Abstract
During the inflammatory response, activation of G-protein coupled receptors (GPCRs) by inflammatory mediators rapidly leads to inhibition of gap junction intercellular communication (GJIC); however, the steps that lead to this inhibition are not known. Combining high-resolution fluorescence microscopy and functional assays, we found that activation of the GPCRs PAR-1 and ETA/B by their natural inflammatory mediator agonists, thrombin and endothelin-1, resulted in rapid and acute internalization of GJs that coincided with the inhibition of GJIC followed by increased vascular permeability. The endocytosis protein clathrin and the scaffold protein ZO-1 appeared to be involved in GJ internalization, and ZO-1 was partially displaced from GJs during the internalization process. These findings demonstrate that GJ internalization is an efficient mechanism for modulating GJIC in inflammatory response.
Keywords: gap junction, GJIC, G-protein coupled receptors, thrombin, endothelin-1, ZO-1
1. Introduction
GJIC is a fundamental function in nearly all tissues and plays important roles in numerous biological processes including tissue homeostasis, growth and differentiation[1,2] and embryonic development.[3] We recently demonstrated that GJ channel internalization can be achieved by at least two distinct processes: first, cells can rapidly internalize small double-membrane annular GJ vesicles from central portions of the plaques;[4] and second, entire GJ plaques, or large portions thereof, can be internalized in a clathrin-mediated endocytosis-like process.[5,6] While the first process most likely accounts for the continuous replenishment of functional GJ plaque channels, we hypothesized that the latter process may be utilized under various physiological and pathological conditions to down-regulate GJIC and to reduce/abolish physical cell-cell contacts.
Van Zeijl et al. [7] subsequently reported a rapid inhibition of Cx43-based GJIC in response to GPCR activation by thrombin and endothelin-1. They further showed that PIP2 hydrolysis was both necessary and sufficient for GJIC inhibition, with no role for the second messengers DAG or IP3; however, they did not investigate how, mechanistically, inhibition was achieved. Since GJIC inhibition was independent of second messengers that were thought to trigger channel closure, we hypothesized that inhibition of GJIC might have been achieved by the internalization of GJ plaques. Here we report that, in primary pulmonary artery endothelial cells (PAECs), activation of the G-protein coupled receptors (GPCRs), PAR-1 and ETA/B, by their natural inflammatory mediator agonists, thrombin and endothelin-1, resulted in a rapid, acute internalization of GJs that led to inhibition of GJIC followed by increased vascular cell permeability. GJ internalization was also achieved when the receptors were stimulated by the wasp toxin mastoparan, a constitutive activator of Gα, and was effectively inhibited when G-protein activation was blocked by suramin. The endocytosis protein clathrin and the scaffold protein ZO-1 appeared to be involved in GJ internalization, and ZO-1 was partially displaced from GJs during the internalization process. These findings lend direct support to our hypothesis that GJ channel internalization is utilized to modulate GJIC under physiological, as well as pathological conditions.
2. Materials and Methods
2.1 Primary Vascular Endothelial Cell Culture
Primary porcine PAECs were isolated from fresh pulmonary artery obtained through a local slaughterhouse. Arteries were transported to the laboratory in ice cold cord buffer (1.68mM CaCl2, 2.5mM Fe(NO3)3*9H2O, 25mM glucose, 25mM HEPES, 18.8mM inositol, 5.4mM KCl, 44mM KH2PO4, 0.8mM MgSO4*7H2O, 120mM NaCl, 4.2mM NaHCO3, 0.34mM Na2HPO4 anhydrous, 1.0% Pen/Strep, 0.04% Fungizone). Endothelial cells were gently scraped off the lumenal surface and immediately transferred to gelatin-coated tissue culture dishes. Cells were grown at 37°C with 5% CO2 in DMEM (10.0% FBS, 1.0% L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin). Cultures contaminated with smooth muscle cells were visually identified and discarded. Endothelial cells stained positively for PECAM.
2.2 HeLa-22 Stable Transfectant Cell Culture
A stable HeLa-22 cell line allowing regulation of Cx43-YFP expression was cultured as previously described. [8] Cx43-YFP expression was induced by addition of 2μg/mL doxycycline (Sigma) 18-24 hours prior to all experiments.
2.3 Immunostaining and Fluorescence Microscopy
Immunostaining and fluorescence microscopy were performed as previously described.[5] See Supplemental Materials and Methods for antibody dilutions.
2.4 Western Blot Analyses
2.5 Receptor Activation Assays
Porcine PAECs or HeLa-22 Cx43-YFP stable transfectant cells were grown to confluence, either on poly-L-lysine-coated coverslips or on 3.5 cm tissue culture dishes. Normal growth medium was supplemented with thrombin (10U/mL, Sigma), endothelin-1 (50 nM, Sigma), mastoparan (240 nM, Sigma), or suramin (240nM, Sigma) for the indicated times prior to fixation or dye transfer assays.
2.6 Scrape-Loading Dye Transfer
Porcine PAECs were grown to confluence on 3.5 cm culture dishes. Incisions through monolayers were made in the presence of 0.5% lucifer yellow (Sigma). See also, Supplemental Materials and Methods.
2.7 Transendothelial Electrical Resistance (TER) Measurements
Porcine PAECs were grown to confluence on 12 mm polyester transwell filters (0.4 μm pore size) (Corning) and TER measurements were carried out at room temperature using a Square Wave Electroporator (model CUY21EDIT; Nepa Gene). After subtracting TER values of empty filters, recorded values were multiplied by the filter area (1.13 cm2) and normalized to baseline recordings of untreated cells. Results were graphed as normalized TER ± SEM.
