Abstract
Background & Aims
Nonalcoholic fatty liver disease (NAFLD) is extremely common among morbidly obese patients. We assessed the usefulness of plasma caspase-generated cytokeratin 18 (CK-18) fragments as a novel marker for NAFLD in a bariatric cohort.
Methods
The cohort consisted of 99 consecutive patients who underwent liver biopsy at the time of bariatric surgery. CK-18 levels were measured using an enzyme-linked immunosorbant assay before and 6 months after surgery. Patients were subdivided into four histological groups: not NAFLD (normal liver biopsy), NAFL, borderline diagnosis, and definitive nonalcoholic steatohepatitis (NASH).
Results
CK-18 levels were significantly higher in subjects with NASH compared to those with not NAFLD, NAFL, or borderline diagnosis (median [Q25, Q75]: 389 U/L [275, 839] vs. 196 U/L [158, 245], vs. 217 U/L [154, 228], or vs. 200 U/L [176, 274], respectively; P<0.0001). CK-18 levels were significantly higher in subjects with moderate to severe fibrosis versus those with no or mild fibrosis (334.5 U/L [240.5, 896] vs. 207 U/L [175, 275], respectively; P=0.007). A significant decrease in CK-18 levels was observed in most patients 6 months postoperatively. The area under the ROC curve for NASH diagnosis was estimated to be 0.88 (95% CI: 0.77, 0.99). The values with the best combination of sensitivity and specificity were 252 U/L (sensitivity = 82%, specificity = 77%) and 275 U/L (sensitivity = 77%, specificity = 100%).
Conclusion
These results support the potential utility of this test for diagnosis and staging of NAFLD before bariatric surgery.
Keywords: Nonalcoholic fatty liver disease, NASH, apoptosis, biomarker, cytokeratin 18, bariatric surgery, morbid obese
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in the United States and occurs mainly in overweight or obese individuals (1, 2). It is extremely common among patients undergoing bariatric surgery (3, 4). NAFLD encompasses a wide spectrum of conditions ranging from nonalcoholic fatty liver (NAFL) or steatosis to nonalcoholic steatohepatitis (NASH) to cirrhosis (5). NASH is a potentially serious condition since as many as 25% of patients may progress to cirrhosis and experience its complications (6, 7). A liver biopsy remains the only reliable way to differentiate steatosis from NASH and determine the stage and grade of the disease (8). Morbidly obese patients undergoing bariatric surgery are a group at particular risk for NAFLD and for development of the more serious forms of this condition (9, 10). Development of reliable noninvasive biomarkers to diagnose and determine disease severity prior to bariatric surgery and to monitor disease status postoperatively would be of significant clinical utility.
Increased hepatocyte death by apoptosis may play an important role in liver injury and disease progression in NAFLD (11). During the process of apoptosis, effector caspases (mainly caspase 3) are activated and cleave a number of substrates inside the cell including cytokeratin 18 (CK-18), the major intermediate filament protein in the liver, resulting in the characteristic morphologic changes of apoptosis (11). Previously, we demonstrated that the plasma concentration of caspase-generated CK-18 fragments accurately differentiate NASH from NAFL and predicts stage of fibrosis in patients with NAFLD (12, 13). The aim of this study was to assess the utility of this novel biomarker in determining NASH, assessing disease severity and monitoring disease status following bariatric surgery in morbidly obese patients.
PATIENTS AND METHODS
Patient characteristics
The study was approved by the Cleveland Clinic Institutional Review Board. Our cohort consisted of 99 consecutive patients who underwent liver biopsy at the time of bariatric surgery as part of a standard clinical procedure. ’’The diagnosis of NAFLD was based on liver biopsy features as assessed by an experienced hepatopathologist (L.Y.). Patients were subdivided into four histological groups: not NAFLD (normal liver biopsy), NAFL, borderline diagnosis and definitive NASH. The NAFLD NIDDK activity score (14) was applied to each patient (see below). Demographic, clinical and laboratory data were obtained from clinic visits (2–4 wks) prior to surgery. The absence of current excessive alcohol use was defined by an average daily consumption of alcohol of ≤20 grams/day for men and <10 grams/day for women. Prevalence of diabetes, hypertension and hyperlipidemia was assessed by review of past medical history. Prevalence of diabetes was based on past medical history and/or fasting plasma glucose of 126mg/dl or greater.
