingly, one out of the ten control animals survived the challenge. In our previous studies of mice given this challenge, all control mice (n=35) had died by day 4 or 5 post challenge.
Figure 8.
CD4+ cells are required at time of challenge for complete protection against plague challenge. (A) Timeline for the depletion-challenge study. Mice in four groups (A-D) were immunized IM with 109 pfu of VSV-ssLcrV1. Serum sampling was done on day 28, 56 and 82 post immunization. Mice were injected with CD4+/CD8+ depleting MAbs (IP) on days 90 and 91 post immunization followed by challenge on day 91 with 10 LD50 (10,000 cfu) of Y. pestis (CO92 strain). VSV-eGFP1 vaccinated mice were used as the control group. Rat IgG2b isotype MAb was used as a control. (B) Survival profile for mice in the depletion-challenge experiment. VSV-ssLcrV1 vaccinated mice were injected with isotype-matched control (filled square), anti-mCD4 (filled triangle), anti-mCD8 (open diamond) or mCD4+mCD8 (open circle) MAbs. Control animals receiving VSV-eGFP1 (open triangle) were not treated with MAbs.
4. Discussion
In an earlier study, we showed that an intranasal (IN) prime-boost immunization with two different VSV vectors expressing LcrV elicited high-level protection against IN Y. pestis challenge [24]. We report here the development of an rVSV vector (VSV-ssLcrV1), expressing a secreted form of LcrV from the first position of the VSV genome, that when given intramuscularly (IM) as a single dose of 109 pfu, induced high-level anti-LcrV antibody titers in mice, and completely protected animals against subsequent lethal pulmonary Y. pestis challenge. Compared to the VSV-LcrV1 vector (expressing non-secreted LcrV), that we described earlier [24] the vector expressing secreted LcrV (VSV-ssLcrV1) gave significantly better single-dose protection. Immunization with VSV-ssLcrV1 also elicited higher IgG2a relative to IgG1 titers suggestive of a shift toward a Th1 type of immune response to the secreted protein.
Although the basis for protection by LcrV-based vaccines is not completely understood, several studies have shown a correlation between high anti-LcrV titers and protection [15, 16, 25, 26]. Additionally, passive transfer of polyclonal LcrV specific antibody, as well as LcrV monoclonal antibody (MAb) treatment was shown to be protective in mouse models of plague infection that LcrV antibody can be sufficient for protection [22, 23].
In our study, we observed that the pre-challenge LcrV ELISA titers did not correlate completely with protection because animals having even very low titers were protected in some instances. It was also noted in a non-human primate model of plague infection that some animals possessing high LcrV antibody titers succumbed to Y. pestis challenge [54]. Together these results suggested a possible cellular immune component in protection. Recently, immunizations with live replicating Y. pestis have also shown the importance of cell-mediated immunity in protection against plague challenge [55, 56]. However, LcrV did not appear to be a major inducer of T-cell responses in that study [56].
In our studies, induction of increased IgG2a response to LcrV following immunization with VSV-ssLcrV1 indicated an antigen-specific bias toward a Th1 cellular immune response. To determine if we could identify a role for cellular immunity in protection, we performed selective depletion of mouse CD4+ and/or CD8+ T-cells at the time of challenge. Mice completely depleted for CD8+ T-cells prior to challenge were 100% protected from disease indicating that CD8+ T-cells are not required for protection. However, depletion of CD4+ T-cells, with or without depletion of CD8+ T cells, significantly reduced the level of protection. This result suggests that CD4+ T-cells might be involved in protection, although effects on other CD4+ cells cannot be ruled out. Effects on antibody production are unlikely because the depletion is performed just prior to challenge. Consistent with our findings, CD4+ T-cell epitopes have been reported for the LcrV protein in Balb/c mice [57], while CD8+ T-cell epitopes have not been defined.
What role might the CD4+ T-cells be playing in protection against Y. pestis? Vaccine induced CD4+ T-cells are known to secrete phagocyte activating type 1 cytokines like interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), which contribute to intracellular antimicrobial defense by activating macrophages [58, 59]. However, although intracellular niches of Y. pestis exist, pulmonary plague infection is considered predominantly extracellular. Some recent studies suggest that both CD4+ T cells and IFN-γ play critical roles in combating extracellular bacterial infections [60, 61]. Other studies imply important roles for IFN-γ and TNF-α in protection against lethal Y. pestis infections [62, 63]. Thus depletion of vaccine primed CD4+ T-cells leading to abrogation of the cytokine secreting activity, might be reflected in the reduced level of protection that we have observed. In addition, the role of cytotoxic CD4+ T-cells in eliminating infected cells also cannot be ruled out.