2.8 Ultrastructural Analyses
Porcine PAECs were fixed in 3.5% glutaraldehyde in 1× PBS for 1 h at room temperature. Electron microscopy was performed as previously described.[5]
2.9 Statistical Analyses
3. Results
3.1 Activation of G-protein coupled receptors by inflammatory mediators stimulates GJ internalization
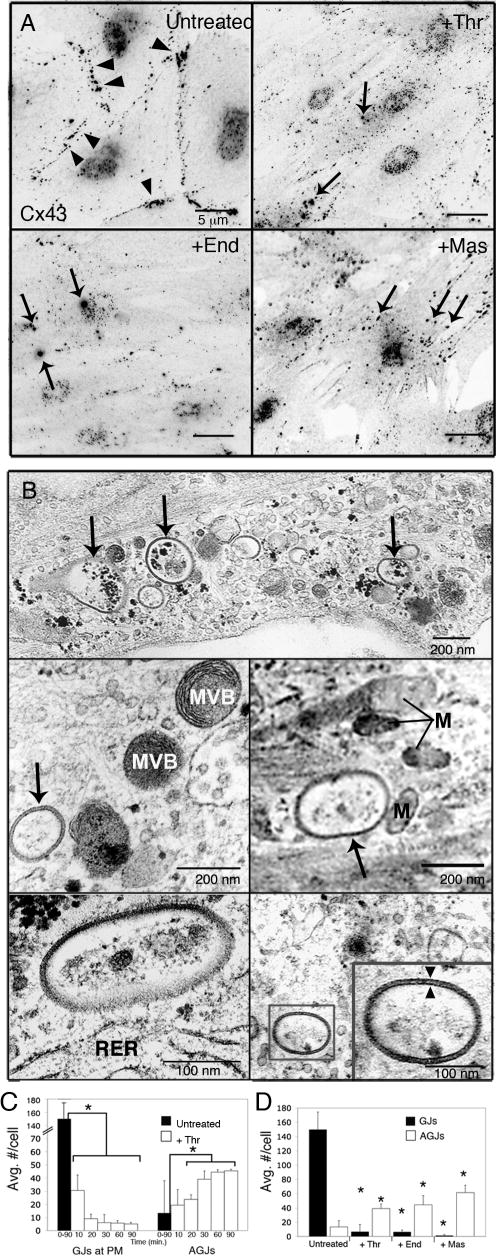
The thrombin PAR-1 receptor and the endothelin-1 receptors, ETA and ETB, are well-characterized GPCRs ubiquitously expressed by vascular endothelial cells [9,10] and it has been reported that activation of these receptors leads to increased endothelial cell permeability and down-regulation of GJIC [11-14]. To investigate the fate of GJs in response to activation of these receptors by their natural inflammatory mediator agonists, primary porcine PAECs were exposed to thrombin and endothelin-1, or the wasp toxin mastoparan, a constitutive activator of Gα. In untreated PAECs expressing endogenous Cx43, immunolabeling for Cx43 revealed the presence of numerous punctate Cx43 GJ plaques at areas of cell-cell apposition (Fig. 1A, Untreated, arrowheads). Activation of GPCRs with thrombin, endothelin-1 or mastoparan resulted in loss of GJs at the plasma membrane within 10 min. of treatment (Fig. 1A, +Thr, +End, +Mas). These observations were accompanied by the appearance of numerous intracellular vesicles with the same intense fluorescence as GJ plaques in treated cells, both near the cell borders and cytoplasmically located (Fig. 1A, arrows). These we recognized as internalized double-membrane GJ vesicles, or annular gap junctions (AGJs), as we have characterized in previous studies.[5,6] Ultrastructural, thin-sectioned analyses of thrombin-treated PAECs by EM confirmed the presence of vesicular cytoplasmic structures near cell borders (Fig. 1B, upper panel, arrows) and localized deep within the cytoplasm in the proximity of multivesicular bodies (Fig. 1B, middle left panel), mitochondria (Fig. 1B, middle right panel) and rough endoplasmic reticulum (Fig. 1B lower left panel). These Cx43-rich vesicles exhibited the double-membrane morphology typical of internalized AGJs (Fig. 1B, lower right panel, see inset).
Figure 1.
Effects of inflammatory mediator activation of GPCRs on GJ localization. Primary porcine PAECs were treated with thrombin (10U/mL), endothelin-1 (50nM) or mastoparan (240nM) for 20 min. (A) Immunofluorescence images (black and white inverted for better visibility) of untreated monolayers showed numerous punctate Cx43-based GJs at cell peripheries (Untreated, arrowheads). Activation of GPCRs with inflammatory mediators, thrombin (+Thr), endothelin-1 (+End) or the wasp toxin, mastoparan (+Mas), resulted in loss of GJ signal at the PM and the appearance of numerous intracellular AGJs (arrows) (B) Ultrastructural analysis of AGJs in thrombin-treated PAECs. AGJs are visible near cell borders (upper panel) and deeper in the cytoplasm (middle and lower panels). AGJs exhibited typical double-membrane morphology (lower right panel, inset, arrowheads). (C) Significant reduction of GJs at the PM was accompanied by a significant increase in AGJs in thrombin-treated PAECs over time (n=45 cells), and (D) after 20-minute incubations with thrombin, endothelin-1 and mastoparan (n=15 cells). Data shown are mean ± SEM; p<0.05 (asterisks). MVB=multivesicular body; M=mitochondria; RER=rough endoplasmic reticulum
Statistical analysis of PAECs treated with thrombin for up to 90 minutes revealed a significant reduction of cell-surface GJs, coupled with a significant increase in cytoplasmic AGJs over time (Fig. 1C). Further statistical analysis showed that when cells were exposed to inflammatory mediators, thrombin and endothelin-1, or the constitutive GPCR activator, mastoparan, for 20 minutes, GJs were significantly reduced by 95.5%, 95.7% and virtually 100% (99.991%), respectively, when compared to untreated PAECs (Fig. 1D). In addition, cells treated for 20 minutes with thrombin contain three times as many AGJs as untreated cells; AGJs are increased more than three times (3.3) in the presence of endothelin-1 and more than four times (4.6) in the presence of mastoparan, all statistically significant increases (Fig. 1D).