Liver histology
The histological diagnosis of NAFLD was established by the study pathologist according to her expertise and following the NAS in a blinded manner regarding the CK-18 fragment measurements and patient’s clinical and laboratory data (14). In this scoring system, the degree of steatosis, liver injury and inflammatory activity is measured using an 8-point scale (steatosis 0–3; lobular inflammation 0–3; ballooning degeneration of hepatocytes 0–2). The NAS is the unweighted sum of steatosis, lobular inflammation and hepatocellular ballooning scores. The stage of fibrosis was similarly measured using a 6-point scale (1a, b = mild (1a)/moderate (1b) zone 3 perisinusoidal fibrosis; 1c = portal fibrosis only; 2 = zone 3 and portal/periportal fibrosis; 3 = bridging fibrosis; 4 = cirrhosis).
Measurement of caspase-generated CK-18 fragments in the blood
CK-18 levels were measured in 86 patients who had plasma available within one week prior to surgery using a sandwich immunoELISA specific for CK-18 fragments. Additionally, CK-18 levels were measured 6 months after bariatric surgery in those patients with available plasma (n = 34). All samples were initially processed to plasma and stored frozen at −80°C. The plasma was subsequently used for quantitative measurement of the apoptosis-associated neo-epitope in the C-terminal domain of CK-18 by the M30-Apoptosense ELISA kit (PEVIVA, Alexis, Grünwald, Germany). All assays were performed in duplicate and the absorbance was determined using a microplate reader (Molecular Devices M2, Sunnyvale, California, US).
Statistical Analysis
Descriptive statistics were computed for all variables. These include means and standard deviations or medians, as well as 25th and 75th percentiles for continuous factors. For categorical variables, frequencies and percentages were estimated. Kruskal-Wallis and Dunn’s tests were used to assess whether CK-18 levels were significantly different between the three subject groups. In addition, Wilcoxon rank sum tests were used to compare CK-18 levels between subjects with moderate to severe fibrosis and those with mild fibrosis. Spearman’s correlation coefficients were used to assess associations between CK-18 levels and histological characteristics. Logistic regression analysis was used to assess the association between plasma levels of CK-18 fragments and the likelihood of having definitive NASH as opposed to simple steatosis. To predict the presence of NASH with optimal sensitivity and specificity, receiver operating characteristic curve analysis was used to estimate potential cutoff values of plasma CK-18 fragments. The same was done to assess the utility of CK-18 levels in the prediction of fibrosis. A P value of 0.05 was considered statistically significant. SAS version 9.1 software (SAS Institute, Cary, NC) and R 2.0.1 software (The R Foundation for Statistical Computing) were used to perform all analyses.
RESULTS
Characteristics of the patient population
The main clinical and laboratory characteristics of the patients are described in Table 1 while the histological characteristics of the liver biopsies are summarized in Table 2. The patients’ age (median 51 years), gender (68% females), and BMI (median 48 kg/m2) did not statistically differ among the four histological groups. There was no difference in the prevalence of diabetes, hypertension or hyperlipidemia among the groups. Serum AST and ALT were within the normal range in most patients, although subjects with NASH tended to have significantly higher AST and ALT levels than both subjects without NAFLD and those with NAFL. In addition, borderline subjects had higher ALT levels than those without NAFLD.
Table 1.
