Abstract
The cortical subventricular zone (SVZ), a proliferative compartment in the forebrain, has a uniquely important role during the second half of intrauterine development in human. This is best observed in numerous neonatal pathologies that result from prenatal SVZ damage. These conditions highlight a need to better understand the contribution of the SVZ to the development of the human cerebral cortex. With this goal in mind, we analyze histological organization, cell proliferation, and molecular diversity in the human fetal SVZ, from 7 to 27 gestational weeks (gw), using light and electron microscopy, immunohistochemistry, and in vitro methods. Complex histological organization distinguishes human cortical SVZ from that of other mammals. In vitro quantification showed that approximately 50% of cells in the VZ/SVZ region are neurons, 30% are astroglia, 15% are nestin+ cells, with other cell types representing smaller fractions. Immunolabeling with BrdU, showed that a considerable number of cells (approximately 10%) are generated in the human cortical SVZ during midgestation (18–24gw), in in vitro conditions. Immunofluorescence with cell type specific markers and BrdU revealed that all major cell types, neural precursors (nestin+), astroglia including radial glia (GFAP+, vimentin+), and oligodendrocyte progenitors (PDGFR-α+), were proliferating. An increase in the ratio of the size of the SVZ to VZ, protracted period of cell proliferation, as well as cellular and histological complexity of the human fetal SVZ, are directly related to the evolutionary expansion of the human cerebral cortex.
Keywords: organotypic slice culture, cortical interneurons, Dlx, Nkx2.1, proliferation, migration
INTRODUCTION
One of the main characteristics of the developing CNS is that the majority of neural cells (neurons and macroglia) originates in the proliferative region near the lumen of the cerebral ventricle, the ventricular zone (VZ), and then migrates to their final destinations. In the human embryonic telencephalon (5–6 gestational weeks, gw), the VZ is the only proliferative zone. At 7–8 gw, the cortical plate appears, and the secondary proliferative zone, designated as the subventricular zone (SVZ), forms above the VZ (Cajal, 1911; Boulder Committee, 1970; Sidman and Rakic, 1982; Zecevic, 1993). In this zone, proliferation continues for the next several months, to the end of 40 gw long intrauterine period. The substantial development of the cerebral cortex in the second half of gestation in primates is mainly related to cell proliferation in the SVZ. Two classes of cortical neurons, subplate and interneurons, continue to be generated during that time. Formation of the transient subplate layer, which achieves maximal size in the primate forebrain (Kostovic and Rakic, 1990), is mainly due to cell proliferation in the SVZ (Smart et al., 2002). Moreover, in contrast to rodents, where almost all cortical interneurons originate in the ganglionic eminence (Anderson et al., 1997; rev. Marin and Rubenstein, 2001), a subpopulation of interneurons might originate locally, in the cortical VZ/SVZ in the human brain (Letinic et al., 2002; Rakic and Zecevic, 2003a; Filipovic and Zecevic, 2004). In addition, two types of macroglia, oligodendrocytes and astrocytes (Back et al., 2001; Rakic and Zecevic, 2003b; Zecevic, 2004; Jakovcevski and Zecevic, 2005), as well as stem-like cells (Flax et al., 1998; Vescovi et al., 1999; Ourednik et al., 2001) are generated and reside in the human fetal SVZ. Simultaneous proliferation, migration, and differentiation of these various cell types within a confined space, provide opportunities for interaction that are not yet fully understood.
The remains of this proliferative zone persist as the subependymal zone, a source of stem-like cells, that contribute to the repair processes of the CNS in the adult brain (e.g., Reynolds and Weiss, 1992, Sanal et al., 2004).
In clinical practice, the cortical SVZ is an important site of common neonatal pathology typically observed in premature babies and neonates (Back et al., 2001; Volpe, 1997, 2001). Abnormal myelination in the forebrain observed in periventricular leukomalacia (PVL) may be related, in part, to selective targeting of oligodendrocyte progenitors by the insult (Volpe, 1997; Back et al., 2001, 2002), whereas cognitive disorders after hypoxic-ischemic insult may be related to selective damage of subplate neurons (McQuillen et al., 2003). Increased cell proliferation, or altered rate of cell death in the SVZ region, may result in childhood tumors (Lewis, 1968).
In addition to its clinical importance, the cellular organization of the SVZ in primates is significantly different than in other animals (Smart et al., 2002). In the human brain, due to its larger size and prolonged development, these differences are more pronounced. Indeed, compared to other mammals, the cortical SVZ increases during evolution and achieves its peak in size and complexity in humans. This prompted us to study the complex cellular and molecular organization of the human fetal SVZ, with emphasis on its contribution to the cerebral cortex.
MATERIALS AND METHODS
Human embryos and fetuses, ranging in age from 7 to 27 gestation weeks (gw), were obtained from abortions and autopsies performed at the University of Belgrade, Serbia and Montenegro, as well as from the Brain Bank, The Albert Einstein College of Medicine, Bronx, NY, USA. Tissue was collected with proper consent and with the approval of the Ethics Committees. Ultrasonic and neuropathological examination found no evidence of disease or developmental abnormalities. The time between collected tissue and fixation was on average 5–15 minutes. The age of embryos and fetuses was estimated on the basis of weeks after ovulation, crown-rump length (Olivier and Pineau, 1962), and anatomical landmarks (O'Rahilly et al., 1987).
Immunofluorescence
Brain blocks were fixed in 4% paraformaldehyde in 0.1M phosphate buffer, pH 7.4, for 8–12 hrs., cryoprotected by immersion in 30% sucrose in 0.1M phosphate buffer (PBS), and frozen in isopentane cooled up to −70°C. Frozen brain blocks were serially sectioned in either coronal or sagittal planes at 14µm, and collected on poly-L-lysine-coated slides. Alternating sections were processed for immunocytochemistry, or stained with Toluidine Blue (Sigma, St. Louis, MO, USA).
Frozen sections were incubated for 30 minutes in blocking serum (1% bovine serum albumin, 5% normal goat serum, and 0.5% Tween in PBS buffer), followed by incubation with appropriate markers or antibodies overnight at room temperature (Table 1). The next day secondary antibodies conjugated with either Fluorescein or Rhodamine (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), were applied for 2 hrs. and sections were counterstained with bisbenzamide (Sigma) to label cell nuclei necessary for quantitative analysis. Sections were viewed and analyzed with a conventional fluorescent microscope (Leitz, Orthoplane 2, Wetzler, Germany) or a confocal laser-scanning microscope (Carl Zeiss, LSM 410). Pictures were adjusted for brightness and contrast with PhotoShop program 6.0 (Adobe Systems, CA, USA). We used controls for antibody specificity described earlier (Saper and Sewchenko, 2003). First, some primary antibodies were tested by Western blot analysis, and only a band at the appropriate molecular weight was demonstrated. Second, incubation of sections only with secondary antibodies resulted in a lack of immunostaining. We also relied on data from the literature that used the same antibodies and showed their specificity (Table 1). In addition, in our double labeling studies antibodies specific for one cell class, such as neurons for example, labeled cells that by their morphology and size corresponded to neurons, and did not label other classes of cells such as oligodendrocytes, radial glia, astrocytes, or microglia.
Table 1.
Primary antibodies used in this study
| Name/type | Labels | Reference | Dilution | Manufacturer | Catalog # |
|---|---|---|---|---|---|
| RADIAL GLIA | |||||
| BLBP-rabbit IgG | Brain lipid binding protein | Feng et al., 1994 | 1:2000 | Gift from Dr. Heintz, Rockefeller University | |
| GFAP-rabbit IgG | Glial fibrillary acidic protein | Bignami and Dahl, 1974 | 1:400 | DAKO, Carpinteria, CA | Z0334 |
| 4A4 mouse IgG2b | Intermediate filament proteins phosphorylated by cdc2 kinase | Kamei et al., 1998 | 1:2000 | MBL, Nagoya, Japan | D076-3 |
| Vimentin-mouse IgG | Intermediate filament potein | Dahl et al.,1981 | 1:50 | Sigma, St. Lois, MO | V6630 |
| NEURONS | |||||
| Glutamate-rabbit IgG | Glu-KLH, Glu-BSA, KLH, L-glutamine, Asp-Glu, Gly-Glu | Cotman and Iversen, 1987 | 1:1000 | Sigma, St. Lois, MO | G6642 |
| SMI-31 mouse IgG | Phosphorylated form of neurofilament protein | Sternberger and Sternberger, 1983 | 1:1000 | Sternberger Monoclonals, Baltimore, MD | SMI-31 |
| Tbr1 rabbit IgG | T-box transcription fctor | Hevner et al., 2001 | 1:1000 | Gift from Dr. Hevner, Univ. Washington Sch. of Med. | |
| β-III-tubulinmouse IgG | Carboxyterminal sequence on human β-tubulin isotype III | Lee et al., 1990 | 1:100 | Sigma, St. Lois, MO | T8660 |
| β-III-tubulinrabbit IgG | Carboxyterminal sequence on human β-tubulin isotype III | 1:100 | Covance Research, Berkley, CA | PRB-435P | |
| PSA-NCAM mouse IgM | Polysialic acid neuronal cell adhesion molecule | Walsh and Doherty, 1993 | 1:100 | Becton Dickinson, San Jose, CA | 556325 |
| INTERNEURONS | |||||
| Calretinin rabbit IgG | Calretinin | Schwaller et al., 1993 | 1:2000 | Swant, Belliziona, Switzerland | 7696 |
| GABA mouse IgG | γ-amino butyric acid | Zecevic and Milosevic, 1997 | 1:1000 | Sigma, St. Lois, MO | A 0310 |
| STEM CELLS | |||||
| Nestin mouse IgG1 | Human nestin fragment (amino acid residues 618–1618) | Hockfield and McKay, 1985 | 1:500 | Neuromics, Northfield, MN | MO15012 |
| MICROGLIA | |||||
| Tomato lectin | Glycoconjugate residues on cell surface of microglia | Andjelkovic at al., 1998 | 1:100 | Vector Laboratories, Burlingame, CA | B-1175 |
| OLIGODENDROCYTE PROGENITOR CELLS | |||||
| PDGFR-α-rabbit IgG | Transmembrane glycoprotein | Pringle et al., 1992 | 1: 500 | Gift from Dr. Stallcup | |
| TRANSCRIPTION FACTORS | |||||
| Dll (pan Dlx)- rabbit IgG | Dll transcription factors | Kohtz et al., 2001 | 1:75 | Gift from Dr. Kohtz | |
| Nkx 2.1-rabbit IgG | Thyroid transcription factor 1 (known as TTF-1 and Nkx2.1) | Anderson et al., 1997 | 1:500 | BIOPAT Immunotech., Piedimonte Matese, Italy | PA0100 |
| S-PHASE MARKER | |||||
| BrdU-mouse IgG | Bromodeoxyuridine | Gratzner, 1982 | 1:50 | Becton Dickinson, San Jose, CA | B2531 |
| BrdU-sheep IgG | Bromodeoxyuridine | 1:200 | Fitzgerald, Concord, MA | 20-BS17 | |
| S, G2, M PHASE MARKERS | |||||
| Ki-67-rabbit IgG | Ki67, nuclear protein in the proliferating cells | Kill, 1996 | 1:500 | ANASPEC Inc., San Jose, CA | 29567 |
Light and electron microscopy
Tissue blocks from the lateral telencephalic wall of embryos (7–9 gw), or frontal and occipital poles from fetuses (15–27 gw) were dissected, fixed by immersion in mixed gluteraldehyde/paraformaldehyde fixative, and embedded either in paraffin or Epon. Other details of light and electron microscopic procedures were described previously (Zecevic, 1993, 1998). Light microscopy was done either using toluidine blue stained l µm thick Epon embedded sections, or 8 µm thick paraffin sections stained with cresyl violet or Nissl stain.