3.2 GJ internalization is followed by increased vascular endothelial cell permeability
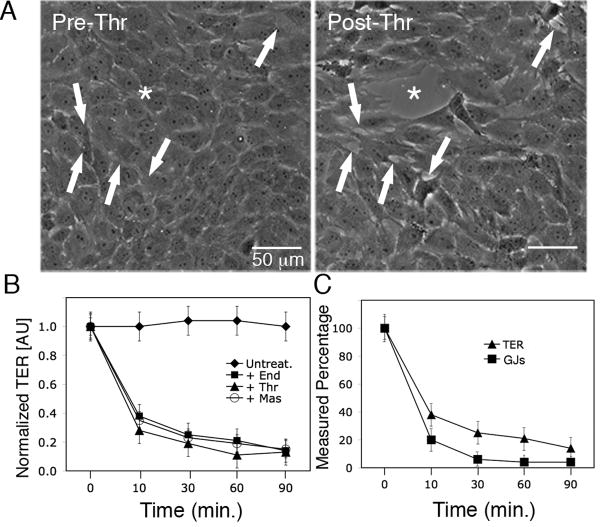
To determine whether the observed GJ internalization resulted in permeability changes in PAECs in response to inflammatory mediators, we measured transendothelial electrical resistance (TER) for up to 90 minutes following activation of GPCRs with endothelin-1, thrombin or mastoparan. Phase contrast images of fully confluent PAEC monolayers incubated with thrombin for 30 minutes showed cells physically separated from one another (Fig. 2A, arrows) and progressive development of visible openings or “gaps” within the monolayer (Fig. 2A, asterisk). Without addition of agonists, TER of fully confluent PAECs remained stable throughout the 90-minute time period (Fig. 2B, Untreated). The cells demonstrated progressive reductions in TER in response to GPCR agonists (Fig. 2B), indicating increasing vascular cell permeability. Overall, during 90-minute incubations with endothelin-1, TER decreased from a mean of 151 ± 4.1 Ω/cm2 to 10.9 ± 7.7 Ω/cm2; with thrombin, TER decreased from a mean of 155.2 ± 17.5 Ω/cm2 to 18.1 ± 6.3 Ω/cm2; and with mastoparan, TER decreased from a mean of 150.3 ± 6.3 Ω/cm2 to 21.1 ± 7.5 Ω/cm2 (See Supplemental Table S1).
Figure 2.
Vascular endothelial cell permeability increases in response to inflammatory mediators. (A) Phase contrast images of a single field of view of a PAEC monolayer pre- and post-thrombin (10U/mL) treatment. Note the development of smaller separations at arrows and a larger “gap” (asterisk) within 30 minutes, as cells physically separated from one another. (B) TER of PAECs was determined over time before and after addition of endothelin-1 (50nM), thrombin (10U/mL) or mastoparan (240nM) Resistance measurements of untreated cells were used to establish a baseline for normalization, and (C) average number of GJs at the PM (n=15 cells) and average TER measurements (n=9) in treated cells were compared to untreated cells and expressed as a percentage. Values shown are mean ± SEM.
When we compared the reduction in number of GJs in the plasma membrane to reduction in TER over time, we found that GJs were reduced by 80% ± 5.9%, while TER was reduced by 62% ± 5.7% over the first 10 minutes following GPCR activation. Over the 30-60-minute time period, GJs were reduced by 96% ± 2.1% and TER was reduced by 75% ± 4.6% over the same time course. The maximum percent reduction in TER measured at 90 minutes post-GPCR activation was 86% ± 6.8% and maximum percent reduction in GJs was 96% ± 3.7% (Figure 2C). These results suggest that GJ internalization precedes the observed increase in endothelial cell permeability.
3.3 GJ internalization coincides with inhibition of GJIC in response to inflammatory mediators
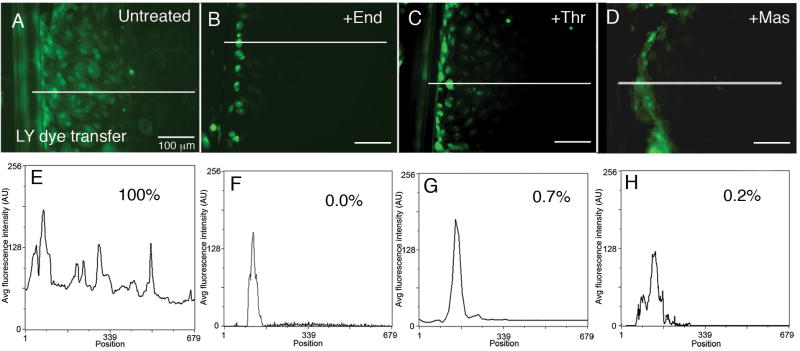
To test the hypothesis that GJIC inhibition is achieved by the internalization of GJ plaques in response to activation of GPCRs, GJIC was assessed by scrape loading dye transfer assays using the GJ-permeable dye, lucifer yellow (LY). In untreated PAECs, within 5 minutes, dye was transferred 10-15 cells distal to the area of the scrape (Fig. 3A). In order to establish a basis for comparison, average fluorescence intensity of LY dye transfer was scanned along a straight line perpendicular to the scrape in untreated cells (Fig. 3E). The area under the curve was measured and was defined as 100% dye transfer. LY dye transfer was then assessed following GPCR activation with endothelin-1 (Fig. 3B), thrombin (Fig. 3C) or mastoparan (Fig. 3D) and average fluorescence intensity was similarly measured (Figs. 3F-H). When dye transfer in the untreated cells was compared to cells exposed to endothelin-1 (Fig. 3F), thrombin (Fig. 3G) and mastoparan (Fig. 3H), dye transfer was reduced to 0.0%, 0.7% and 0.2% respectively, indicating rapid and efficient inhibition of GJIC in response to inflammatory mediators. Figure S1A shows an average of five linescans of dye transfer in treated and untreated cells. When compared to untreated cells, dye transfer, on average, was reduced to <1% following GPCR activation with endothelin-1 (0.14%±0.001), thrombin (0.13%±0.001) or mastoparan (0.17%±0.0002).