Demograhic and Clinical Characteristics of Subjects who Underwent Bariatric Surgery
| Factor | All (N = 86) | Not NAFLD (N = 21) | NAFL (N = 13) | Borderline (N = 30) | NASH (N = 22) | P value |
|---|---|---|---|---|---|---|
| Age (yr) | 51.0 (41.0, 56.0) | 51.0 (41.0, 57.0) | 46.0 (42.0, 51.0) | 52.5 (42.0, 56.0) | 50.0 (40.0, 56.0) | 0.68 |
| BMI (kg/m2) | 48.0 (43.0, 54.0) | 48.0 (46.0, 54.0) | 48.0 (45.0, 51.0) | 48.0 (42.0, 54.0) | 47.5 (42.0, 55.0) | 0.93 |
| AST (U/L) | 23.0 (18.0, 29.0) | 19.0 (16.0, 24.0) | 20.0 (17.0, 23.0) | 23.5 (19.0, 29.0) | 28.5 (23.5, 50.5) | 0.01 |
| ALT (U/L) | 21.5 (16.0, 33.0) | 17.0 (11.0, 19.0) | 17.0 (15.0, 20.0) | 26.0 (19.0, 34.0) | 33.5 (22.0, 61.5) | 0.001 |
| Gender | 0.98 | |||||
| Male (%) | 18 (20.9) | 4 (19.1) | 3 (23.1) | 7 (23.3) | 4 (18.2) | |
| Female (%) | 68 (79.1) | 17 (81.0) | 10 (76.9) | 23 (76.7) | 18 (81.8) | |
| Ethnicity | 0.04 | |||||
| Caucasian(%) | 70 (81.4) | 15 (71.4) | 8 (61.5) | 26 (86.7) | 21 (95.5) | |
| Other (%) | 16 (18.6) | 6 (28.6) | 5 (38.5) | 4 (13.3) | 1 (4.6) | |
| Diabetes | 0.92 | |||||
| No (%) | 51 (59.3) | 12 (57.1) | 8 (61.5) | 19 (63.3) | 12 (54.6) | |
| Yes (%) | 35 (40.7) | 9 (42.9) | 5 (38.5) | 11 (36.7) | 10 (45.5) | |
| HTN | 0.7 | |||||
| No (%) | 28 (32.6) | 9 (42.9) | 4 (30.8) | 9 (30.0) | 6 (27.3) | |
| Yes (%) | 58 (67.4) | 12 (57.1) | 9 (69.2) | 21 (70.0) | 16 (72.7) | |
| Dyslipidemia | 0.96 | |||||
| No (%) | 37 (43.0) | 9 (42.9) | 5 (38.5) | 14 (46.7) | 9 (40.9) | |
| Yes (%) | 49 (57.0) | 12 (57.1) | 8 (61.5) | 16 (53.3) | 13 (59.1) |
Statistics include number (%) or median (25th and 75th percentiles)
Table 2.
Histological Characteristics of the patient population (n = 86)
| Factor | Number (%) |
|---|---|
| Steatosis | |
| <5% | 31 (36) |
| 5–33% | 30 (35) |
| 34–65% | 14 (16) |
| >=66% | 11 (13) |
| Lobular Inflammation | |
| None | 34 (39) |
| <2 under 20x | 36 (42) |
| 2–4 under 20x | 16 (19) |
| Ballooning | |
| None | 28 (33) |
| Few | 22 (26) |
| Many | 36 (42) |
| Fibrosis | |
| 0 | 51 (61) |
| 1 | 21 (25) |
| 2 | 9 (11) |
| 3 | 3 (4) |
| NAS | |
| 0 | 21 (24) |
| 1 | 10 (12) |
| 2 | 3 (3.5) |
| 3 | 14 (16) |
| 4 | 16 (19) |
| 5 | 9 (10.5) |
| 6 | 5 (6) |
| 7 | 8 (9) |
CK-18 fragments are markedly increased in patients with definitive NASH
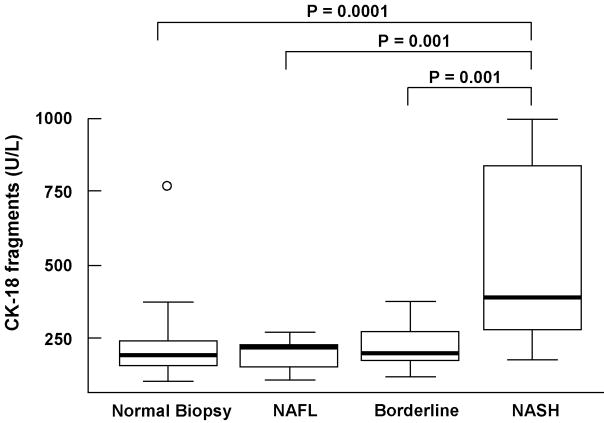
Plasma levels of CK-18 fragments ranged from 103 to 1000 U/L (Median (Q25, Q75): 226 U/L (177, 298)). Compared to either subjects with not NAFLD, NAFL or borderline diagnosis, CK-18 levels were significantly higher in subjects with NASH (median (Q25, Q75): 196 (158, 245) vs. 217 (154, 228) vs. 200 (176, 274) vs. 389 (275, 839), respectively; P<0.0001) (Fig. 1). On the other hand, there was no evidence to suggest a difference in CK-18 levels between subjects without NAFLD, and those with NAFL or borderline diagnosis (P>0.40).