In vitro methods
Brain tissue collected from fetuses at midgestation (18–24 gw) was placed in a medium containing modified Hank’s Balanced Salt Solution (HBSS) (Sigma, St. Louis, MO), 2 mM glutamine (Invitrogen, Carlsbad, CA), 10 mM HEPES buffer (Sigma, St. Louis, MO), 20 µg/ml gentamycine sulfate, and shipped on ice. Upon arrival, the tissue was immediately used for dissociated cell cultures or organotypic slice cultures.
Organotypic slice cultures
Frontally cut fetal hemispheres were used to obtain tissue blocks (1×1×0.5 cm) containing the VZ/SVZ. These blocks were embedded in 3% low-melted agarose (Invitrogen), and cut into 400 µm-thick slices using a Vibroslice (World Precision Instruments). Slices were transferred onto 30mm membrane inserts (0.4µm porosity, Millipore Corp, Bedford, MA), and placed in 6-well plates containing Dulbecco’s modified eagle medium (DMEM) (Sigma) with 0.24% D-glucose, 2mM L-glutamine, N2 (Invitrogen), 5% fetal bovine serum (FBS). Using the interface technique (Stoppini et al., 1991), slices were kept in a humidified incubator at 37°C with 5% CO2 for three days. Cell proliferation in slice culture was studied by incorporation of BrdU (see bellow), upon which slices were fixed in the 4% paraformaldehyde for 6 hrs., cryoprotected with 30% sucrose over night, and frozen at −70°C. Each slice was re-sectioned on cryocut into 14–20 µm thick sections.
Acutely dissociated cell culture
Acute cell cultures (n=6) of the VZ/SVZ region at midgestation were established according to a modified method of Hartfuss et al. (2001).
Human fetal brains kept in HBSS were cut into approximately 1cm coronal slices, and meninges were carefully discarded. A tissue band approximately 2000µm above the VZ surface, cut to include the SVZ, was enzymatically degraded at 37°, in 1.3mg/ml trypsin-EDTA (Invitrogen) and 0.67mg/ml hyaluronidase (Sigma), triturated through a fire-polished pipette coated with fetal calf serum (FCS, Sigma), resuspended in serum-free defined growth medium containing 5µg/ml of insulin, 100µg/ml of transferrin, 20nM progesterone, 100µM putrescine and 30 nM selenium (Bottenstein and Sato, 1979), and seeded into four or eight well chamber slides pre-coated with poly-L-lysine at a concentration of 1 × 105 cells/ml or 4 × 104 cells/ml, respectively. Cells were incubated at 37° with 5% CO2 for 4–6 h. and then fixed in 4% paraformaldehyde for 20 min. and processed for immunohistochemistry (see bellow). The total number of viable cells, assessed by trypan blue exclusion, was 90.8±8.5%.
BrdU incorporation
Thymidine analogue, BrdU (5-bromodeoxyuridine) in a dose of 50µM was added to organotypic slice cultures for 18hrs., or to dissociated cell cultures for 4hrs. in order to label cells in S phase of the mitotic cycle. After fixation in a mixture of ethanol and acetic acid (95:5) for 2 min at −20°, slices or cells were denaturated in 2N HCL for 2 min, followed by incubation with sodium borate buffer (0.1M, pH 8.5) for 10 min. After that, sections were incubated overnight with mouse monoclonal anti-BrdU antibody (Becton Dickinson or Fitzgerald) diluted 1:50 in PBS containing 3% natural goat serum. After rinsing, sections were incubated for 2hrs. at room temperature with secondary antibody (1:400, Alexa Fluor 555, Molecular probes, Eugene, OR), and then cover-slipped with Vectashield (Vector Laboratories).
Cell counting
Cells in dissociated (n=4) or slice cultures (n=3) labeled with various markers and antibody to BrdU, were expressed as a percentage of the total number of cells viewed with nuclear stain (bisbenzimide). Ten adjacent optical fields (surface of one field = 264 µm2) were examined at 20x magnification in each culture. Means and standard errors of the mean (±SEM) were calculated and compared with those from control cultures to determine and correlate statistical differences, trends and associations. Values of p<0.05 were considered significant.
RESULTS
Histological organization of the human fetal SVZ
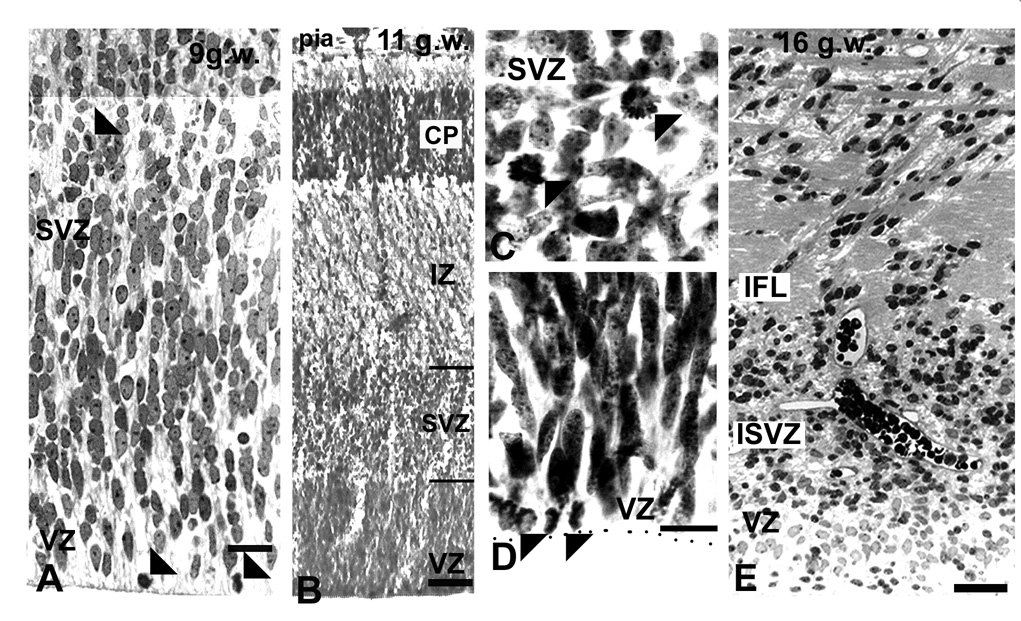
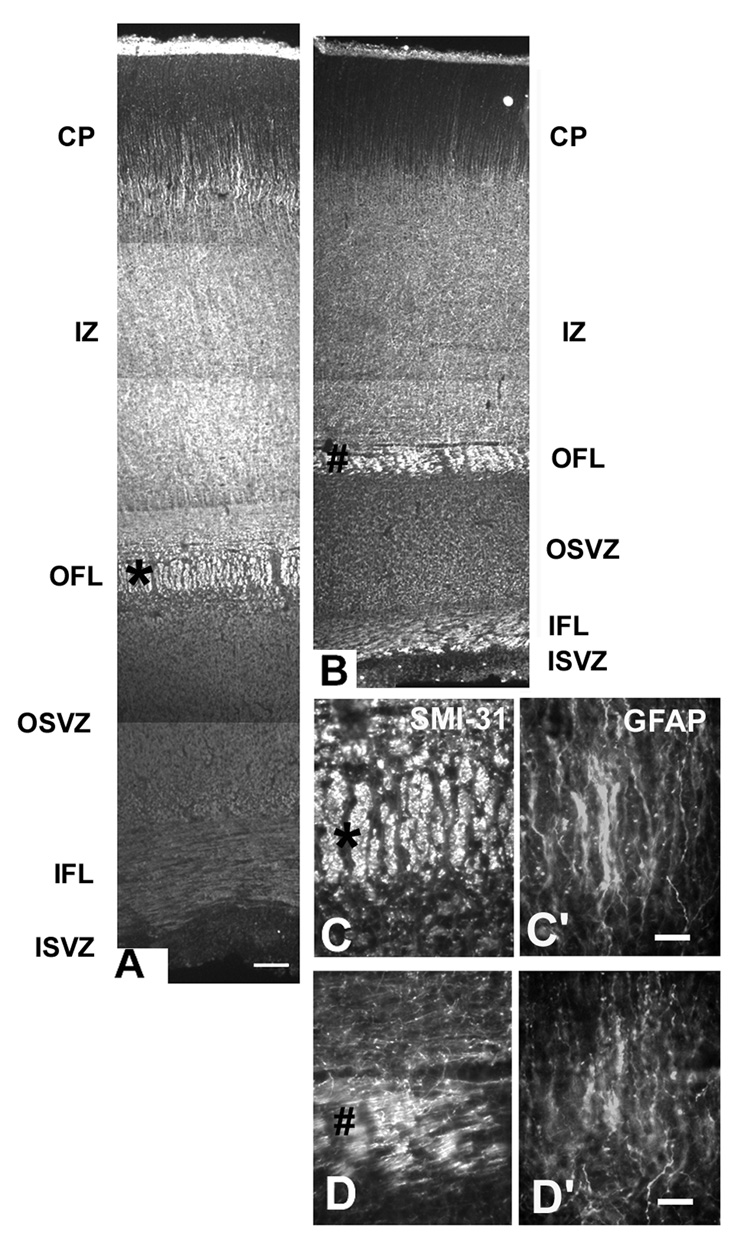
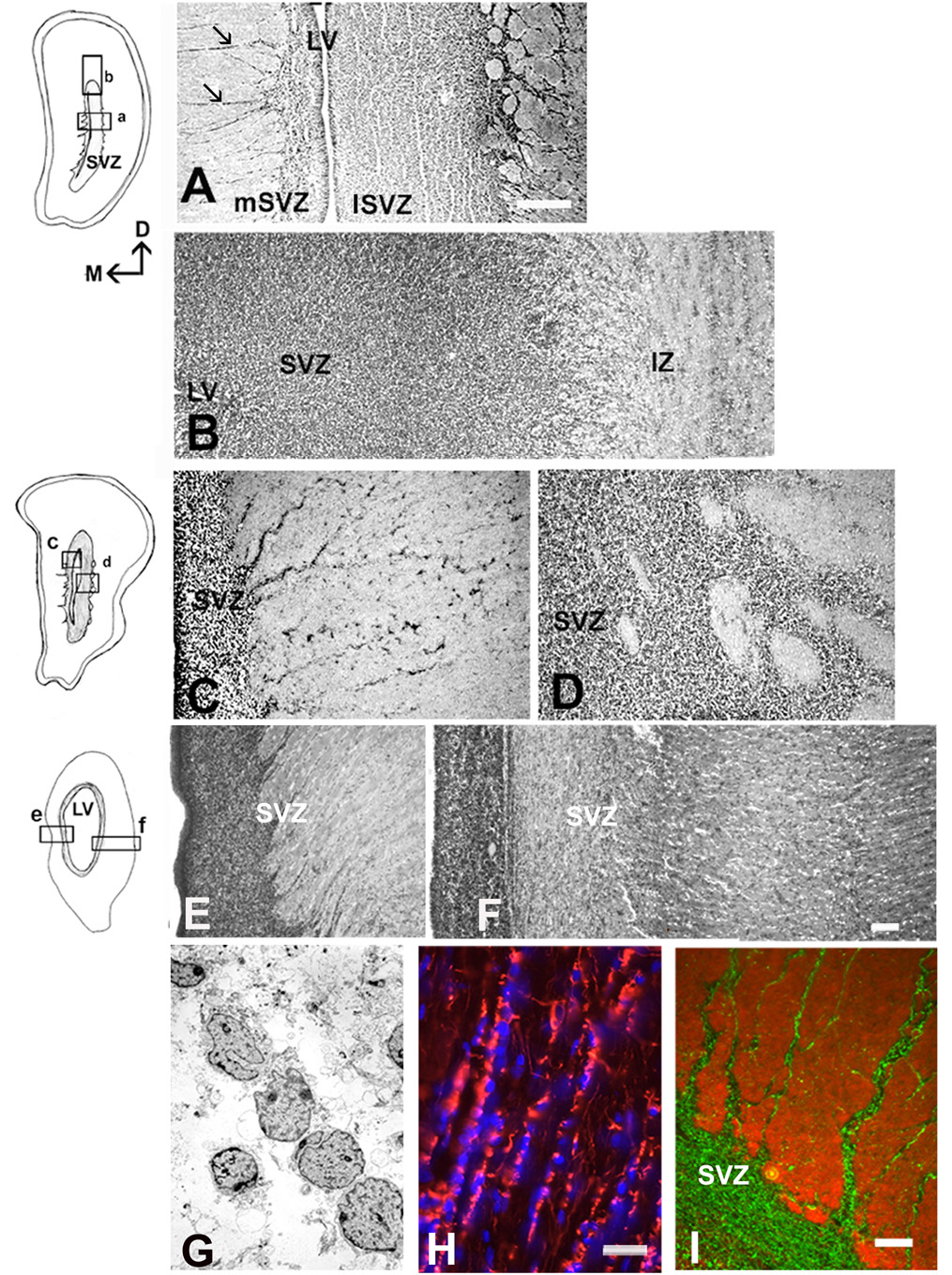
