Abstract
Melanoma patients may exhibit a TH2-skewed cytokine profile within blood and tumor infiltrating lymphocytes (TIL). Therapies that induce beneficial TH1-type tumor-specific immune responses, therefore, are highly desirable. Dendritic cells (DC) are widely used as immune adjuvants for cancer. Prior to their administration, DC are generally induced to mature with a cocktail of recombinant cytokines (IL1β, TNF-α and IL-6), and Prostaglandin E-2 (PGE-2), which is added to preserve the ability of DC to migrate to draining lymph nodes. However, PGE-2 suppresses the production of IL-12p70, a cytokine essential for differentiation of TH1 responses. In this study, human DC were transfected with IL-12p70 mRNA and tested for their ability to alter the TH2 type bias manifested by blood T cells of patients with melanoma. Transfected DC secreted high levels of bioactive IL-12p70, as indicated by their capacity to enhance NK cell activity, skew TH1 responses in allogeneic MLRs through reduction of IL-4 and IL-5, and prime CD8+ T cells to the melanoma associated antigen Melan A/MART-1. Furthermore, T cell lines primed in vitro from the blood of melanoma patients showed strong type 2 skewing that was dramatically reversed by IL-12p70 transfection of autologous DC. Thus, IL-12p70 transfection of clinical DC preparations may enhance type 1 anti-tumor responses, and may thereby contribute to effective immune-based therapy.
Keywords: Dendritic Cells, Melanoma, Vaccines, IL-12, immunotherapy
Introduction
Substantial evidence exists to indicate that T cell responses can mediate the suppression and elimination of tumors in animal models and in human subjects (1-3). Tumors in subjects with progressive cancer may evade these responses through various mechanisms, including antigenic variation and expression of immune-suppressive factors such as IL-10, VEGF, TGF beta and PD-L1 (4). Additionally, the cytokine milieu in the tumor-bearing host can determine the particular type of immune response mounted, and thus the efficiency of the anti-tumor response in eradicating tumor cells.
Certain tumors, including melanoma, may be associated with production of IL-5, IL-4, and IL-13 by CD4 and CD8 T cells (TH2 type immune responses), thereby adding an additional immunomodulating step that can repress host anti-tumor responsiveness (5-9). McCarter et al showed that conditioned medium from in vitro cultures of melanoma cells could drive T cell lines toward a TH2 phenotype in co-culture with MDDC (7). Notably in a clinical trial in which human subjects with melanoma received autologous MDDC transfected with mRNA encoding tumor antigen, antigen-specific T cells produced a significantly greater amount of IL-5 and IL-13 after vaccination, while there was no significant increase in IFN-γ or TNF-α production (Type I (TH1) T cell response) in the same patients (10). Thus effective tumor vaccines should induce T cell responses to a broad range of epitopes derived from essential oncogenic proteins, while promoting pro-inflammatory functions to overcome tumor-associated immune deviation. A widely investigated method of therapeutic anti-tumor vaccination is dendritic cell (DC) immunotherapy (11). DC in their various forms are the most potent antigen- presenting cells for the induction of immune responses in animals and humans. DC may be loaded with tumor antigens by exposure to recombinant tumor proteins or tumor lysate, or by DNA transfection, retroviral transduction, or mRNA transfection. In particular, transfection with mRNA is a promising method for the delivery of multiple tumor antigens and immunomodulatory molecules to DC in a safe and efficient manner (12).
The type of DC most widely used for immunotherapy is the monocyte-derived DC (MDDC), which is differentiated in vitro from peripheral blood monocyte precursors in the presence of IL-4 and GM-CSF. Following differentiation, DC must undergo a maturation step before they gain the ability to induce and enhance anti-tumor T cell responses (13, 14). For clinical production of MDDC, maturation is usually induced by a cytokine cocktail that includes recombinant TNF-α, IL-1β, and IL-6. PGE-2 is often added (15), as it preserves the migratory ability of maturing MDDC as demonstrated in in vitro studies (16). However PGE-2 inhibits the production of bioactive IL-12p70 (17-19). IL-12p70 is a heterodimeric cytokine that plays an essential role in innate and adaptive immune responses that mediate anti-tumor resistance (20). It functions in the regulation of the helper T cell response to optimally induce, activate and expand a TH1 response (21). IL-12p70 also enhances the CD8+ T cell cytolytic response (22), and its' anti-angiogenic properties further contribute to an important role in anti-tumor responses (20, 23). Many experimental mouse models have illustrated the effectiveness of IL-12p70 in inhibiting tumor growth, improving survival of tumor-bearing mice and inducing effective tumor-specific immunity (20). Experiments in vitro and in vivo have opened the way for clinical trials using systemic recombinant human IL-12p70 as adjuvant anti-tumor therapy (24-26). The trials to date have been promising and have shown some evidence of an effective immune response- including enhancement of natural killer (NK) cell activity, T cell proliferation, and IFN-γ production- and clinical regression of tumors with partial and complete responses (20, 24, 26). Systemic administration of IL-12p70, however, has inherent and considerable toxicities (27). Furthermore, lack of availability of clinical grade rHu IL-12p70 may pose a limitation to additional trials. Therefore new approaches to improve the immunogenicity of PGE-2-treated MDDC are urgently required.