Figure 1. CK-18 fragments in morbid obese patients undergoing bariatric surgery.
Vertical axis is plasma CK-18 levels in U/L and horizontal axis patient groups. The box represents the interquartile range (the 25th and 75th percentiles) from the median (the horizontal line), the bars the 95% confidence interval. CK-18 fragment levels were significantly increased in patients with NASH as compared to patients with normal liver biopsy, NAFL and borderline diagnosis.
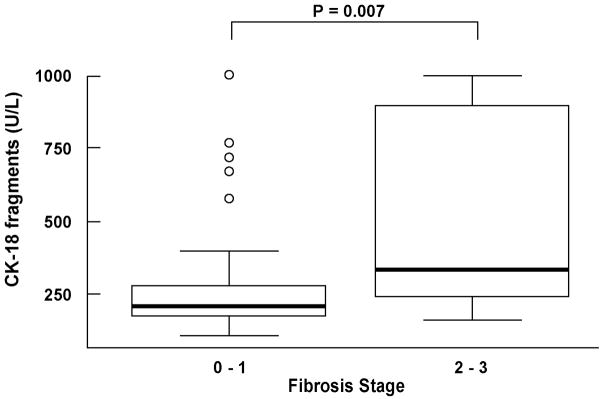
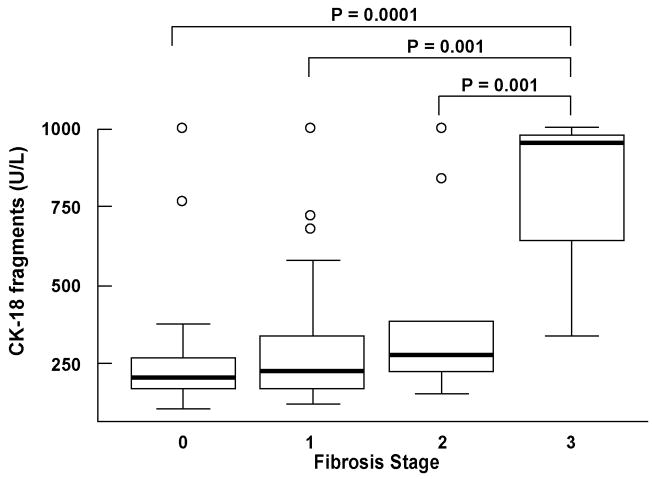
CK-18 fragment levels showed a significant positive correlation with NAS, the individual NAS components, as well as with fibrosis (Table 3). The majority of patients had no or mild fibrosis; nevertheless, CK-18 levels were significantly higher in subjects with moderate to severe fibrosis (stage 2–3) than in those with no or mild fibrosis (stage 0 -1) (median (Q25, Q75): 334.5 (240.5, 896) vs. 207 (175, 275), respectively; P=0.007) (Fig. 2, and Fig. 3). Moreover, we further performed a restricted analysis looking only at patients with NASH or borderline diagnosis and found similar results: CK-18 levels were significantly higher in those patients with borderline diagnosis or NASH with moderate to severe fibrosis than in those with no or mild fibrosis (334.5 (240.5, 896) vs. 234.5 (181.5, 346), respectively; P=0.047).
Table 3.
Correlation Between CK-18 Levels and Histological Characteristics
| Factor | rho | 95% CI | P value |
|---|---|---|---|
| NAS | 0.44 | (0.24,0.63) | <0.001 |
| Steatosis | 0.4 | (0.20,0.60) | <0.001 |
| Lobular Inflammation | 0.45 | (0.25,0.64) | <0.001 |
| Ballooning | 0.33 | (0.12,0.53) | 0.002 |
| Fibrosis | 0.27 | (0.06,0.48) | 0.013 |
Figure 2. CK-18 fragments are increased with the severity of fibrosis on liver biopsy.
Vertical axis is plasma CK-18 levels in U/L and horizontal axis is the grade of fibrosis. The box represents the interquartile range (the 25th and 75th percentiles) from the median (the horizontal line), the bars the 95% confidence interval. CK-18 fragment levels were significantly higher in patients with moderate to severe fibrosis (stage 2–3) compared to those patients with no or mild fibrosis (stage 0–1).
Figure 3. CK-18 fragments positively correlates with stage of fibrosis on liver biopsy.