Figure 3.
Inhibition of GJIC coincides with GJ internalization in response to GPCR activation by inflammatory mediators. (A-D) GJIC was assessed by scrape loading dye transfer in untreated PAEC monolayers (A); and following 30-min. incubations with 50nM endothelin-1 (B), 10U/mL thrombin (C), or 240nM mastoparan (D). (E-H) Scans of fluorescence intensity measured along lines indicated in A-D. The area under the curve for untreated cells was set as 100% dye transfer, and the areas of the curves for treated cells were expressed as a percentage of untreated control.
3.4 Blocking GPCR activation prevents GJIC inhibition, GJ internalization and increased vascular cell permeability in the presence of inflammatory mediators
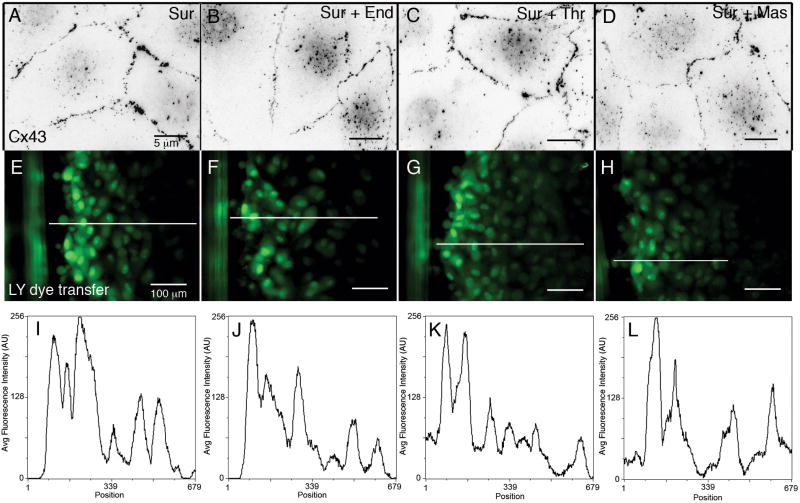
In order to confirm that these results were due to activation of GPCR pathways and to rule out secondary effectors, we chose to block receptor activation with suramin, a polysulfonated naphthylurea which has been described as a GPCR inhibitor that both disrupts association of the Gα subunit and prevents guanine nucleotide exchange.[15,16] We incubated PAECs in serum-free medium supplemented with suramin (240 nM) and repeated our immunofluorescence and functional analyses. Cells treated with endothelin-1, thrombin or mastoparan in the presence of suramin (Figs. 4B-D), and cells treated with suramin alone (Fig. 4A) all had numerous intact GJ plaques at cell-cell contacts when immunolabeled for Cx43 30 minutes after treatment. Moreover, when GJIC was assessed using LY scrape loading dye transfer assays, GJIC was measured at the same levels in cells treated with agonists in the presence of suramin (Figs. 4F-H, J-L) as compared to suramin alone (Figs. 4E,I), and quantified for 5 independent linescans in Fig. S1B. These results lend direct support to our hypothesis that GPCR activation initiates GJ channel internalization.
Figure 4.
Effects of blocking GPCR activation in the presence of inflammatory mediators. (A-D) Immunofluorescence images of Cx43-based GJs showed that neither GJ internalization nor cell separation occurred in PAECs treated with 240nM suramin alone (A), or with suramin plus 50nM endothelin-1 (B), 10U/mL thrombin (C), or 240nM mastoparan (D) for 30 min. (E-H) GJIC was assessed by scrape loading dye transfer in PAECs treated under the same conditions as in A-D. (I-L) Linescans of average fluorescence intensity measured along the lines indicated in E-H.