Why addition of an amino terminal signal sequence for secretion of the LcrV protein enhances protection is unclear, but this was also noted previously for multi-dose DNA vaccine expressing secreted LcrV [26]. The authors of that study suggested that aggregation of the secreted protein might be involved in greater induction of antibody. The inclusion of the signal sequence, which we show here causes efficient secretion of LcrV, could also be responsible for enhancing CD4+ T-cell responses if the secreted protein were more efficiently directed into the MHC class II presentation pathway.
Acknowledgments
This work was supported by Northeast Biodefense Center grant AI-0571158.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perry RD, Fetherston JD. Yersinia pestis--etiologic agent of plague. Clin Microbiol Rev. 1997;10(1):35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer KF. Pneumonic plague. Bacteriol Rev. 1961;25:249–61. doi: 10.1128/br.25.3.249-261.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galimand M, Guiyoule A, Gerbaud G, Rasoamanana B, Chanteau S, Carniel E, et al. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N Engl J Med. 1997;337(10):677–80. doi: 10.1056/NEJM199709043371004. [DOI] [PubMed] [Google Scholar]
- 4.Hinnebusch BJ, Rosso ML, Schwan TG, Carniel E. High-frequency conjugative transfer of antibiotic resistance genes to Yersinia pestis in the flea midgut. Mol Microbiol. 2002;46(2):349–54. doi: 10.1046/j.1365-2958.2002.03159.x. [DOI] [PubMed] [Google Scholar]
- 5.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense Jama. 2000;283(17):2281–90. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 6.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev Vaccines. 2008;7(2):209–21. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Titball RW, Williamson ED. Yersinia pestis (plague) vaccines. Expert Opin Biol Ther. 2004;4(6):965–73. doi: 10.1517/14712598.4.6.965. [DOI] [PubMed] [Google Scholar]
- 8.Benner GE, Andrews GP, Byrne WR, Strachan SD, Sample AK, Heath DG, et al. Immune response to Yersinia outer proteins and other Yersinia pestis antigens after experimental plague infection in mice. Infect Immun. 1999;67(4):1922–8. doi: 10.1128/iai.67.4.1922-1928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philipovskiy AV, Cowan C, Wulff-Strobel CR, Burnett SH, Kerschen EJ, Cohen DA, et al. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect Immun. 2005;73(3):1532–42. doi: 10.1128/IAI.73.3.1532-1542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heath DG, Anderson GW, Jr, Mauro JM, Welkos SL, Andrews GP, Adamovicz J, et al. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine. 1998;16(11–12):1131–7. doi: 10.1016/s0264-410x(98)80110-2. [DOI] [PubMed] [Google Scholar]
- 11.Anderson GW, Jr, Leary SE, Williamson ED, Titball RW, Welkos SL, Worsham PL, et al. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect Immun. 1996;64(11):4580–5. doi: 10.1128/iai.64.11.4580-4585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyles JE, Williamson ED, Spiers ID, Alpar HO. Protection studies following bronchopulmonary and intramuscular immunisation with Yersinia pestis F1 and V subunit vaccines coencapsulated in biodegradable microspheres: a comparison of efficacy. Vaccine. 2000;18(28):3266–71. doi: 10.1016/s0264-410x(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 13.Leary SE, Williamson ED, Griffin KF, Russell P, Eley SM, Titball RW. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect Immun. 1995;63(8):2854–8. doi: 10.1128/iai.63.8.2854-2858.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powell BS, Andrews GP, Enama JT, Jendrek S, Bolt C, Worsham P, et al. Design and testing for a nontagged F1-V fusion protein as vaccine antigen against bubonic and pneumonic plague. Biotechnol Prog. 2005;21(5):1490–510. doi: 10.1021/bp050098r. [DOI] [PubMed] [Google Scholar]
- 15.Williamson ED, Eley SM, Griffin KF, Green M, Russell P, Leary SE, et al. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol Med Microbiol. 1995;12(3–4):223–30. doi: 10.1111/j.1574-695X.1995.tb00196.x. [DOI] [PubMed] [Google Scholar]
- 16.Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine. 1997;15(10):1079–84. doi: 10.1016/s0264-410x(96)00303-9. [DOI] [PubMed] [Google Scholar]
- 17.Williamson ED, Eley SM, Stagg AJ, Green M, Russell P, Titball RW. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine. 2000;19(4–5):566–71. doi: 10.1016/s0264-410x(00)00159-6. [DOI] [PubMed] [Google Scholar]
- 18.Williamson ED, Sharp GJ, Eley SM, Vesey PM, Pepper TC, Titball RW, et al. Local and systemic immune response to a microencapsulated sub-unit vaccine for plague. Vaccine. 1996;14(17–18):1613–9. doi: 10.1016/s0264-410x(96)00151-x. [DOI] [PubMed] [Google Scholar]