Vertical axis is plasma CK-18 levels in U/L and horizontal axis is the stage of fibrosis. The box represents the interquartile range (the 25th and 75th percentiles) from the median (the horizontal line), the bars the 95% confidence interval.
CK-18 fragments as an independent predictor of NASH
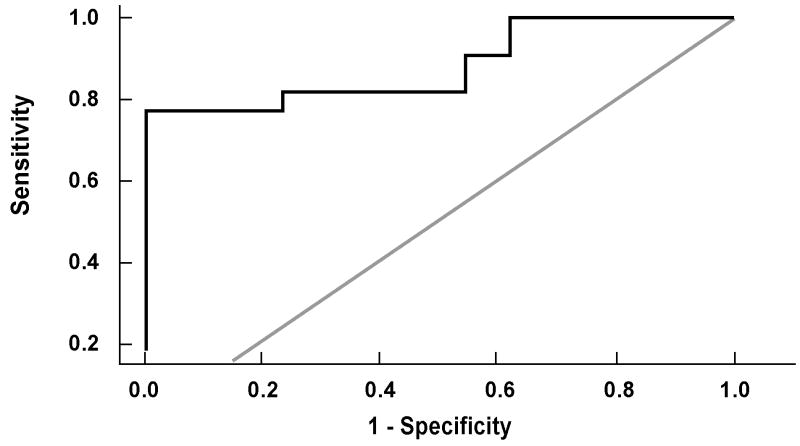
The risk of having definitive NASH on liver biopsy increased with increasing CK-18 fragment levels. For every 50 U/L increase in the plasma level of CK-18, the likelihood of having NASH increased 2.45 times (OR (95% CI): 2.45 (1.20, 5.00)). Using the area under the receiver operating characteristic (ROC) curve approach we next calculated potential cutoff values to separate patients with “definitive NASH” from those with simple steatosis or borderline diagnosis (Fig. 4). The area under the ROC curve was estimated to be 0.88 (95% CI: 0.77, 0.99) and was found to be significantly higher than 0.5 (i.e. better than chance assignment). The values with the best combination of sensitivity and specificity were 252 U/L (sensitivity=82% and specificity=77%) and 275 U/L (sensitivity=77% and specificity=100%). The positive and negative predictive values with a CK-18 level of 252 U/L were 85.7% and 71.4%, respectively, and with a CK-18 level of 275 U/L were 100% and 72.2%, respectively.
Figure 4. CK-18 fragment levels accurately diagnose NASH in morbid obese patients undergoing bariatric surgery.
The area under the ROC curve for NASH diagnosis was estimated to be 0.88 (95% CI: 0.77, 0.99) and was found to be significantly higher than 0.5 (i.e. better than chance assignment). The values with the best combination of sensitivity and specificity were 252 U/L (sensitivity=82% and specificity=77%) and 275 U/L (sensitivity=77% and specificity=100%).
Changes in CK-18 fragment levels following bariatric surgery
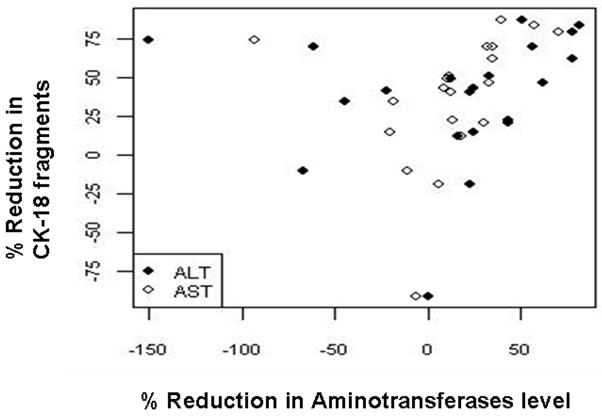
CK-18 fragment levels were measured at 6 months after bariatric surgery in 34 patients (8 with not NAFLD, 5 with NAFL, 11 with borderline diagnosis and 10 with NASH). The baseline and 6-month laboratory and clinical features of these patients are summarized in Table 4. Of the 34 patients, 3 (8.8%) had an increase in CK-18 levels and 31 (91.2%) had a decrease. CK-18 decreases ranged between 13% and 88% of the original value with a median value of 44%. Initial CK-18 fragment concentration was found to be significantly correlated to the percent change in CK-18 fragment levels (rho (95% CI): 0.59 (0.30, 0.88)). Subjects with NASH had a significantly greater decrease in CK-18 values than those without NASH (Median (Q25, Q75): 70 (50, 80) vs. (40 (20, 50); p=0.003). In addition, the percent change in CK-18 fragment levels was positively correlated to changes in both ALT and AST levels (Fig. 5).