3.5 The endocytosis coat-protein, clathrin and the scaffold protein, ZO-1 are involved in the internalization of GJs
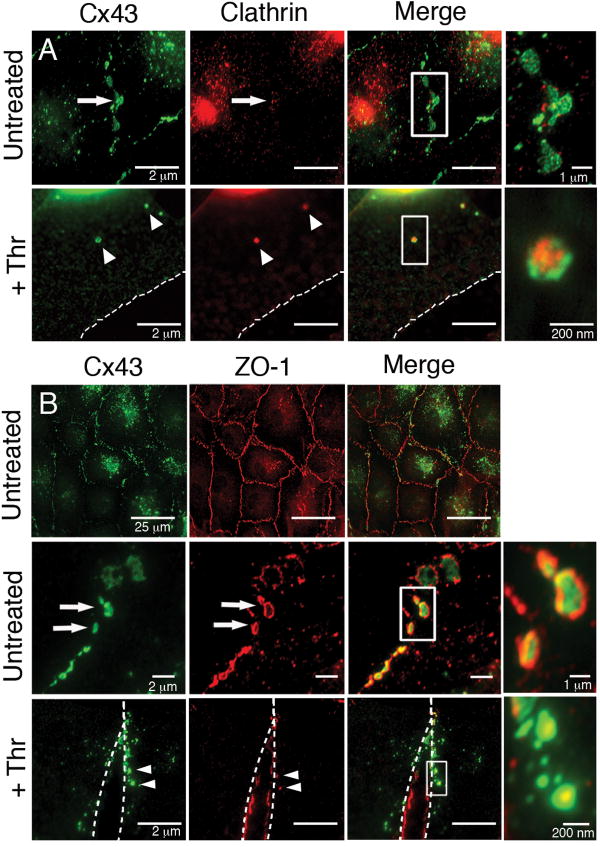
We next turned our attention to identifying the proteins involved in GJ internalization in this pathway. We have previously reported a clathrin-mediated endocytosis-like process for observed GJ internalization in cultured cells.[5,6] We thus investigated whether clathrin may also be involved in GJ internalization in response to activation of GPCRs using immunofluorescence colocalization studies. A patchy distribution of clathrin was detected at Cx43-based GJ plaques in untreated PAECs (Fig. 5A, Untreated, see inset). Following PAR-1 activation with thrombin, numerous intracellular vesicles that we recognized as internalized AGJs according to the criteria described above were colocalized with clathrin (Fig. 5A, +Thr, see inset). Similar results were achieved with endothelin-1 and mastoparan (data not shown). These results support our previous findings and suggest that GJ internalization in response to activation of thrombin and endothelin-1 receptors by their naturally-occurring agonists is a clathrin-mediated process.
Figure 5.
Colocalization of Cx43 with the endocytic coat protein clathrin and the scaffold protein ZO-1. (A) Immunofluorescence colocalization showed a patchy distribution of clathrin (red) at Cx43-based GJ plaques (green) in PAECs (Untreated, arrows; see merged inset) and colocalized with internalized Cx43 AGJs following 20-min. incubations with 10U/mL thrombin (+Thr, arrowheads; see merged inset). (B) Immunofluorescence colocalization showed robust colocalization of ZO-1 (red) at virtually all Cx43-based GJ plaques (green) in untreated PAECs (Untreated, upper panel) particularly at plaque peripheries (Untreated, lower panel, arrows; see merged inset). In PAECs treated 20 min. with thrombin, ZO-1 remained localized at the PM of separating cells (+Thr, dashed outlines), and appeared to be localized preferentially at the center of Cx43 AGJs (+Thr, arrowheads; see merged inset).
Since van Zeijl, et al. (2007)[7] reported that ZO-1 was required for the down-regulation of GJIC they observed in response to thrombin and endothelin-1, we also investigated a possible role for ZO-1 in PAECs by immunofluorescence colocalization studies. In untreated cells, ZO-1 showed robust colocalization at virtually all Cx43-based GJ plaques and appeared to be mainly concentrated at the plaque peripheries as well as at plaque-free areas of PM (Fig. 5B, Untreated, see inset). Upon stimulation with thrombin, endothelin-1 or mastoparan, ZO-1 was displaced from internalizing plaques and remained mostly at the plasma membranes of adjacent cells, but also appeared to be localized at the center of Cx43 AGJs (Fig 5B, +Thr, see inset).
To confirm that ZO-1 is required for inflammatory mediater-induced GJ internalization, we chose to perform a series of experiments in HeLa-22 cells stably expressing Cx43-YFP.[8] It has been described that Cx43 interacts with the second PDZ domain of the ZO-1 protein via the last four amino acids of the extreme C-terminal tail of Cx43.[17,18] It is also known that PDZ binding domains do not bind to internal sequences, but rather to exposed C-terminal tails of proteins that insert into a binding pocket formed by the PDZ domain.[19] Indeed, tagging of the Cx43 C-terminus with myc or GFP abolishes ZO-1 binding,[17,20] presumably because such tags interfere with binding in the pocket. Therefore, we do not expect ZO-1 to bind Cx43-YFP in these cells.
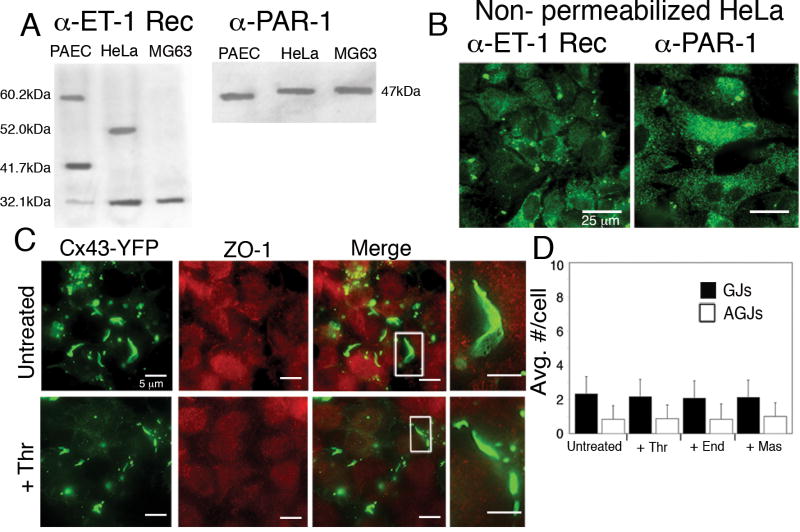
We first confirmed that HeLa-22 stable transfectants expressed both the endothelin-1 (ET-1) receptors and the thrombin PAR-1 receptor by Western blot (Fig. 6A). Note that different ET-1 receptor sub-types are expressed in different cell types. Immunofluorescence imaging of non-permeabilized HeLa-22 cells using antibodies directed against each of the receptors also confirmed the results obtained by Western blot (Fig. 6B).