In this study, we investigated the effect of transfecting human MDDC with a single synthetic mRNA encoding IL-12p70. We evaluated the ability of transfected MDDC to promote pro-inflammatory functions crucial for the elimination of tumors in vivo. These include NK cell activation, priming and expansion of tumor-specific T cell responses, and type 1 skewing of T cells derived from melanoma patients. Our results suggest that IL-12p70 mRNA transfection of PGE-2-treated MDDC promotes innate immunity and reverses tumor-associated immune evasion by enhancing the production of IFN-γ, and simultaneously inhibiting the production of the type 2 cytokines IL-4, IL-5, and IL-13. Significantly, these effects are demonstrable at both the CD4+ and CD8+ T cell levels.
Materials and Methods
Human Subjects
Subjects with melanoma were recruited from the New York University (NYU) Cancer Institute, and peripheral blood cell samples were acquired by leukapheresis. HLA- A*0201 positive stage IIB, IIC and/or III melanoma patients that were clinically free of disease but at high risk for recurrence were included. Blood samples from healthy subjects were acquired by venipuncture or leukapheresis. Leukaphereses from normal healthy subjects were obtained from BRT Laboratories (Baltimore, MD). Approval was obtained from the NYU School of Medicine Institutional Review Boards (IRB) for these studies. For natural killer cell studies described in Figure 2, healthy donors were recruited at the Massachusetts General Hospital (MGH); the MGH IRB approved the study, and each subject gave informed consent for participation in the study. In all cases informed consent was provided according to the Declaration of Helsinki.
Figure 2. IL-12p70 produced by transfected MDDC is bioactive and enhances target-specific responses of NK cells.
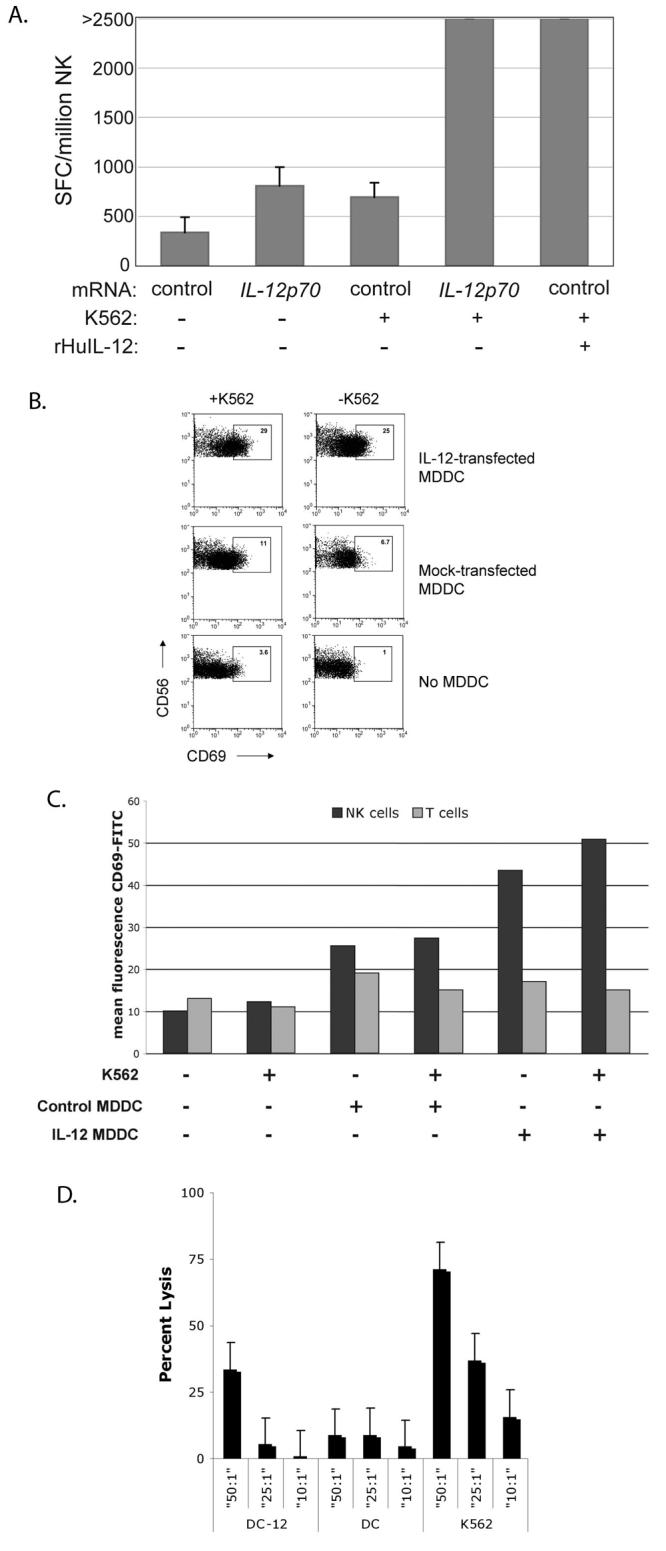
A. NK cells isolated from PBMC of a normal donor were incubated with autologous mock-transfected or IL-12p70-transfected MDDC in the presence or absence of class I-negative K562 targets. rHuIL-12 was added to co-cultures of NK cells and mock-transfected MDDC as an additional control. IFN-γ production, a measure of NK cell activation, was subsequently measured in an overnight ELISpot assay.
B. PBMC from a normal donor were co-cultured overnight with autologous IL-12p70-transfected MDDC in the presence or absence of K562 targets as shown. The degree of NK cell activation was determined by measuring CD69 expression on CD3negCD56+ lymphocytes with FACS. The percent CD69+ cells is shown within the gate.