Before the emergence of the cortical plate at 7–8 gw, dividing cells were observed at considerable distance from the VZ, indicating the incipient SVZ (Fig. 1, Zecevic, 2004). For the first time, at 9 gw, a distinct, although small, SVZ could be recognized between the highly proliferative VZ and the non-proliferative intermediate zone (Fig. 1A). The lower border of the SVZ appeared as the continuation of radially arranged compact VZ cells, whereas the upper border with the IZ was loosely organized. (Fig. 1A). Over the next several months (10–24 gw), the initial compact cellular appearance of the SVZ changed considerably due to penetration of tangentially oriented fibers from subcortical regions, as well as fibers crossing through the corpus callosum (Fig. 1C). Incoming fibers were best observed at later fetal stages (see bellow, Fig. 3). The appearance of the SVZ changed along the rostro-caudal axis within each fetal brain, as could be illustrated on coronally cut, Nissl stained serial sections of the 22 gw fetal brain (Fig. 2). At any rostro-caudal level, the lateral side of the SVZ was several times wider than the medial side facing the interhemispheric fissure. For example, in the frontal pole, this ratio was approximately 3:1, as the lateral SVZ was 1400µm and the medial was 450µm thick (Fig. 2A). In the lateral SVZ, at the interface with the intermediate zone, a characteristic circular arrangement was formed by fiber bundles and interposed cells (Fig. 2A). At midgestation multiple cell bands radiated from the SVZ through the intermediate zone (Rakic and Zecevic, 2003a). Occasionally, bands stretched for more than 2000µm to reach the overlaying subplate layer, and were more pronounced in the medial than in the lateral SVZ (Fig. 2A,C,E). Cells in these bands were closely linked as observed on the electromicrographs (Fig. 2G). Various glial and neuronal markers labeled these cells (Fig. 2H,I), which often were dividing as we showed by PCNA labeling (Rakic and Zecevic, 2003a). Interestingly, although SVZ cell bands were reminiscent of chain migration in the rat rostral migratory stream (Luskin, 1993), they were not labeled with PSA-NCAM (Fig. 2I). In the occipital cortex at 20–4 gw, the characteristic feature of the SVZ in the occipital pole was that incoming fibers partitioned the cell rich SVZ into the inner and the outer SVZ. This complex histological organization was particularly well demonstrated in 22gw fetus on sections immunolabeled with the antibody to phosphorylated neurofilament protein (SMI-31, Fig. 3). The inner layer of closely packed subventricular cells was located close to the VZ, followed by the inner fiber bundle, and then the outer SVZ arranged in parallel rows of fibers separated by radially aligned cells (Fig. 3C). This radial arrangement was originally named “palisades” in the fetal monkey occipital cortex (Smart et al., 2002). The uniformly running radial GFAP+ fibers were demonstrated throughout the entire human SVZ, consistent with the idea that “palisades” were formed by neuronal cell bodies and fibers, and not by glia fibers (Fig. 3C’). In contrast, the medial SVZ in the occipital pole was thinner and without a specific outer fiber layer (Fig. 3D).
Fig. 1.
VZ/SVZ region in the fetal human brain as viewed by light microscopy. A: At 9 gw Nissl stained section, the VZ merges with the SVZ without a sharp border; dividing cells (arrowheads) can be observed in both the VZ and SVZ. B: At 11 gw, in the lateral cortex, the SVZ/VZ border as well as the upper border with the intermediate zone (IZ) are clearly pronounced (lines on the right); C and D: Higher magnification of proliferating cells at 11 gw in C: the SVZ, and D: the VZ (arrowheads). E: In the occipital cortex at 17 gw, afferent fibers in the SVZ form the inner fiber layer (IFL). Scale bars= A, C, D -10µm, B-50µm, E-100µm.
Fig. 3.
Occipital cortex at midgestation immunostained with SMI-31 antibody to display inner fiber layer (IFL) close to the inner SVZ, and the outer fiber layer (OFL) between the IZ and the outer SVZ (OSVZ). A: lateral and B: medial side of the hemisphere. A much narrower medial cerebral cortex demonstrates a smaller SVZ. IZ- the intermediate zone, CP-cortical plate. C-C”: Higher magnification of the outer fiber layer in the lateral SVZ labeled with an asterisk on A. Double labeling with SMI-31 (C) and GFAP (C’) reveals characteristic “palisades” of radially aligned SMI-31+ cells and GFAP+ fibers. D,D’: In the medial SVZ, in contrast, this palisade organization is not present; D: SMI-31, and D’: GFAP. Scale bars= A, B-100µm; C,D- 10µm.
Fig. 2.