- 19.Pettersson J, Holmstrom A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, et al. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol Microbiol. 1999;32(5):961–76. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 20.Sarker MR, Neyt C, Stainier I, Cornelis GR. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J Bacteriol. 1998;180(5):1207–14. doi: 10.1128/jb.180.5.1207-1214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill J, Leary SE, Griffin KF, Williamson ED, Titball RW. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect Immun. 1997;65(11):4476–82. doi: 10.1128/iai.65.11.4476-4482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill J, Copse C, Leary S, Stagg AJ, Williamson ED, Titball RW. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersinia pestis. Infect Immun. 2003;71(4):2234–8. doi: 10.1128/IAI.71.4.2234-2238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motin VL, Nakajima R, Smirnov GB, Brubaker RR. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect Immun. 1994;62(10):4192–201. doi: 10.1128/iai.62.10.4192-4201.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palin A, Chattopadhyay A, Park S, Delmas G, Suresh R, Senina S, et al. An optimized vaccine vector based on recombinant vesicular stomatitis virus gives high-level, long-term protection against Yersinia pestis challenge. Vaccine. 2007;25(4):741–50. doi: 10.1016/j.vaccine.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Une T, Brubaker RR. Roles of V antigen in promoting virulence and immunity in yersiniae. J Immunol. 1984;133(4):2226–30. [PubMed] [Google Scholar]
- 26.Wang S, Heilman D, Liu F, Giehl T, Joshi S, Huang X, et al. A DNA vaccine producing LcrV antigen in oligomers is effective in protecting mice from lethal mucosal challenge of plague. Vaccine. 2004;22(25–26):3348–57. doi: 10.1016/j.vaccine.2004.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiuchiolo MJ, Boyer JL, Krause A, Senina S, Hackett NR, Crystal RG. Protective immunity against respiratory tract challenge with Yersinia pestis in mice immunized with an adenovirus-based vaccine vector expressing V antigen. J Infect Dis. 2006;194(9):1249–57. doi: 10.1086/507644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Do Y, Park CG, Kang YS, Park SH, Lynch RM, Lee H, et al. Broad T cell immunity to the LcrV virulence protein is induced by targeted delivery to DEC-205/CD205-positive mouse dendritic cells. Eur J Immunol. 2008;38(1):20–9. doi: 10.1002/eji.200737799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose JK, Whitt MA. Rhabdoviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields’ Virology. Phhiladelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 30.Roberts A, Kretzschmar E, Perkins AS, Forman J, Price R, Buonocore L, et al. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J Virol. 1998;72(6):4704–11. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A. 1995;92(10):4477–81. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnell MJ, Buonocore L, Whitt MA, Rose JK. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J Virol. 1996;70(4):2318–23. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egan MA, Chong SY, Rose NF, Megati S, Lopez KJ, Schadeck EB, et al. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res Hum Retroviruses. 2004;20(9):989–1004. doi: 10.1089/aid.2004.20.989. [DOI] [PubMed] [Google Scholar]
- 34.Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ, et al. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78(8):3930–40. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–49. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 36.Kretzschmar E, Buonocore L, Schnell MJ, Rose JK. High-efficiency incorporation of functional influenza virus glycoproteins into recombinant vesicular stomatitis viruses. J Virol. 1997;71(8):5982–9. doi: 10.1128/jvi.71.8.5982-5989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73(5):3723–32. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dietzschold B, Rupprecht CE, Fu ZF, Koprowski H. Rhabdoviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fields’ Virology. Philadelphia: Lippincott-Raven; 1996. pp. 1137–59. [Google Scholar]
- 39.Zinkernagel RM, Adler B, Holland JJ. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp Cell Biol. 1978;46(1–2):53–70. doi: 10.1159/000162882. [DOI] [PubMed] [Google Scholar]