C. Mean fluorescence intensity (MFI) of CD69 on CD3negCD56+ NK cells and CD3+CD56neg T cells. Enhancement of activation by IL-12p70 was specific for CD3negCD56+ NK cells, as there was no change in the activation state of CD3+CD56neg T cells. Results in this figure are a representation of three similar experiments.
D. IL-12 production by immature MDDCs increases the cytolytic activity of autologous NK cells. Immature MDDC were transfected with IL-12p70 or control mRNA, labeled with 51Cr and incubated with fresh autologous NK cells for four hours in a chromium release assay; untransfected K562 targets were used as a positive control for cytolytic activity, The percent lysis was calculated as [((sample count−spontaneous release)/(maximal release−spontaneous release))×100].
Cell culture
PBMC were purified by Ficoll-Hypaque density gradient centrifugation, washed and frozen in sterile aliquots in medium supplemented with 10% human albumin USP and 10% DMSO. All cells were grown in RPMI medium (Cellgro, Herndon, VA) supplemented with 1mM HEPES (Gibco, Rockville, MD) and 20 μg/mL gentamicin (Gibco). RPMI with 1% pooled human plasma (Valley Biomedical, Winchester, VA) was used for culture of DC, and RPMI with 5% pooled human serum (PHS; ValleryBiomedical) was used for all other cell assays. For lymphoblastoid B-cell lines (BLCLs), 10% fetal bovine serum (FBS; Gibco) was used. T-cell lines were grown and assayed in 5% PHS.
DC Preparation
Peripheral blood mononuclear cells (PBMC) were obtained from buffy coats (New York Blood Center- NY, NY) or leukapheresis products (BRT labs-MD) by Ficoll-Paque Plus gradient centrifugation (Pharmacia, Uppsala, Sweden). PBMC were plated in tissue culture plates (Falcon; Becton Dickinson, NJ) at 35 × 106 cells/ plate in RPMI 1640 supplemented with 20 μg/ml of gentamicin, 10 mmol/l HEPES, and 1% human plasma. Cells were allowed to adhere for 2 hrs at 37° and 5% CO2. Non-adherent cells were then removed by several washes. The monocyte-enriched fraction was supplemented with 116 IU/ml rHu GM-CSF (Immunex, Seattle, WA) and 15-300 IU/ml rHu IL-4 (R&D, Minneapolis, MN) on days 2 and 4 of culture. Immature DC were harvested on days 5 of culture. DC were matured using a cocktail consisting of 5ng/mL IL-1β, 5 ng/ml TNF-α, 150 ng/ml IL-6 (all from R&D), and 1 μg/ml PGE-2 (Sigma), which was added directly to DC on day 5 of culture for 24 hrs.