Changing appearance of the SVZ along the rostro-caudal axis in the fetal brain at midgestation (22 gw). Drawings represent Nissl stained coronal sections; small quadrants mark areas represented on the photographs. A: In the frontal pole, the lateral SVZ (lSVZ) is five times wider than the medial SVZ (mSVZ) facing the interhemispheric fissure. Note the cell bands on the medial side (arrows). B: Dorsal SVZ from the same section. VZ is to the left, pia to the right. Fiber bundles are running at the border of the SVZ with the intermediate zone (IZ). C, D: More caudally, at the level of the internal capsule (IC), medial (C) and lateral SVZ (D). E, F: At the occipital pole level, the medial SVZ (E) with cell bands that stretch to the subplate layer; the lateral SVZ (F) has complex organization (see Fig.3 for details). G: On the electron micrograph of the SVZ at 22 gw, the cells in bands are closely linked. H: Cell nuclei counterstained with bis-benzimide (blue) aligned along GFAP+ fibers (red) radiating from the SVZ. I: Double-labeling with GFAP (green) and PSA-NCAM (red) reveals that these two markers do not overlap in the SVZ cell bands. Scale bars= A-500µm, B–F (in F)-100µm; G-Direct Mg.2000X, H-20µm, I- 50µm.
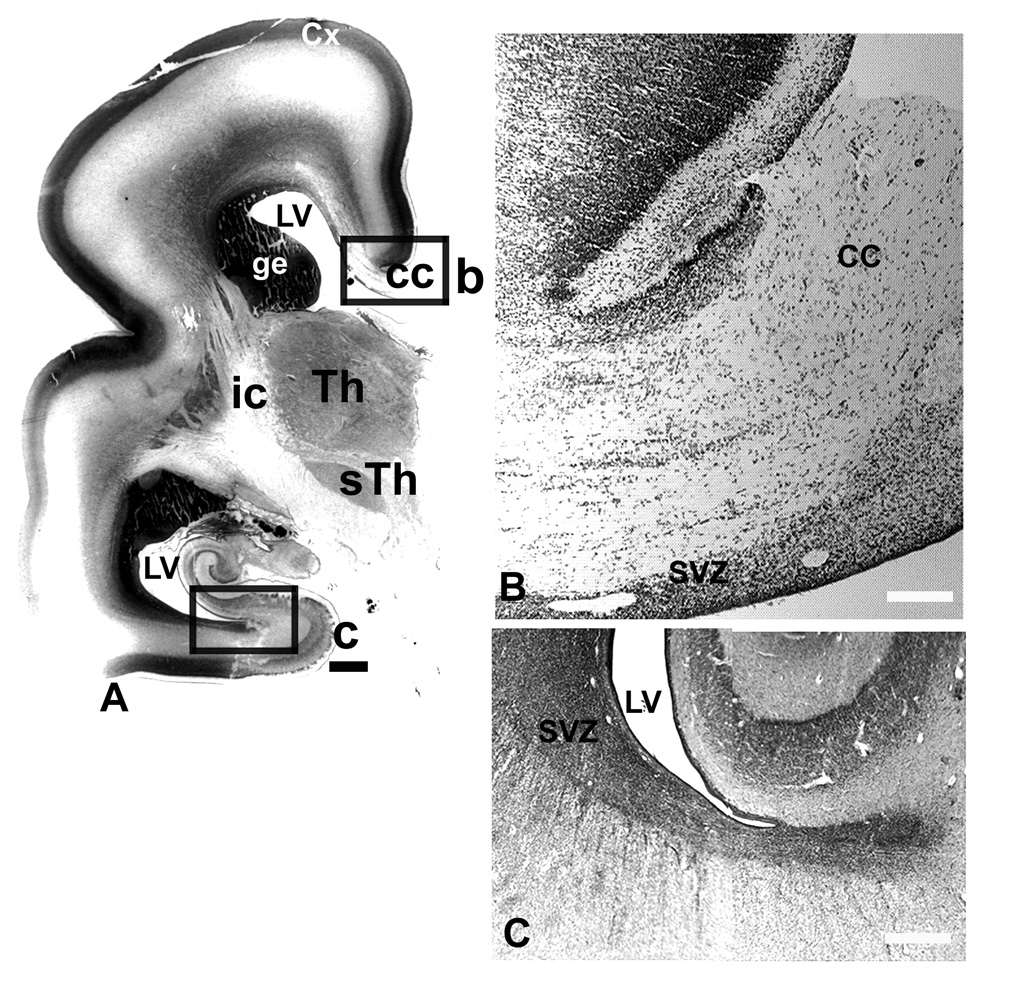
In a 25–7 gw fetal brain, the latest fetal age studied here, the VZ had been reduced to one cell-thick ependymal layer, while SVZ was still present around lateral ventricle (Fig. 4). The thin medial SVZ close to the corpus callosum had characteristic shape with obliquely oriented cell bands intruding between the axons of the corpus callosum, as seen on coronal sections through the hemisphere at the level of the thalamus (Fig. 4B). The posterior SVZ displayed a characteristic stream of cells that extended to the white matter of the temporal lobe (Fig. 4C).
Fig. 4.
Nissl stained frontal section through the mid-brain level at 25gw. A: Contact picture with boxed areas which are represented on corresponding photographs. B: Medial SVZ in the intrahemispheric fissure, with cell bands extending into the corpus callosum (cc). C: Posterior SVZ with cell stream invading the white matter of the temporal pole. SVZ-subventricular zone, Th-thalamus, sTh- subthalamic nucleus, LV-lateral ventricle, ge-ganglionic eminence, cc-corpus callosum, ic-internal capsule, Cx-cerebral cortex. . Scale bars= A-1mm, B, C-100µm.
Cellular composition of the human fetal SVZ
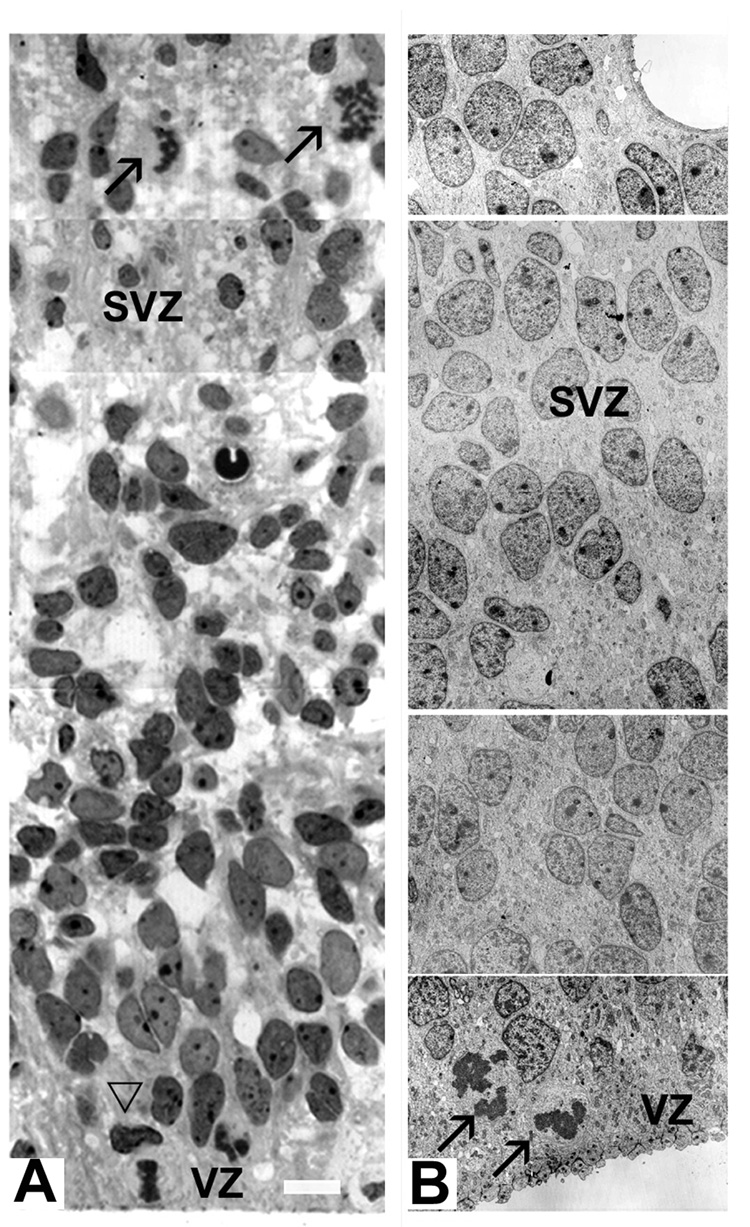
All SVZ cells had immature morphology, as could be observed on both light and electromicrographs (Fig. 5). However, two subclasses of cells could be distinguished: one cell class had regularly shaped, pale and larger nuclei, whereas the other had irregular, darker and smaller nuclei on Nissl stained sections (Fig. 5A). Similarly, on the electron microscope, one cell subpopulation had smooth, elongated nuclei and small amount of dark cytoplasm with numerous ribosomes, whereas other had irregular nuclei with clumps of chromatin, and more cytoplasm (Fig. 5B). These morphological differences among the SVZ cells become even more noticeable around 22–24 gw. However, cell immaturity made classification of cells solely on the basis of their morphology and nuclear staining difficult. Various cell types became apparent when cell-type specific immunomarkers were used. This approach revealed antigenetic differences in SVZ cells already at early fetal stages (8–9 gw).
Fig. 5.
Various shape and staining properties of SVZ cells on the light and electron microscopic level. A: On a 1µm toluidine blue stained section of the 18 gw fetus, diverse staining properties of SVZ cells could be seen, along with horizontal and vertical mitotic spindles on the VZ surface (empty arrowhead) and in the SVZ (arrows). B: On the electron micrograph at 12 gw darker and lighter cell nuclei are dispersed through the SVZ, whereas two mitoses are observed at the ventral surface (arrows). Scale bars= A-10µm; B - direct Mg. 2000X.