- 40.Garbutt M, Liebscher R, Wahl-Jensen V, Jones S, Moller P, Wagner R, et al. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J Virol. 2004;78(10):5458–65. doi: 10.1128/JVI.78.10.5458-5465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geisbert TW, Jones S, Fritz EA, Shurtleff AC, Geisbert JB, Liebscher R, Grolla A, Stroher U, Fernando L, Daddario KM, Guttieri MC, Mothe BR, Larsen T, Hensley LE, Jahrling PB, Feldmann H. Development of a new vaccine for the prevention of lassa fever. PLOS Medicine. 2006;2(6):537–45. doi: 10.1371/journal.pmed.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11(7):786–90. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 43.Kahn JS, Roberts A, Weibel C, Buonocore L, Rose JK. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J Virol. 2001;75(22):11079–87. doi: 10.1128/JVI.75.22.11079-11087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K, Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174–82. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Natuk RJ, Cooper D, Guo M, Calderon P, Wright KJ, Nasar F, et al. Recombinant vesicular stomatitis virus vectors expressing herpes simplex virus type 2 gD elicit robust CD4+ Th1 immune responses and are protective in mouse and guinea pig models of vaginal challenge. J Virol. 2006;80(9):4447–57. doi: 10.1128/JVI.80.9.4447-4457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuter JD, Vivas-Gonzalez BE, Gomez D, Wilson JH, Brandsma JL, Greenstone HL, et al. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J Virol. 2002;76(17):8900–9. doi: 10.1128/JVI.76.17.8900-8909.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts A, Reuter JD, Wilson JH, Baldwin S, Rose JK. Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein. J Virol. 2004;78(6):3196–9. doi: 10.1128/JVI.78.6.3196-3199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlereth B, Rose JK, Buonocore L, ter Meulen V, Niewiesk S. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J Virol. 2000;74(10):4652–7. doi: 10.1128/jvi.74.10.4652-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schnell MJ, Johnson JE, Buonocore L, Rose JK. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell. 1997;90(5):849–57. doi: 10.1016/s0092-8674(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz JA, Buonocore L, Roberts A, Suguitan A, Jr, Kobasa D, Kobinger G, et al. Vesicular stomatitis virus vectors expressing avian influenza H5 HA induce cross-neutralizing antibodies and long-term protection. Virology. 2007;366(1):166–73. doi: 10.1016/j.virol.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, et al. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J Virol. 2005;79(24):15043–53. doi: 10.1128/JVI.79.24.15043-15053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuerst TR, Niles EG, Studier FW, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986;83(21):8122–6. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iverson LE, Rose JK. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981;23(2):477–84. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- 54.Bashaw J, Norris S, Weeks S, Trevino S, Adamovicz JJ, Welkos S. Development of in vitro correlate assays of immunity to infection with Yersinia pestis. Clin Vaccine Immunol. 2007;14(5):605–16. doi: 10.1128/CVI.00398-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, et al. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect Immun. 2005;73(11):7304–10. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Philipovskiy AV, Smiley ST. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect Immun. 2007;75(2):878–85. doi: 10.1128/IAI.01529-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shim HK, Musson JA, Harper HM, McNeill HV, Walker N, Flick-Smith H, et al. Mechanisms of major histocompatibility complex class II-restricted processing and presentation of the V antigen of Yersinia pestis. Immunology. 2006;119(3):385–92. doi: 10.1111/j.1365-2567.2006.02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerksiek KM, Pamer EG. T cell responses to bacterial infection. Curr Opin Immunol. 1999;11(4):400–5. doi: 10.1016/S0952-7915(99)80067-3. [DOI] [PubMed] [Google Scholar]
- 59.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–89. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 60.Pilione MR, Harvill ET. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect Immun. 2006;74(2):1043–9. doi: 10.1128/IAI.74.2.1043-1049.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005;102(13):4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elvin SJ, Williamson ED. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Microb Pathog. 2004;37(4):177–84. doi: 10.1016/j.micpath.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 63.Nakajima R, Brubaker RR. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61(1):23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]