Preparation of IL-12 construct
A single IL-12p70 open reading frame encoding the IL-12 p40 and p35 subunits joined by an elastin linker was subcloned by PCR from the pORF-hIL-12 vector (Invivogen) into the XhoI and PacI sites of transcriptional template vector psp73-Sph/A64 (28). PCR was performed using primers 5′ TATATACTCGAGAGGAGGGCCACCATGGGT and 5′ GCGCGCTTAATTAACCATTAGGAAGCATTCAGATAGC.
Transfection of APCs
For transfection, antigen-presenting cells (APCs) were washed 3 times and resuspended to a concentration of 3.3 × 106/mL in OptiMEM medium (Invitrogen, Carlsbad, CA). APCs (106) were incubated with mRNA on ice for 10 minutes and then electroporated in a 0.2-cm cuvette with a GenePulser Xcell (BioRad, Hercules, CA) at 400 V/0.75 ms, square wave protocol.
NK Cell experiments
MDDC were prepared from the blood of normal donors, and transfected with IL-12p70 mRNA or irrelevant control mRNA. Transfected MDDC were co-cultured with autologous purified NK cells or autologous whole PBMC as described. Untouched NK cells were negatively isolated from PBMC with a RosetteSep NK kit (StemCell Technologies, Inc, Vancouver, CA). For the Elispot assay, NK cells and MDDC were co-cultured at a ratio of 1:1 overnight. For the flow-based assay, MDDC were co-cultured with autologous PBMC overnight in 5% human serum without addition of Brefeldin A. PBMC were then stained for CD3 and CD56, fixed, and analysed by FACS. To measure cytolytic activity, immature MDDC were transfected with IL-12p70 or control mRNA, and rested at 37° for two hours. K562 and transfected MDDC targets were labeled with Na2(51Cr04) for 1 h at 37 °C and incubated with autologous NK effectors at the indicated ratios for four hours at 37°. The percent lysis was calculated as [((sample count−spontaneous release)/(maximal release−spontaneous release × 100].
Isolation and Quantification of mRNA from T cell lines
Total RNA was extracted using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. Complementary DNA was generated using an oligo dT primer and Powerscript RT enzyme (BD Clontech, Palo Alto, CA) at 42° C for 60 minutes. Gene expression was quantified by real- time PCR on an Mx3005P thermocycler (Stratagene, La Jolla, CA) using Full Velocity SYBR Green 2x mix (Stratagene) and gene-specific primers (Table I, supplemental materials). Following denaturation (10 minutes, 95°C), DNA was amplified using 40 cycles at 95°C, 60°C, and 72°C (20 seconds each). Primer pairs were designed to span intron boundaries and were tested to verify specificity for mRNA products and not genomic DNA, based on size and sequencing of each product. All primers were purchased from Invitrogen. Results were normalized to HPRT using a 2(DDCt) method and are displayed as relative expression values in arbitrary units.
Intracellular Cytokine Staining
T cell lines generated from priming cultures were stimulated using 2uM of MART-1 21-35 peptide (YTTAEEAAGIGILTV). After 1 hr, 10ug/mL Brefeldin A (Sigma, St. Louis, MO) was added to the stimulated cells. 5 hrs later, cells were stained for surface markers (CD3, CD4, CD8, Becton Dickinson) then fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin, and stained for intracellular cytokines. Cells were acquired using FACScaliber and LSRII and analyzed by CellQuest and FlowJo.
Allogeneic Mixed lymphocytes reactions
Immature or cytokine cocktail matured DC were either mock electroporated or electroporated with 10μg/106 cells IL-12p70 mRNA (as above), and then mixed in triplicate with allogeneic CD4+ naive T cells derived by magnetic depletion of CD19, CD14, CD56, CD45RO (Miltenyi Biotech, Auburn, CA). rHu IL-12p70 (R&D Systems) was added to cultures on D0. After 5-7 days at 37 °C, after rigorous washing, CD3/CD28 beads (Dynal, Invitrogen) were added to the cultures for 14-24 hrs. Supernatants from stimulated cells were harvested and analyzed for cytokines secreted using the Th1/Th2 cytokine bead array (BD).