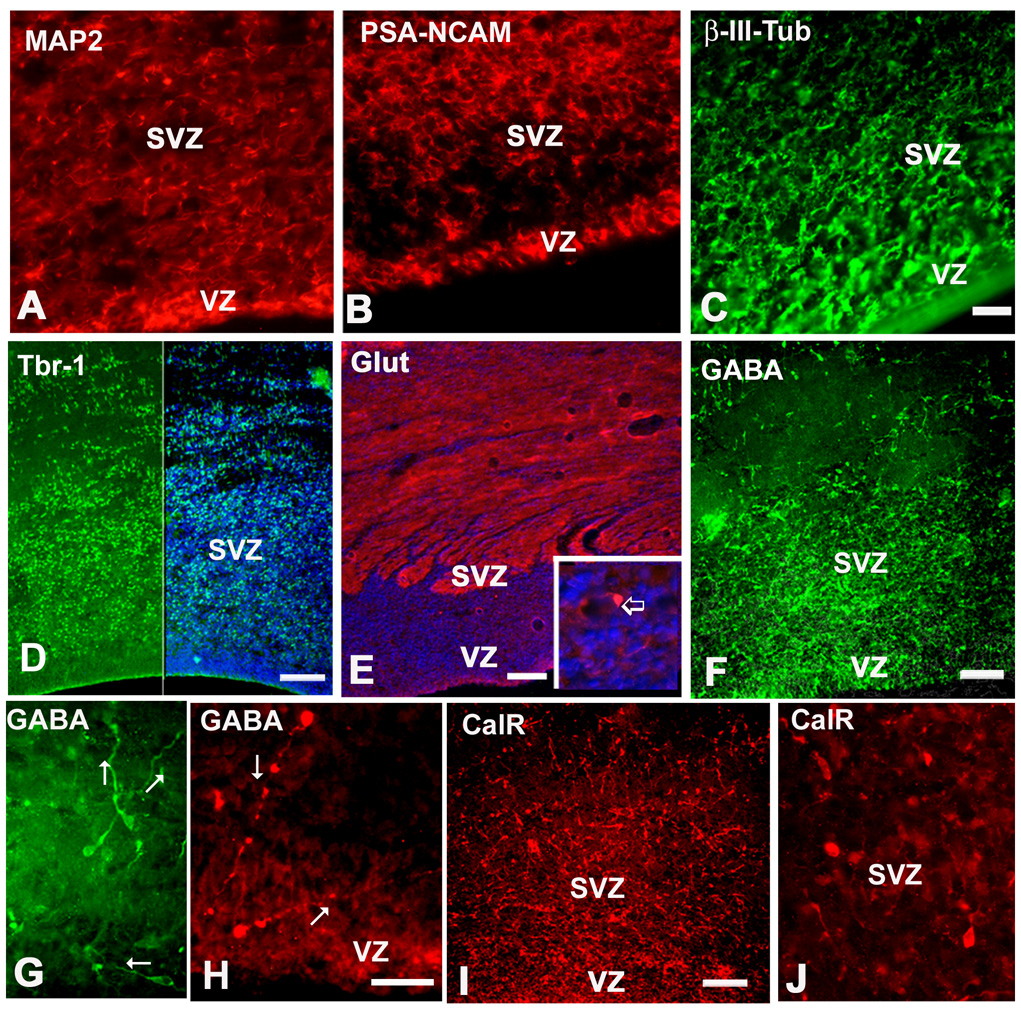
Immature neurons, labeled with β-III-tubulin, PSA-NCAM, as well as MAP2, were present in the fetal SVZ at all ages studied (Fig. 2I, 6A–C). Antibodies to the transcription factor Tbr-1 and glutamate labeled a subpopulation of projection neurons, which probably radially migrated to upper cortical layers (Fig. 6D,E). Other neuronal markers, such NeuN (neuronal nuclear antigen) and NSE (neuronal specific enolase) labeled predominantly neurons that already migrated away from the SVZ, and were observed above the SVZ, in the subplate, the cortical plate and layer I (not shown). GABA, calretinin and calbindin immunolabeled interneurons in the SVZ, in all cases studied (7–22 g.w). GABAergic cells had simple uni- or bipolar morphology that suggested their migration (Fig. 6F–I). We considered bipolar neurons with one leading and one trailing process on the opposite side of the cell body to have migratory morphology. Leading processes of these cells were directed randomly, either towards the pia, or the VZ, or oriented horizontally (Fig. 6H–J). We further characterized these cells in slice culture (see bellow).
Fig. 6.
Immunohistochemical labeling of different cortical neuron subtypes in the SVZ at midgestation. A: MAP2, B: PSA-NCAM, C: β-III-tubulin, D: Tbr-1 (green), right half is the combination of Tbr-1 and bisbenzimide (blue), E: Glutamate labeling is mostly expressed in neuronal processes; immunopositive cell in the inset (arrow). F, G: GABAergic cells in the VZ/SVZ at 17 gw, G: higher magnification of GABAergic cells with leading processes in various directions (arrows); pia is towards the top; H: At 11 gw two GABAergic cells close to the ventricular surface, with long leading processes (arrows). I: Calretinin (CalR) in the same 17 gw fetus as shown for GABA on F. J: Higher magnification of CalR+ cells. Scale bars- A–C=20µm, D–F, I=100µm, G, H, J=50µm.
The astroglial cell population, abundant in the cortical SVZ (Fig. 2H), has been previously described (Rakic and Zecevic, 2003a, Zecevic, 2004, Howard et al., submitted) and will not be discussed here.
Cortical interneuron progenitors in the slice culture of the fetal SVZ
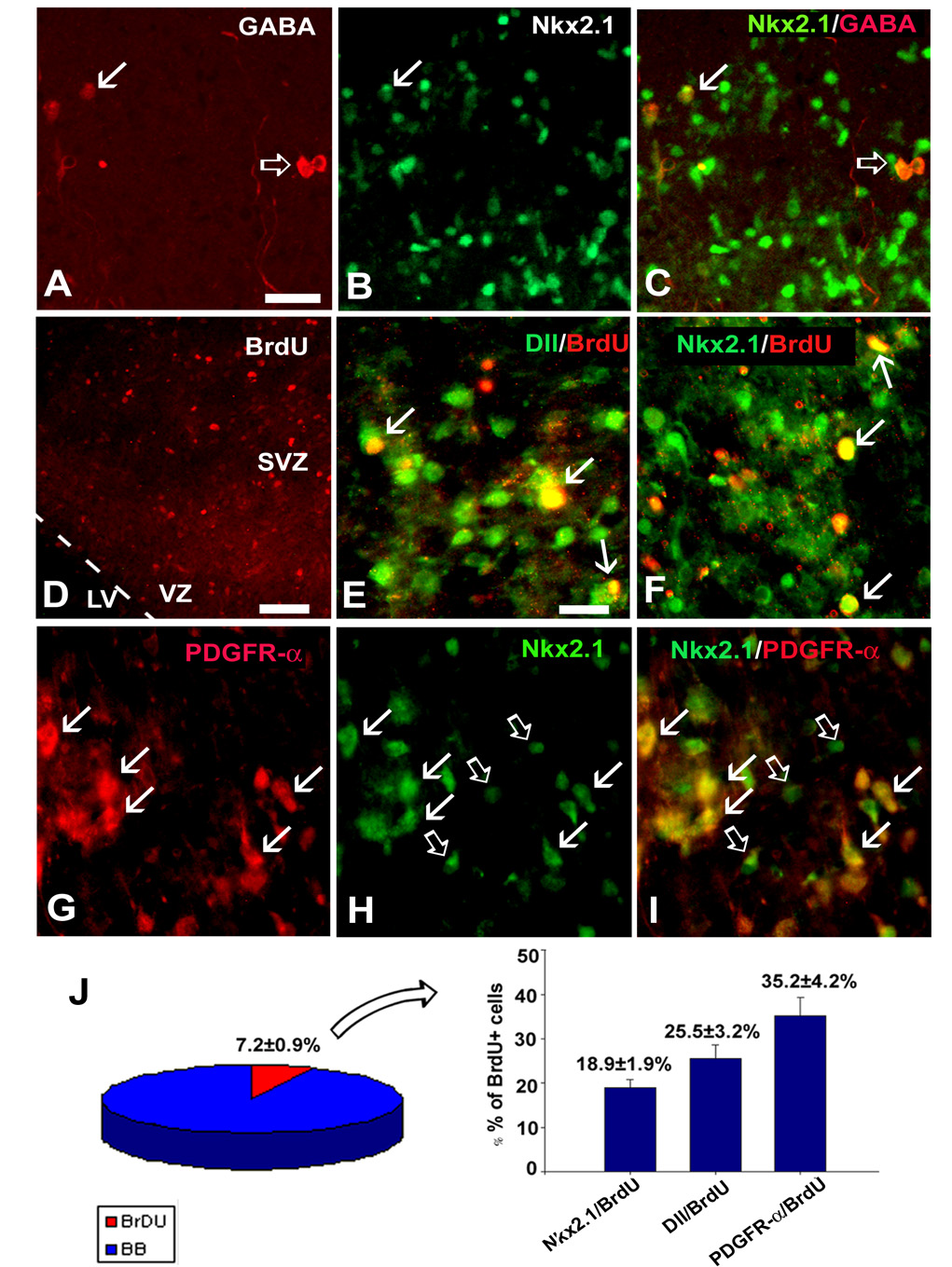
Previously, we have shown that numerous cells in the fetal SVZ expressed ventral transcription factors, Dlx and Nkx2.1, which is characteristic for cells of ganglionic eminence (GE) origin (Rakic and Zecevic, 2003a,b). At the same time numerous interneurons, labeled with either GABA or calretinin, were demonstrated in the SVZ (Fig. 6F–J). Furthermore, in slice cultures from the VZ/SVZ region (22 gw, n=3), GABA+ interneurons occasionally co-expressed transcription factor Nkx 2.1 (Fig. 7A–C). To determine whether these cells migrated from the GE to the cortical SVZ, or were generated locally, we studied the proliferation of Nkx2.1+ and Dlx+ cells in the cortical SVZ. From all cells in the SVZ, a fraction were labeled with BrdU and therefore were proliferating (Fig. 7D,J). Although numerous GABAergic interneurons were present in the SVZ, none of these cells incorporated either BrdU or expressed proliferation marker, Ki67 (not shown, n=3). In contrast, a fraction of cells that expressed Dlx (pan-Dlx antibody) or Nkx-2.1, were co-labeled with BrdU (Fig. 7E, F). Of the total BrdU+ cells in the SVZ one quarter (25.5±3.2%) were Dlx/BrdU+, whereas 18.95±1.9% were Nkx2.1/BrdU+ (Fig. 7J). However, not all of these cells were neurons. Double staining showed that 55.2±6.5% of Dlx+ and 80.1±7.2% of Nkx2.1+ cells present in slice cultures (n=3) were double-labeled with PDGFR-α, an early oligodendrocyte progenitor marker (Fig. 7G–I). From all dividing, BrdU+ cells, 35.2±4.2 % were PDGFRα+ oligodendrocyte progenitors (Fig. 7).