Figure 6.
ZO-1-Cx43 interaction is required for GJ internalization in response to inflammatory mediators. (A, B) Western blot (A) and immunofluorescence (B) of HeLa-22 cells stably expressing Cx43-YFP confirmed the expression of both endothelin-1 receptors (α-ET-1 Rec) and thrombin receptors (α-PAR-1). (C) Immunofluorescence colocalization of ZO-1 (red) with Cx43-YFP (green) showed no colocalization in untreated HeLa22 cells (Untreated; see merged inset) or in HeLa22 cells treated 20 min. with thrombin (+Thr; see merged inset). (D) No increase in GJ internalization in response to thrombin, endothelin-1 or mastoparan treatment for 20 min. was observed in the absence of ZO-1 binding in HeLa22 cells (n=15 cells). Values shown are mean ± SEM.
Colocalization of Cx43-YFP with ZO-1 was determined by immunofluorescence. Untreated HeLa-22 stable transfectant cells contained numerous, large GJ plaques; however, ZO-1 did not colocalize with Cx43 at the plasma membrane (Fig. 6C, Untreated, see inset). When cells were treated with thrombin, endothelin-1 or mastoparan, localization of both Cx43-YFP and ZO-1 remained separated as in untreated cells and no increase in GJ internalization was observed (Fig. 6C, +Thr). As expected, due to the lack of ZO-1 binding, GJs were larger in size than in PAECs; and thus, GJs and AGJs were fewer in number than in PAECs. When untreated HeLa-22 stable transfectant cells were compared to those treated with thrombin, endothelin-1 or mastoparan for up to 30 minutes, statistical analyses revealed no significant changes in either number of GJs or number of AGJs present in treated cells over time (Fig. 6D). These results strongly suggest that ZO-1-Cx43 interaction is required for GJ internalization in this pathway.
4. Discussion
Although the signaling pathways that control GJ internalization are poorly understood, the phenomenon seems to be widespread. Since the late 1970s, ultrastructural analyses have identified AGJs in diverse tissue types including chick otocyst epithelium,[21] rabbit granulosa cells,[22] and equine epidermis.[23] GJ internalization is also frequently seen in cardiovascular pathologies including cardiomyopathies and cardiac ischemic events.[24-26] In the present study, we investigated the fate of GJs in response to inflammatory mediators in vascular endothelial cells. Following GPCR activation with inflammatory mediators, primary PAECs began to internalize GJs within minutes, followed by increased endothelial cell permeability as assessed by decreased TER (Figs. 1A and 2). Upon GPCR activation, the average number of GJ plaques at the plasma membrane was reduced from approx. 150 to 7 (thrombin), 6 (endothelin-1) and 1 (mastoparan) within 20 min. after treatment. Simultaneously, the average number of cytoplasmically localized AGJs significantly increased from approx. 13 (in untreated cells) to 39, 44 and 62 respectively (Fig. 1D). While the number of AGJs counted in treated cells was lower than the equivalent number of previously present GJ plaques, it is likely that (I) smaller internalized plaques were excluded due to our stringent criteria for counting AGJs, and (II) that some AGJs were already degraded at the time AGJs were counted. In addition, our functional assays confirmed complete inhibition of GJIC concomitant with the observed increase in internalization of GJs (Fig. 3). It is important to note that our ultrastructural EM analyses showed internalized GJ vesicles that often were localized deep within the cytoplasm (Fig. 1B, middle and bottom panels). This would tend to rule out the possibility that these structures are tubular invaginations of PM, since such structures would, necessarily, be very large (>2μm). Moreover, tubular GJ invaginations would also be detectable by immunofluorescence and these structures were never observed.
Although GJ plaques were significantly reduced within 10 minutes of GPCR activation, on average, approximately 6 GJ plaques per cell still remained following 20-minute incubations with thrombin and endothelin-1 (Fig.1D). Interestingly, those GJ channels that remained at cell contact areas were impermeable, as indicated by our functional dye transfer assays (Fig. 3) suggesting that channels were closed. Given that there is some evidence that Cx43 phosphorylation by c-Src plays a role in regulating GJIC in response to GPCR activation,[14,27] it is plausible that channel closure via Cx phosphorylation either precedes or occurs simultaneously with initiation of GJ internalization.
It is known that cell contractility via actomyosin cross-bridging contributes to increased vascular endothelial cell permeability in response to inflammatory mediators;[11,28] however, to our knowledge, we are the first to provide evidence that GJs are internalized prior to cell retraction. Our data support that three events occur in sequence in PAECs in response to GPCR activation by inflammatory mediators: (1) rapid channel closure (within 1-2 minutes);[7] (2) GJ internalization (within 10-20 minutes)(Figs. 1A,C,D and 2C); (3) followed by cell retraction (within 20-30 minutes)(Fig. 2A-C). In addition, when receptor activation was blocked with suramin, all three downstream events (GJIC inhibition, GJ internalization and increased vascular permeability) were prevented (Fig. 4) indicating that GPCR activation initiates the process.
Our previous studies have elucidated a method for GJ internalization that involves the clathrin-mediated endocytosis machinery.[5,6] In the present study, we found that, in PAECs, clathrin colocalized with endogenous Cx43-based GJ plaques and AGJs (Fig 5A), indicating that clathrin is involved in this pathway as well. Ongoing studies will elucidate whether additional clathrin-mediated endocytosis proteins are also involved.