Table 4.
CK-18 fragment levels, BMI, AST and ALT values at baseline and 6 month post-surgery
| Baseline | 6 months | |
|---|---|---|
| CK-18 fragment levels | 248 (183, 338) | 133.8 (102.9, 157.5) |
| BMI | 50 (44, 54) | 38.5 (33, 43) |
| ALT | 22 (14, 34) | 13 (10, 24) |
| AST | 21 (16, 30) | 17 (14, 21) |
Statistics include median (25th and 75th percentiles)
Figure 5. CK-18 fragment levels significantly decrease following bariatric surgery and the percent change positively correlates with changes in aminotransferases levels.
A positive correlation between changes in CK-18 fragment levels and changes in activities of ALT and AST is shown. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
DISCUSSION
Obesity is major public health problem worldwide (15) and it is strongly associated with NAFLD, an increasingly recognized form of chronic liver disease that can progress to cirrhosis and end-stage liver disease (16, 17). Morbidly obese patients are a population at particular risk for developing NAFLD (18, 19) and recent studies assessing the histological characteristics of liver biopsies from these patients at the time of bariatric surgery have demonstrated that NAFLD is almost universally present (3, 20–22). Thirty to 50% of these patients may have NASH and close to 50% some degree of fibrosis, while about 10% may have severe fibrosis (3). Moreover, increasing evidence suggests that bariatric surgery is an effective weight loss treatment that rapidly corrects many of the metabolic complications of obesity including NAFLD (10, 23). At the present time, an invasive liver biopsy is the only reliable way to diagnose NASH and assess the severity of liver damage (8). A reliable non-invasive test to not only assess for NASH and disease severity prior to surgery but also allow for frequent monitoring of disease status after bariatric surgery would be of great clinical utility.
Emerging data suggest that hepatocyte apoptosis may be a key component of the “second hit” involved in the progression of NAFLD to the more severe forms of this disease (11, 24–27). A central consequence of the apoptotic process is the activation of the effector caspases (mainly caspase 3) which cleave a number of different substrates inside the cell including cytokeratin 18 (CK-18), the major intermediate filament protein in the liver, resulting in the characteristic morphologic changes of apoptosis. We have demonstrated in a small pilot study using a specific immunoELISA assay that these fragments are strikingly increased in the serum of patients with NASH as compared to both patients with NAFL and normal liver biopsies (13). Using this novel approach in a recent study, we were able to demonstrate that determination of CK-18 fragments in the blood accurately identifies the presence of NASH and the severity of fibrosis on liver biopsy in adult patients with well-characterized NAFLD (12). Our current data extend these observations by demonstrating that determination of CK-18 fragment levels in the blood accurate identifies the presence of NASH and disease severity in morbidly obese patients undergoing bariatric surgery. Using the AUC approach, two cutoff values were identified: the first one to minimize the rate of false positive results (275 U/L) with a specificity of 100% and a sensitivity of 77%, and a second one to minimize the false negative rates (252 U/L) with a specificity of 77% and a sensitivity of 82%. Finally, bariatric surgery resulted in a dramatic decrease in CK-18 levels 6 months following surgery. These changes were greater in those subjects with NASH and positively correlated with changes in transaminases, suggesting that measuring CK-18 fragment levels in the blood may be a useful test to monitor disease status postoperatively. A limitation of the current study in this regard is that we do not have follow up liver biopsies to assess histological changes after surgery. However, previous data (10, 23) clearly demonstrate a dramatic effect of bariatric surgery to achieve profound weight loss, normalize hyperlipidemia, resolve hyperglycemia and improve NAFLD. Therefore, to further assess the utility of this biomarker to monitor disease status following surgery and as a prognostic marker in this population, we are in the process of planning a larger longitudinal prospective study with baseline and 1-year follow up liver biopsies. Thus, the CK-18 test appears to have several unique features that fulfill many of the requirements for an ideal biomarker for NAFLD including that the test is simple, easy to measure and handle, and is reproducible. It not only identifies the presence of NASH but also the risk of associated fibrosis, and it allows for monitoring disease progression over time.