Fig. 7.
Interneurons in slice cultures from the human fetal SVZ at midgestation. Double-labeling with A: GABA and B: Nkx2.1 antibodies. C: Overlay image shows Nkx 2.1 in some GABA+ interneurons (arrow) and absence in the others (empty arrow). D: Proliferating cells in the VZ/SVZ region are labeled with anti-BrdU antibody. E: Pan-Dlx (antibody Dll, green) labels nuclei of SVZ cells, some of which are proliferating as seen by their co-labeling with BrdU (arrows, yellow color). Similar to this, F: several Nkx 2.1 (green) cells in the SVZ are co-labeled with BrdU (arrows). G-I: OPCs labeled with G: anti-PDGFR-α antibody, H: Nkx 2.1 in the same section. I: Overlay image shows that Nkx2.1 co-localized with PDGFR-α (red) in most cells (arrows). Empty arrows point to Nkx2.1+ (green) cells only. J: Quantification of immunolabeled cells demonstrates that 7.2±0.9% of all SVZ+ cells are BrdU+. Of all BrdU+ cells 18.9±1.9% are Nkx2.1+ cells, 25.5±3.2% are Dll/BrdU+ cells, while 35.2±4.2% are PDGFR-α+ cells. Scale bars= 40µm (in A for A–C), 75 µm (D), 25µm (in E for E–I).
Quantification of VZ/SVZ cells in vitro
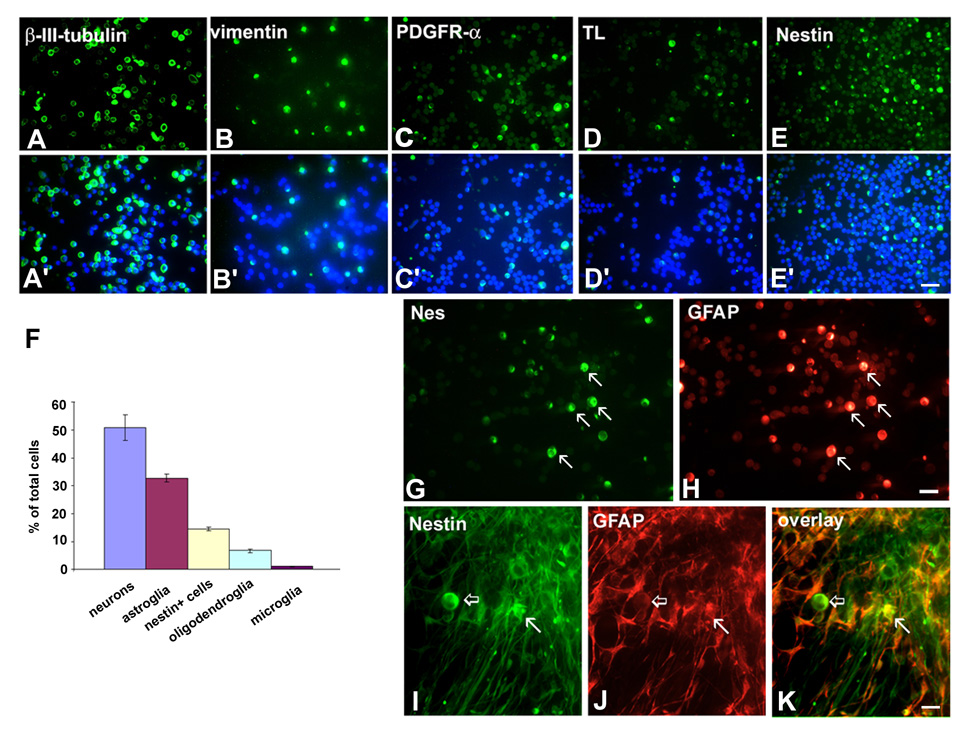
To determine the contribution of particular cell types to the entire population present in the SVZ, we did a quantitative study using acutely dissociated cell culture from VZ/SVZ at midgestation (Fig. 8, n=6). In this type of acute culture, cells were kept in vitro for only 4 hrs. Their viability, tested with Trypan Blue staining, was 90.8±8.5%. This approach showed that approximately 50% of all SVZ cells were neurons, labeled with either MAP2 (50.9±4.6%) or β-III-tubulin (51.7±3.8%) (Fig. 8B). Transcription factor Tbr-1, a marker of pyramidal neurons, was expressed by 35.0±2.6% of SVZ cells, whereas GABAergic cortical interneurons represented 19.2±3.2%. One third of SVZ cells were astroglia labeled with vimentin (32.7±1.5%) or GFAP (31.9±3.0%), with both markers co-localized in the same cells (Fig. 8B). Other cell types, accounted for smaller percentages, such as nestin+ cells (14.6±0.9%), PDGFR-α+ oligodendrocyte progenitor cells (6.7+0.6%), and lectin+ microglia/macrophages (1.1±0.1%) (Fig. 8). In acute cell culture the fraction of BrdU+ cells was calculated to be 11.0±0.2% of total cells present in SVZ (Howard et al., 2004), which is slightly higher than the percentage obtained in slice culture at the comparable midgestational fetal age.
Fig. 8.
Acute and chronic dissociated cell culture of VZ/SVZ region at midgestation. A–E: Examples of immunostaining with cell-specific antibodies and A’–E’: counterstaining with bisbenzimide (blue) for cell nuclei. F: Graphical representation of morphometric results for main cell groups present in the VZ/SVZ. Percentages are calculated in respect to the total number of cell nuclei in the field of view and averaged for four fetuses (n=4) (for more details see the text); G–H: Examples of cells in VZ/SVZ acutely dissociated cell culture which co-express nestin (G) and GFAP (H, arrows). I–K: In chronic cell culture (10DIV), nestin and GFAP are co-expressed in astrocytes (arrows), but not in round cells (empty arrows) that are only nestin+. Scale bars= A–E- 50µm; G–H and I–K-20µm.
Glial and stem-like cell populations in the human VZ/SVZ
Vimentin, GFAP and 4A4 antibodies labeled radial glia (RG) cells in the human fetal brain in all stages of development studied, from the earliest embryonic stages to midgestation (Zecevic, 2004, Howard et al., in prep.) At midgestation, numerous fibers that radiated from the VZ/SVZ towards the cerebral cortex could still be labeled with both vimentin and GFAP antibodies (Zecevic, 2004). Cell nuclei, revealed with bis-benzimide, were aligned along labeled glia fibers (Fig. 2H), consistent with the role of RG in neuronal migration (e.g., Rakic, 1972).
In acutely dissociated cell cultures from the same age VZ/SVZ, nestin and GFAP were co-expressed in a subset of round cells (Fig. 8 G,H). After ten days in vitro (10DIV), nestin was expressed in two morphologically different cell types: round cells without processes, which did not express GFAP, and in astrocytes, where it co-localized with GFAP (Fig. 8 I–K). Round nestin+ cells represented a distinct cell population that resembled neuronal precursors or stem-like cells.
Other cells types, such as oligodendrocytes and numerous microglia/macrophages, were described previously in the human fetal SVZ (Andjelkovic et al., 1998; Filipovic et al., 2003; Rakic and Zecevic, 2003a,b; Jakovcevski and Zecevic, 2005). Moreover, cell death, which is very prominent in the human fetal SVZ, has been described earlier (Rakic and Zecevic, 2000).
DISCUSSION
In this study, we provide details of histological organization, molecular characteristics, and tempo of cell differentiation in the human SVZ from 7–27 gw. Single and double immunohistochemistry demonstrate various cell types present in the SVZ, whereas in vitro methods provide details about their proliferation and more accurate quantification of different cell types. The question of cortical interneuron origin was addressed, and although conclusive results cannot be provided, we demonstrate that cells expressing ventral transcription factors (Dlx2, Nkx2.1) proliferate in the cortical SVZ at midgestation. Their antigen phenotype is consistent with either oligodendrocyte progenitors or neurons. Taken together with previously published results (Letinic et al., 2002; Rakic and Zecevic, 2003), these results support the notion that a subpopulation of cortical interneurons may be generated locally, in the cortical SVZ.
Proliferation and migration in the fetal SVZ
The human cortical SVZ, during the examined fetal period (7 to 27 gw), is a highly proliferative and heterogeneous zone that contributes to both neurogenesis and gliogenesis. Around midgestation (18–24 gw) approximately 10% of SVZ cells were proliferating in in vitro experiments. Such late cell proliferation is important for the generation of upper cortical layers, when the SVZ is the only proliferative zone. Thus, cell proliferation in the cortical SVZ is one of the major contributors to the expansion of the brain in the last trimester of the intrauterine development, when a three fold increase in the number of cells take place (Badsberg Samuelsen et al., 2003).
Various modes of migration, including tangential, radial, and multipolar migration have been demonstrated by time lapse photography on organotypic slice cultures in the rodent SVZ (Kakita and Goldman, 1999; rev. Marin and Rubenstein, 2001; Nadarajah et al., 2002; Ang et al., 2003; Tabata and Nakajima, 2003). Since our results are mainly based on the fixed human fetal tissue, we did not directly observe migration of SVZ cells. However, the orientation of the leading processes of SVZ cells suggests that similar types of migration are present in the human SVZ. While RG fibers provide the anatomical framework for the radial migration of projection neurons (e.g., Rakic 1972; Sidman and Rakic, 1982), tangential fiber bundles in the SVZ help migration of cortical interneurons (rev. Marin and Rubenstein, 2001; Kriegstein and Noctor, 2004), and oligodendrocyte progenitors (He et al., 2001; Marshall and Goldman, 2002; Marshall et al., 2003; Rakic and Zecevic, 2003b). Several fiber systems, such as cortico-cortical, cortico-thalamic (corticofugal) and thalamocortical (corticopetal) fibers in the cortical SVZ (Miller et al, 1993; Molnar and Blakemore, 1995), may assist in tangential migration. The “palisade” organization of the outer SVZ, typical for primates, is probably formed in the occipital pole by geniculostriate fibers (Smart et al., 2002). Vimentin and GFAP labeled glial fibers oriented transversally together with radially oriented glial fibers and migrating neurons outline a striking 3D mesh organization, both in the fetal monkey (Zecevic and Rakic, 2001) and human lateral SVZ (Rakic and Zecevic, 2003a; Zecevic, 2004).