Priming Experiments
DC were grown from melanoma patients' PBMC as described above. Cytokine cocktail matured DC were harvested and transfected with 15 μg LysoMART-1 mRNA or 15 μg LysoMART-1 mRNA plus 10 μg IL-12p70 mRNA. The DC were then irradiated at 35 gray. DC were co-cultured with autologous non-adherent PBMC fractions after magnetic depletion of CD25+ cells (Miltenyi Biotech) in the presence of IL-6 (1000U/mL) (R&D Systems). Extra DC were frozen and used for restimulation. The cultures were re-stimulated every 7-10 days in 10 U/mL IL-2 and 5ng/mL IL-7 (final concentration) using the same DC conditions and tested by ICS or CBA for peptide- specific cells, or were stimulated with PMA plus Ionomycin or CD3/CD28 beads (Dynal) for cytokine secretion assays.
Cytokine Bead Array
Stimulated T cells were incubated at approximately 1 million / mL overnight, and samples of supernatants were acquired and frozen for future analysis. Thawed samples were tested with the human TH1/TH2 Cytokine Bead Array (BD Pharmingen, San Diego, CA) and used to measure IL-4, IL-5, IFNγ, TNF-α after T cell stimulations.
IL-12p70 measurement by ELISA
Cytokine cocktail matured DC were electroporated with 10μg IL-12p70 or GFP mRNA as negative control. DC were resuspended in RPMI with 1% human plasma (1% plasma) at 1 million cells/mL and cultured at 37°C, 5% CO2 with 95% humidity. At the indicated time points, supernatants were harvested and replaced with fresh, warm 1% plasma. Cumulative concentration of IL-12p70 was determined by culturing DC separately for various lengths of time prior to harvesting supernatants for ELISA (IL-12p70 ELISA kit, BD).
LysoMART-1 mRNA production
A transcriptional template for chimeric lysosome-targeted Melan-A/MART-1 (LysoMART-1) was constructed as described previously for other antigens (29, 30). We used a vector (pLyso) containing the following sequential elements: a T7 polymerase promoter, a Kozak ribosome binding motif including the start codon of the open reading frame (ORF), the endoplasmic reticulum (ER)–targeting translocation signal of heat shock protein gp96, an antigen insertion locus, the lysosomal targeting motif of LAMP-1, a stop codon, and a polyA tail sequence. Synthetic mRNA was prepared by transcription from a linear DNA template with the Message Machine kit (Ambion, Austin, TX).
Deriving Tumor Infiltrating Lymphocytes (TIL)
To isolate melanoma TIL, fresh tumor tissue was minced under sterile conditions and plated in R10 medium (RMPI-1640 supplemented with 10% heat-inactivated human AB serum) at 2ml/well in wells of a well plate, adding one or two 1 mm cubes of tissue per well. Wells were supplemented with IL-2 (Roche, NJ, USA) to a final concentration of 150 U/ml to grow TIL. Cells were then cultured at 37°C, 5% CO2, replenishing IL-2 twice per week, and wells were split when necessary. TIL were harvested once 24 confluent wells were obtained and were then counted and frozen in aliquots in R10-PHS/10% DMSO supplemented with 150 U/ml IL- 2 and stored in a liquid nitrogen freezer.
Results
MDDC secrete IL–12p70 upon transfection with IL–12p70 mRNA
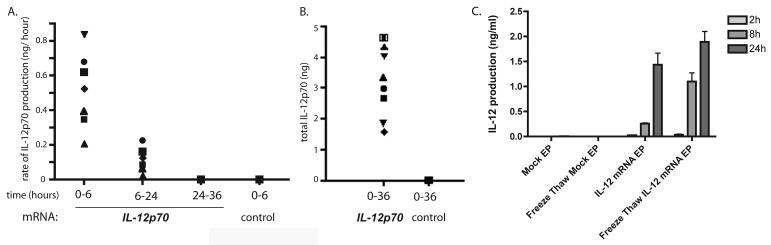
Previous reports have shown that transfection with mRNA is an efficient way to modify MDDC to express antigens or costimulatory molecules (31). We constructed a template vector encoding a single open reading frame representing the p35 and p40 subunits of IL–12p70 joined together by an elastin linker under the control of the T7 polymerase promoter. We used this template to synthesize mRNA in vitro, and transfect MDDC by electroporation. As shown in Figure 1, MDDC secreted significant levels of IL–12p70 upon transfection with IL–12p70 mRNA, but not GFP RNA. The majority of the secretion occurred within six hours after transfection. Additionally, MDDCs frozen following transfection with IL-12p70 retained IL-12 secretory ability after thawing (Figure 1C). Consistent with previous reports (24, 27), we found that mRNA capped with anti-reverse cap analog (ARCA) induced prolonged secretion compared to conventional cap analogs (not shown).