It has been reported that Cx43-ZO-1 interactions regulate GJ plaque size, and changes in Cx43-ZO-1 interactions have been observed in diverse cell types, suggesting a role for ZO-1 in GJ internalization.[29-32] In untreated PAECs, we observed patterns of Cx43-ZO-1 colocalization that were strikingly similar to those observed in cardiomyocytes by Hunter, et al.[31] (Fig. 5B, Untreated). Notably, when GJs were internalized in response to thrombin or endothelin-1, ZO-1 appeared to be localized in the center of AGJ vesicles, but not on their outer surface (Fig. 5B, +Thr; see merged inset). Recently, we and our collaborators reported a novel role for ZO-1 in GJ internalization in response to the non-genomic carcinogen lindane in 42GPA9 Sertoli cells. There, it was shown that ZO-1 was displaced from that side of the GJ plaque into which the plaque was internalized, and that this mechanism was c-Src dependent.[33] Our results here support a similar pattern of ZO-1 displacement in response to inflammatory mediators in PAECs and future experiments will determine whether c-Src is involved in this pathway as well.
In contrast, in Cx43-YFP-expressing cells, GJ plaque size was markedly increased, with no detectable Cx43-ZO-1 colocalization (Fig. 6C,D). These results support previous reports that tagging Cx43 with fluorescent proteins abolishes ZO-1 binding and increases GJ plaque size.[20] In these cells, no increase in GJ internalization was observed in response to activation of GPCRs (Fig. 6D), providing further evidence that Cx43-ZO-1 binding plays a role in this pathway. Interestingly, it is known that GJ internalization can occur in the absence of ZO-1 binding, as we and others have observed;[4-6,31,34] however, our observations here report, for the first time, a novel role for ZO-1 in mediating GJ internalization in response to inflammatory mediator-induced activation of GPCRs.
Supplementary Material
Table S1. Effect of inflammatory mediators on PAEC transendothelial electrical resistance (TER). TER of PAECs was measured over time following exposure to 50nM endothelin-1 (End), 10U/mL thrombin (Thr) or 240nM mastoparan (Mas). Resistance measurements are expressed in Ω/cm2. Values shown are mean ± SEM. For all measurements, p<0.05 vs. untreated (n=3 independent measurements).
Figure S1. GJIC inhibition in response to GPCR activation is effectively prevented when receptor activation is blocked. Comparison of lucifer yellow dye transfer as assessed by linescans in PAECs treated with 50nM endothelin-1 (+End), 10U/mL thrombin (+Thr) or 240nM mastoparan (+Mas) (A) show that GJIC is restored to virtually 100% when receptor activation is blocked with the Gα inhibitor suramin (240nM) (B). Values shown are expressed as mean ± SEM; p<0.05 (asterisks) (n=5 linescans).
Acknowledgments
We thank M.K. Iovine and A. Hoptak-Solga for providing equipment and training for TER measurement; L. Lowe-Krentz and D. Kanyi for providing initial instruction in the harvesting and culturing of primary cell cultures used in these experiments; and P. Baker for providing training on Adobe Photoshop.
Sources of Funding: Supported by NIH, National Institute of General Medical Sciences Grant GM-55725 and the Bioengineering and Bioscience 2020 Funds.
Abbreviations
- AGJ
annular gap junction
- Cx43
connexin43
- GJ
gap junction
- GJIC
gap junction intercellular communication
- GPCR
G-protein coupled receptor
- PAECs
pulmonary artery endothelial cells
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mehta PP, Bertram JS, Loewenstein WR. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986;44:187–96. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- 2.Loewenstein WR, Rose B. The cell-cell channel in the control of growth. Semin Cell Biol. 1992;3:59–79. doi: 10.1016/s1043-4682(10)80008-x. [DOI] [PubMed] [Google Scholar]
- 3.Guthrie SC, Gilula NB. Gap junctional communication and development. Trends Neurosci. 1989;12:12–6. doi: 10.1016/0166-2236(89)90150-1. [DOI] [PubMed] [Google Scholar]
- 4.Falk M, Baker SM, Segretain D, Buckheit RW., III Gap junction turnover is achieved by the release of small endocytic double-membrane vesicles that coincides with channel accrual. PNAS. 2008 doi: 10.1091/mbc.E09-04-0288. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM. Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell. 2007;18:337–47. doi: 10.1091/mbc.E06-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gumpert A, Varco JS, Baker SM, Piehl M, Falk MM. Double-membrane gap junction internalization requires the clathrin-mediated endocytic machinery. FEBS Lett. 2008;582:2887–92. doi: 10.1016/j.febslet.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Zeijl L, et al. Regulation of connexin43 gap junctional communication by phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2007;177:881–91. doi: 10.1083/jcb.200610144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci U S A. 2002;99:10446–51. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Major CD, Santulli RJ, Derian CK, Andrade-Gordon P. Extracellular mediators in atherosclerosis and thrombosis: lessons from thrombin receptor knockout mice. Arterioscler Thromb Vasc Biol. 2003;23:931–9. doi: 10.1161/01.ATV.0000070100.47907.26. [DOI] [PubMed] [Google Scholar]
- 10.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 11.Bogatcheva NV, Garcia JG, Verin AD. Molecular mechanisms of thrombin-induced endothelial cell permeability. Biochemistry. 2002;67:75–84. doi: 10.1023/a:1013904231324. [DOI] [PubMed] [Google Scholar]