In addition, prominent cell bands, characteristic for the human SVZ (Rakic and Zecevic, 2003a), may represent an additional migratory path, similar to the rostral migratory stream (RMS) in rodents (Luskin, 1993; Lois and Alvarez-Buyilla, 1994). However, cells in SVZ bands likely have different adhesion properties, as suggested by the lack of PSA-NCAM labeling, which is typical for RMS.
Cell diversity in the human SVZ
One of the main characteristics of the SVZ is that all major cell types (cortical neurons, astrocytes, oligodendrocytes) are being generated in situ during intrauterine development. On the light microscopic level, various cell subpopulations can be distinguished on the basis of their nuclear shape, chromatin density, and cytoplasmic appearance, while some similarities with cell types A (neuronal) and B (astrocytes/stem-cells) described in the mouse (Doetsch et al., 1997) are noted.
Of special interest is that several subpopulations of cortical neurons: pyramidal, later born subplate and olfactory neurons, originate in the human cortical SVZ. A large number of Tbr-1+ and glutamate+ cells observed in the SVZ at midgestation show that projecting, pyramidal neurons do not yet migrate out of this proliferating zone by that time. These late generated Tbr-1+ and glutamate+ cells, are probably destined for upper cortical layers (Hevner et al., 2001, Englund et al., 2005). Subplate neurons are initially generated in the cortical VZ (Marin-Padilla, 1978; Kostovic and Rakic, 1990; Zecevic et al., 1999; Meyer et al., 2000). However, the increase in the size of this layer between 22 and 34 gw in human (Kostovic and Rakic, 1990; Kostovic et al., 2002), and at the corresponding time in the monkey, is due to proliferation in the SVZ (Smart et al., 2002).
In rodents, asymmetric radial glia (RG) divisions in the cortical VZ, and later symmetric divisions of intermediate progenitors (IPC) in the SVZ, produce pyramidal neurons (Noctor et al., 2004). Similarly, in the human VZ/SVZ, we have reported that RG cells are proliferating in embryonic and fetal brains (Zecevic, 2004; Howard et al., 2004) and that some of these cells might be neurogenic (Howard et al., submitted).
Our in vitro study demonstrates that a subpopulation of cells labeled with GE ventral markers, Dlx and Nkx2.1, are co-labeled with GABA, indicating that a fraction of cortical interneurons are generated locally. This is consistent with previous findings on embryonic and fetal human brains (Letinic et al., 2002; Rakic and Zecevic, 2003). In contrast, in vitro studies in rodents do not show proliferation of GE cells tangentially migrating through the cortical SVZ (Polleux et al., 2002), although this may change with the stage of development. In fact, in the mouse Dlx1 expressing cells are proliferating in the cortical SVZ in later development (Anderson et al., 2001). In addition, a small fraction of cortical GABAergic cells express Emx1, a homeobox gene characteristic for cortical SVZ cells and pyramidal neurons (Chan et al., 2001). Later born cortical interneurons are calretinin+ and may represent interneuron subpopulation that originates locally (Xu et al., 2003). The fact that at midgestation, almost all GABAergic neurons could be co-labeled with calretinin (Rakic and Zecevic, 2003a), suggests that, in contrast to rodents, where majority of cortical interneurons are parvalbumin+, in human a higher percentage of cortical interneurons are calretinin+ and may be generated locally. The observed differences between rodent and human brain in relation to cortical interneuron origin may be due to evolutionary changes where the much larger primate brain needs various sources of neurons that include both the GE and the cortical VZ/SVZ.
The finding that the majority of Nkx2.1+ and Dlx+ cells the SVZ at midgestation are co-labeled with PDGFR-α is consistent with the described tangential migration of oligodendrocyte progenitors from the GE to the cortical SVZ, both in rodent (He et al., 2001; Marshall and Goldman, 2002) and human fetal brains (Rakic and Zecevic, 2003b).
Progenitor cells in the human fetal SVZ
The existence of multipotential progenitor cells in the human fetal forebrain has been documented by several laboratories (e.g., Flax et al., 1998; Vescovi et al., 1999; Ourednik et al., 2001; Suslov et al., 2002). Our results show that SVZ cells can be labeled with a combination of markers, which indicates their multipotentcapacity (Jakovcevski and Zecevic, 2002; Rakic and Zecevic, 2003b). In addition, in long-term cell culture, nestin+ cells have round morphology and no processes, consistent with precursor or stem-like cells, although a lineage analysis is necessary to confirm this. Nestin is expressed in undifferentiated cells and RG, as well as in immature astrocytes (Hockfelt and McKay, 1993), and therefore is not cell type specific as much as it is marker of immature cells. RG cells, with their neurogenetic and gliogenetic capabilities, have qualities of multipotential progenitor cells. In the human fetal brain, RG proliferate first in the VZ and subsequently in the SVZ. In fact, the majority of cells that incorporated BrdU in the VZ/SVZ at midgestation were vimentin+ RG cells (Howard et al., 2004).
Clinical relevance
Cortical developmental disorders range from lissencephaly, cortical ectopias with epilepsy (Gleeson and Walsh, 2000), to complex psychiatric disorders, such as schizophrenia, autism or bipolar disorder (Akbarian et al., 1995; Lewis, 2000). Due to the prolonged period of intrauterine development, combined with an apparent developmental immaturity of blood flow to the periventricular region, the SVZ may be very susceptible to environmental influences.
Two pathological conditions in particular, periventricular leukomalacia (PVL) and intracerebral hemorrhage, which are often the cause of motor deficit and mental retardation, may be the result of damage to specific cell types in the SVZ (Volpe, 2001). The number of subplate neurons may be reduced during period of particular vulnerability in preterm brain development. Transient subplate neurons are necessary for the initial organization and later normal function of the cerebral cortex (e.g., Shatz et al., 1988; Kostovic and Rakic, 1990), including the structural plasticity after congenital brain lesions (Kostovic et al., 1989; Volpe, 1997; Kostovic and Judas, 2002)
In animal models, the ablation of the subplate compromises the formation of thalamocortical connections, and visual function (Ghosh et al., 1990; Kanold et al., 2003). Moreover, cognitive disorders and cortical visual loss can result from hypoxic-ischemic insult and death of subplate progenitor cells (McQuillen et al., 2003, Cioni et al., 1997; Piecuch et al., 1997). Oligodendrocyte progenitors may also be deleted in the SVZ lesions, which affect myelination (Back et al., 2001, 2002; Volpe, 1997).
More subtle damages to the SVZ during development may go unrecognized clinically, but depletion of stem cells may result in compromised repair processes later in life (e.g.Reynolds and Weiss, 1992; Morshead et al., 1994; Sanal et al., 2004).
In summary, the human cortical SVZ is larger, has a more complex histological organization, and persists longer than in other mammals. Due to prolonged period of proliferation, the cortical SVZ contributes uniquely to the expansion of the human cerebral cortex.
ACKNOWLEDGEMENTS
We thank Drs. J. Kohtz and Stallcup for generous gifts of antibodies, Dr. S. Back for very helpful comments on the manuscript, and Brian Howard for editorial help. Some samples of human fetal brain tissue were obtained from Dr. B. Poulos at the Albert Einstein College of Medicine, Bronx, NY, USA.
Support: NIH grant R01NS41489-03 and MS-RG grant 3083-A.