In summary, our findings support the usefulness of this test as a noninvasive NASH biomarker in the care of morbidly obese patients undergoing bariatric surgery.
Acknowledgments
This work was supported by the Cleveland Clinic General Clinical Research Center (M01 RR-018390) and by NIH grant (DK076852) and the AGA Research Scholar Award (RSA) to AEF.
Footnotes
Financial Disclosures: None; no conflict of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Wieckowska A, Feldstein AE. Nonalcoholic fatty liver disease in the pediatric population: a review. Curr Opin Pediatr. 2005;17:636–641. doi: 10.1097/01.mop.0000172816.79637.c5. [DOI] [PubMed] [Google Scholar]
- 3.Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45:600–606. doi: 10.1016/j.jhep.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Kroh M, Liu R, Chand B. Laparoscopic bariatric surgery: what else are we uncovering? Liver pathology and preoperative indicators of advanced liver disease in morbidly obese patients. Surg Endosc. 2007 doi: 10.1007/s00464-007-9351-4. [DOI] [PubMed] [Google Scholar]
- 5.Brunt EM, Tiniakos DG. Pathological features of NASH. Front Biosci. 2005;10:1475–1484. doi: 10.2741/1632. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 8.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and Future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. NAFLD, obesity, and bariatric surgery. Gastroenterology. 2006;130:1848–1852. doi: 10.1053/j.gastro.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 10.Mathurin P, Gonzalez F, Kerdraon O, Leteurtre E, Arnalsteen L, Hollebecque A, Louvet A, et al. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130:1617–1624. doi: 10.1053/j.gastro.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Feldstein AE, Gores GJ. Apoptosis in alcoholic and nonalcoholic steatohepatitis. Front Biosci. 2005;10:3093–3099. doi: 10.2741/1765. [DOI] [PubMed] [Google Scholar]
- 12.Wieckowska A, Lopez AR, Zein NN, McCullough AJ, Feldstein AE. Noninvasive assessment of hepatocyte apoptosis in nonalcoholic fatty liver disease: A multi-center validation study. Gastroenterology. 2007;132:A-729. [Google Scholar]
- 13.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 14.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 15.Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- 16.Machado M, Cortez-Pinto H. Non-alcoholic steatohepatitis and metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2006;9:637–642. doi: 10.1097/01.mco.0000241677.40170.17. [DOI] [PubMed] [Google Scholar]
- 17.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 18.Haynes P, Liangpunsakul S, Chalasani N. Nonalcoholic fatty liver disease in individuals with severe obesity. Clin Liver Dis. 2004;8:535–547. viii. doi: 10.1016/j.cld.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004;71:657–664. doi: 10.3949/ccjm.71.8.657. [DOI] [PubMed] [Google Scholar]
- 20.Moretto M, Kupski C, Mottin CC, Repetto G, Garcia Toneto M, Rizzolli J, Berleze D, et al. Hepatic steatosis in patients undergoing bariatric surgery and its relationship to body mass index and co-morbidities. Obes Surg. 2003;13:622–624. doi: 10.1381/096089203322190853. [DOI] [PubMed] [Google Scholar]
- 21.Harnois F, Msika S, Sabate JM, Mechler C, Jouet P, Barge J, Coffin B. Prevalence and predictive factors of non-alcoholic steatohepatitis (NASH) in morbidly obese patients undergoing bariatric surgery. Obes Surg. 2006;16:183–188. doi: 10.1381/096089206775565122. [DOI] [PubMed] [Google Scholar]
- 22.Dallal RM, Mattar SG, Lord JL, Watson AR, Cottam DR, Eid GM, Hamad G, et al. Results of laparoscopic gastric bypass in patients with cirrhosis. Obes Surg. 2004;14:47–53. doi: 10.1381/096089204772787284. [DOI] [PubMed] [Google Scholar]
- 23.Mattar SG, Velcu LM, Rabinovitz M, Demetris AJ, Krasinskas AM, Barinas-Mitchell E, Eid GM, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610–617. 618–620. doi: 10.1097/01.sla.0000179652.07502.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 25.Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 26.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 27.Feldstein AE, Werneburg NW, Li Z, Bronk SF, Gores GJ. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1339–1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]