- 12.D'Hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest Ophthalmol Vis Sci. 2007;48:120–33. doi: 10.1167/iovs.06-0770. [DOI] [PubMed] [Google Scholar]
- 13.Kawamura H, Oku H, Li Q, Sakagami K, Puro DG. Endothelin-induced changes in the physiology of retinal pericytes. Invest Ophthalmol Vis Sci. 2002;43:882–8. [PubMed] [Google Scholar]
- 14.Spinella F, Rosano L, Di Castro V, Nicotra MR, Natali PG, Bagnato A. Endothelin-1 decreases gap junctional intercellular communication by inducing phosphorylation of connexin 43 in human ovarian carcinoma cells. J Biol Chem. 2003;278:41294–301. doi: 10.1074/jbc.M304785200. [DOI] [PubMed] [Google Scholar]
- 15.Chung WC, Kermode JC. Suramin disrupts receptor-G protein coupling by blocking association of G protein alpha and betagamma subunits. J Pharmacol Exp Ther. 2005;313:191–8. doi: 10.1124/jpet.104.078311. [DOI] [PubMed] [Google Scholar]
- 16.Stein CA, LaRocca RV, Thomas R, McAtee N, Myers CE. Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol. 1989;7:499–508. doi: 10.1200/JCO.1989.7.4.499. [DOI] [PubMed] [Google Scholar]
- 17.Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–4. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 18.Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem. 1998;273:12725–31. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- 19.Songyang Z, et al. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–7. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 20.Hunter AW, Jourdan J, Gourdie RG. Fusion of GFP to the carboxyl terminus of connexin43 increases gap junction size in HeLa cells. Cell Commun Adhes. 2003;10:211–4. doi: 10.1080/cac.10.4-6.211.214. [DOI] [PubMed] [Google Scholar]
- 21.Ginzberg RD, Gilula NB. Modulation of cell junctions during differentiation of the chicken otocyst sensory epithelium. Dev Biol. 1979;68:110–29. doi: 10.1016/0012-1606(79)90247-1. [DOI] [PubMed] [Google Scholar]
- 22.Larsen WJ, Hai N. Origin and fate of cytoplasmic gap junctional vesicles in rabbit granulosa cells. Tissue Cell. 1978;10:585–98. doi: 10.1016/s0040-8166(16)30351-2. [DOI] [PubMed] [Google Scholar]
- 23.Leach D, Oliphant L. Annular gap junctions of the equine hoof wall. Acta Anat. 1983;116:1–9. doi: 10.1159/000145719. [DOI] [PubMed] [Google Scholar]
- 24.Beardslee MA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000;87:656–62. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 25.Saffitz JE. Dependence of electrical coupling on mechanical coupling in cardiac myocytes: insights gained from cardiomyopathies caused by defects in cell-cell connections. Ann N Y Acad Sci. 2005;1047:336–44. doi: 10.1196/annals.1341.030. [DOI] [PubMed] [Google Scholar]
- 26.Saffitz JE, Laing JG, Yamada KA. Connexin expression and turnover : implications for cardiac excitability. Circ Res. 2000;86:723–8. doi: 10.1161/01.res.86.7.723. [DOI] [PubMed] [Google Scholar]
- 27.Postma FR, Hengeveld T, Alblas J, Giepmans BN, Zondag GC, Jalink K, Moolenaar WH. Acute loss of cell-cell communication caused by G protein-coupled receptors: a critical role for c-Src. J Cell Biol. 1998;140:1199–209. doi: 10.1083/jcb.140.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131–42. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]
- 29.Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res. 2002;90:317–24. doi: 10.1161/hh0302.104471. [DOI] [PubMed] [Google Scholar]
- 30.Duffy HS, Ashton AW, O'Donnell P, Coombs W, Taffet SM, Delmar M, Spray DC. Regulation of connexin43 protein complexes by intracellular acidification. Circ Res. 2004;94:215–22. doi: 10.1161/01.RES.0000113924.06926.11. [DOI] [PubMed] [Google Scholar]
- 31.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–98. doi: 10.1091/mbc.E05-08-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segretain D, Fiorini C, Decrouy X, Defamie N, Prat JR, Pointis G. A proposed role for ZO-1 in targeting connexin 43 gap junctions to the endocytic pathway. Biochimie. 2004;86:241–4. doi: 10.1016/j.biochi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Gilleron J, Fiorini C, Carette D, Avondet C, Falk M, Segretain D, Pointis G. Molecular reorganization of the connexin 43, Zonula occludens-1 and c-Src complexes during gap junction plaque endocytosis in response to a non genomic carcinogen. J Cell Science. 2008 doi: 10.1242/jcs.033373. in press. [DOI] [PubMed] [Google Scholar]
- 34.Jordan K, Chodock R, Hand AR, Laird DW. The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci. 2001;114:763–73. doi: 10.1242/jcs.114.4.763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effect of inflammatory mediators on PAEC transendothelial electrical resistance (TER). TER of PAECs was measured over time following exposure to 50nM endothelin-1 (End), 10U/mL thrombin (Thr) or 240nM mastoparan (Mas). Resistance measurements are expressed in Ω/cm2. Values shown are mean ± SEM. For all measurements, p<0.05 vs. untreated (n=3 independent measurements).
Figure S1. GJIC inhibition in response to GPCR activation is effectively prevented when receptor activation is blocked. Comparison of lucifer yellow dye transfer as assessed by linescans in PAECs treated with 50nM endothelin-1 (+End), 10U/mL thrombin (+Thr) or 240nM mastoparan (+Mas) (A) show that GJIC is restored to virtually 100% when receptor activation is blocked with the Gα inhibitor suramin (240nM) (B). Values shown are expressed as mean ± SEM; p<0.05 (asterisks) (n=5 linescans).