REFERENCES
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Martin O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migration from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Andjelkovic A, Nikolic B, Pachter J, Zecevic N. Microglia/macrophages-like cells in human central nervous system during development: An immunocytochemical study. Brain Res. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Ang ES, Jr, Haydar TF, Gluncic V, Rakic P. Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex. J.Neurosci. 2003;23:5805–5815. doi: 10.1523/JNEUROSCI.23-13-05805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic A, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglia cells in human central nervous system during development: an immunohistochemical study. Brain Res. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21(4):1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthaudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neuroscience. 2002;22(2):455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badsberg Samuelsen G, Bonde Larsen K, Bogdanovic N, Laursen H, Gream N, Larsen JF, Pakkenberg B. The changing number of cells in the human fetal forebrain and its subdivisions: A stereological analysis. Cer Cortex. 2003;13:115–122. doi: 10.1093/cercor/13.2.115. [DOI] [PubMed] [Google Scholar]
- Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal RS. Histologie du Systeme Nerveux de l’Homme et des Vertebres. Maloine, Paris: 1911. [Google Scholar]
- Chan CH, Godinho N, Thomaidou D, Tan SS, Gulisan M, Parnavelas JG. Emx1 is a marker for pyramidal neurons of the cerebral cortx. Cer Cortex. 2001;11:1191–1198. doi: 10.1093/cercor/11.12.1191. [DOI] [PubMed] [Google Scholar]
- Cioni G, Fazzi B, Coluccini M, Bartalena L, Boldrini A, van Hof-van Duin J. Cerebral visual impairment in preterm infants with periventricular leukomalacia. Pediatr Neurol. 1997;17:331–338. doi: 10.1016/s0887-8994(97)00152-5. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Iversen LL. Excitatory amino acids in the brain - focus on NMDA receptors. Trends Neurosci. 1987;10(7):263–265. [Google Scholar]
- Dahl D, Rueger DC, Bignami A, Weber K, Osborn M. Vimentin, the 57.000 molecular weight protein of fibroblast filaments, is the major cytoskeleton component in immature glia. Eur J Cell Biol. 1981;24:191–196. [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17(13):5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25(1):247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Hatten ME, Heintz N. Brain-lipid binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron. 1994;12:895–908. doi: 10.1016/0896-6273(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Filipovic R, Jakovcevski I, Zecevic N. Expression pattern of GRO-α and CXCR2 in the human fetal brain and MS lesions. Dev Neurosci. 2003;25:279–290. doi: 10.1159/000072275. [DOI] [PubMed] [Google Scholar]
- Filipovic R, Zecevic N. Are proliferative cells in human fetal subventricular zone interneurons? Soc Neurosci Abst. 2004 : 493.4. [Google Scholar]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, Jendoubi M, Sidman RL, Wolfe JH, Kim SU, Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nature Biotech. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McCornell SK, Schatz CJ. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 1990;347:179–181. doi: 10.1038/347179a0. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Walsh CA. Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci. 2000;23:352–359. doi: 10.1016/s0166-2236(00)01607-6. [DOI] [PubMed] [Google Scholar]
- Gratzner HG. Monoclonal antibody to 5-bromo and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hartfuss E, Galli R, Heins N, Götz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- He W, Ingraham C, Rising L, Goderie S, Temple S. Multipotent stem cells from the mouse basal forebrain culture contribute GABAergic neurons and oligodendrocytes to the cerebral cortex during embryogenesis. J Neurosci. 2001;21:8854–8862. doi: 10.1523/JNEUROSCI.21-22-08854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y-P, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JLR. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B, Chen Y-H, Zecevic N. Cortical Progenitor Cells in the Human Fetal Brain. Soc Neurosci Abst. 2004 : 493.3. [Google Scholar]
- Jakovcevski I, Zecevic N. Stem-like cells in the subventricular zone of the human developing telencephalon. Soc Neurosci Abst. 2002 : 726.13. [Google Scholar]
- Jakovcevski I, Zecevic N. Sequence of oligodendrocyte development in the human fetal telencephalon. Glia. 2005;49:480–491. doi: 10.1002/glia.20134. [DOI] [PubMed] [Google Scholar]
- Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron. 1999;23:461–472. doi: 10.1016/s0896-6273(00)80800-4. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Inagaki N, Nishizawa M, Tsutsumi O, Taketani Y, Inagaki M. Visualization of mitotic radial glial lineage cells in the developing rat brain by Cdc2 kinase-phosphorylated vimentin. Glia. 1998;23(3):191–199. doi: 10.1002/(sici)1098-1136(199807)23:3<191::aid-glia2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kanold P, Kara P, Reid RC, Shatz CJ. Role of subplate neurons in functional maturation of visual cortical columns. Scence. 2003;301:521–525. doi: 10.1126/science.1084152. [DOI] [PubMed] [Google Scholar]
- Kohtz JD, Lee HY, Gaiano N, Segal J, Ng E, Larson T, Baker DP, Garber EA, Williams KP, Fishell G. N-terminal fatty-acylation of sonic hedgehog enhances the induction of rodent ventral forebrain neurons. Development. 2001;128:2351–2363. doi: 10.1242/dev.128.12.2351. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Judas M. The role of the subplate zone in the structural plasticity of the developing human cerebral cortex. Neuroembryology. 2002;1:145–153. [Google Scholar]
- Kostovic I, Judas M, Rados M, Hrabac P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cer Cortex. 2002;12:536–544. doi: 10.1093/cercor/12.5.536. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Lukinovic N, Judas M, Bogdanovic N, Mrzljak L, Zecevic N, Kubat M. Structural basis of the developmental plasticity in the human cerebral cortex: The role of the transient subplate zone. Metab Brain Dis. 1989;4:17–23. doi: 10.1007/BF00999489. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J Comp Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kill IR. Localisation of the Ki-67 antigen within the nucleolus. Evidence for a fibrillarin-deficient region of the dense fibrillar component. J Cell Science. 1996;109(6):1253–1263. doi: 10.1242/jcs.109.6.1253. [DOI] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27(7):392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lee MK, Tuttle JB, Rebhun LL, Clevelend DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific b-tubulin isotype during chick embryogenesis. Cell Motil Cytoskelet. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417(6889):645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Lewis DA. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Rev. 2000;31:270–276. doi: 10.1016/s0165-0173(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Lewis PD. Mitotic activity in the primate subependymal layer and the genesis of gliomas. Nature. 1968;217:974–975. doi: 10.1038/217974a0. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;1:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat Embryol. 1978;152:109–126. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- Marshall CA, Goldman JE. Subpallial Dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CA, Suzuki SO, Goldman JE. Gliogenic and neurogenic progenitors of the Subventricular Zone: Who are they, where they come from, and where are they going? Glia. 2003;43:52–61. doi: 10.1002/glia.10213. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability and subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23(8):3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, Goffinet AM. Embryonic and early fetal development of the human neocortex. J Neurosci. 2000;20:1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B, Chou L, Finlay BL. The early development of thalamocortical projections. J Comp Neurol. 1993;335:16–41. doi: 10.1002/cne.903350103. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5:218–224. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Olivier G, Pineau H. Horizons de Streeter et age embrionnaire. Bull Ass Anat. 1962;47e:573–576. [Google Scholar]
- O'Rahilly RF, Müller F, Hutchins GM, Moore GW. Computer ranking of the sequence of appearance of 73 features of the brain and related structures in staged human embryos during sixth week of development. Am J Anat. 1987;180:69–86. doi: 10.1002/aja.1001800106. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J, Flax JD, Zawada WM, Hutt C, Yang C, Park KI, Kim SU, Sidman RL, Freed CR, Snyder EY. Segregation of human neural stem cells in the developing primate forebrain. Science. 2001;293(5536):1820–1824. doi: 10.1126/science.1060580. [DOI] [PubMed] [Google Scholar]
- Piecuch RE, Leonard CH, Cooper BA, Kilpatrick SJ, Schlueter MA, Sola A. Outcome of infants born at 24–26 weeks’ gestation, II: neurodevelopmental outcome. Obstet Gynecol. 1997;90:809–814. doi: 10.1016/S0029-7844(97)00429-8. [DOI] [PubMed] [Google Scholar]
- Polleux F, Witford KL, Dijkhuizen PA, Vitalis T, Ghosh A. Control of cortical interneuron migration by neurotrophines and PI3-kinase signaling. Development. 2002;129:3147–3160. doi: 10.1242/dev.129.13.3147. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey cortex. J Comp Neurol. 1972;145:61–84. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Emerging complexity of cortical layer I in humans. Cer Cortex. 2003a;13:1072–1083. doi: 10.1093/cercor/13.10.1072. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Early oligodendrocyte progenitor cells in the human fetal telencephalon. Glia. 2003b;41:117–127. doi: 10.1002/glia.10140. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Sanal N, Tramontin AD, Qulnones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Garcia Verdugo JM, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Saper C, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465(2):161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Buchwald P, Blumcke I, Celio MR, Hunziker W. Characterization of a polyclonal antiserum against the purified human recombinant calcium-binding protein calretinin. Cell Calcium. 1993;14:639–648. doi: 10.1016/0143-4160(93)90089-o. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Chun JJM, Luskin MB. The role of the subplate in the development of the mammalian telencephalon. In: Peters A, Jones EG, editors. Cerebral Cortex, Vol 7. Development and Maturation of Cerebral Cortex. New York: Plenum Press; 1988. pp. 35–58. [Google Scholar]
- Sternberger LA, Sternberger NH. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci USA. 1983;80:6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini LA, Buchs D, Muller A. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Smart IHM, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cer Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. PNAS. 2003;99(22):14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman RL, Rakic P. Development of the human central nervous system. Springfield, IL: CC Thomas Pub.; 1982. [Google Scholar]
- Tabata H, Nakajima K. Multipolar migration: The third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23(31):9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, Wanke E, Frolichsthal-Schoeller P, Cova L, Arcellana-Pantililo M, Colombo A, Galli R. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenic stimulation. Exp Neurol. 1999;156:71–83. doi: 10.1006/exnr.1998.6998. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Brain injury in the premature infant. Neuropathology, clinical aspects, pathogenesis, and prevention. Clin Perinatol. 1997;24:567–587. [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50(5):553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Factors regulating the expression and function of calcium-independent cell adhesion molecules. Current Opinion in Cell Biol. 1993;5:791–796. doi: 10.1016/0955-0674(93)90027-n. [DOI] [PubMed] [Google Scholar]
- Xu Q, de la Cruz E, Anderson SA. Cortical interneuron fate determination: diverse sources for distinct subtypes? Cer Cortex. 2003;13:670–676. doi: 10.1093/cercor/13.6.670. [DOI] [PubMed] [Google Scholar]
- Zecevic N. Cellular composition of the telencephalic wall in human embryos. Early Human Dev. 1993;32:131–149. doi: 10.1016/0378-3782(93)90007-h. [DOI] [PubMed] [Google Scholar]
- Zecevic N. Synaptogenesis in Layer I of the Human Cerebral Cortex in the First Half of Gestation. Cerebral Cortex. 1998;8(3):245–252. doi: 10.1093/cercor/8.3.245. [DOI] [PubMed] [Google Scholar]
- Zecevic N. Specific characteristics of radial glia in the human fetal telencephalon. Glia. 2004;48(1):27–35. doi: 10.1002/glia.20044. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A. Initial development of γ-aminobutyric acid immunoreactivity in the human cerebral cortex. J Comp Neurol. 1997;380:495–506. doi: 10.1002/(sici)1096-9861(19970421)380:4<495::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Milosevic A, Rakic S, Marin-Padilla M. Origin, early development and composition of the primordial plexiform layer in the human neocortex. An immunohistochemical study. J Comp Neurol. 1999;412:241–254. [PubMed] [Google Scholar]
- Zecevic N, Rakic P. Development of Layer I neurons in the Primate Cerebral Cortex. J Neurosci. 2001;21(15):5607–5619. doi: 10.1523/JNEUROSCI.21-15-05607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]