Figure 1. IL-12p70mRNA transfected MDDC secrete high amounts of IL-12.
Cytokine cocktail-matured DC were electroporated with 10μg IL-12p70 or control GFP mRNA and cultured at a density of 106 cells/ml. Concentrations of IL-12p70 were measured by ELISA. A. Rate of IL-12 secretion was determined by collecting supernatants from cultures at 6, 24 and 36 hours post-electroporation and replacing with fresh, warm medium. Rate (ng/ hour) was calculated by dividing the amount of IL-12p70 produced per 106 DC by the number of hours in the time interval. Symbols represent distinct transfected APC populations (DC and BLCL).
B. Cumulative concentration of IL-12 was determined by culturing several preparations of DC or BLCL for 36 hrs and collecting supernatants at the end of the culture period.
C. IL-12p70 mRNA transfected MDDC retain IL-12 secretory ability upon freeze-thawing. MDDC were transfected with IL-12p70 and frozen after 1 hr of culture at 37 degrees. Frozen MDDC were subsequently thawed and IL-12p70 concentrations measured following thawing. Results are representative of 4 separate experiments.
Transfection of MDDC with IL–12p70 mRNA enhances natural killer cell response to class I-negative targets
In order to determine the bioactivity of the secreted IL-12p70 itself, we tested whether immature DC transfected with IL-12p70 were able to enhance NK cell activation. NK cells were isolated by negative selection from PBMC, and transfected MDDC were mixed 1:1 with NK cells in the presence or absence of K562 target cells. As shown in Figure 2A, IL–12p70 transfected DC enhanced K562-induced IFN-γ secretion by autologous NK cells to a significantly greater extent than mock transfected DC.
As an alternative way to measure NK cell activation, we co-cultured transfected immature MDDC from a normal donor with autologous PBMC in the presence or absence of K562 targets. After 16 hours of co-culture, activation of NK cells was determined by FACS staining for CD69, an activation antigen. As shown in Figure 2B, IL-12p70 transfected MDDC induced a 2-3 fold increase in CD69hi NK cells compared to mock-transfected MDDC. The increased activation was specific to NK cells, as the mean fluorescence intensity (MFI) of CD69 staining specifically increased on NK cells (CD56+CD3−) but not on T cells (CD56−CD3+) (Figure 2C). Furthermore, transfection of immature MDDC with IL-12p70 produced a modest enhancement in cytolytic activity of autologous NK effectors (Figure 2D).
Transfection of MDDC with IL-12 p70 mRNA enhances priming and expansion of antigen-specific T cells
An important function of IL-12p70 is to skew primary immune responses towards TH1-type and away from TH2 type responses. Therefore we tested whether IL-12p70 transfection of immature MDDC could skew the priming of naïve T cells in vitro. We first used immature DC which by themselves do not efficiently induce TH1 responses. Naive (CD45ROneg) CD4+ T cells were co-cultured with allogeneic MDDC previously transfected with IL-12p70 or with control mRNA. In some co-cultures, recombinant IL-12p70 was added as shown (Figure 3). After 5-7 days, T cells were stimulated with anti-CD3 antibody, and the production of representative TH1 (TNF-α and IFN-γ) and TH2 (IL-4, IL-5) cytokines was measured using a cytokine bead array (CBA). In contrast to control DC alone, IL-12p70 produced by transfected-DC or added as recombinant protein, inhibited production of the TH2 cytokine IL-5 (Figure 3A). IFN-γ production was unchanged or increased in the presence of transfected or exogenous IL-12 respectively, indicating that the decrease in IL-5 production was specific and not a result of T cell death secondary to the effects of IL-12 (Figure 3Bii). The experiments were repeated using cytokine cocktail-matured MDDC. Similar results were obtained confirming that IL-12 enhances TH1 skewing by inhibiting the production of the TH2 type cytokine IL-5, while enhancing TH1 cytokine expression. To confirm the importance of IL-12 in skewing the T cell response away from TH2, MDDC were matured by the TLR agonists Poly I:C and LPS, cytokine cocktail or left immature. IL-12 secretion was measured by these various MDDC. Only Poly I:C-matured MDDC produced IL-12p70 and were able to inhibit IL-5 secretion of T cells in an allogeneic MLR (Figure 3C).
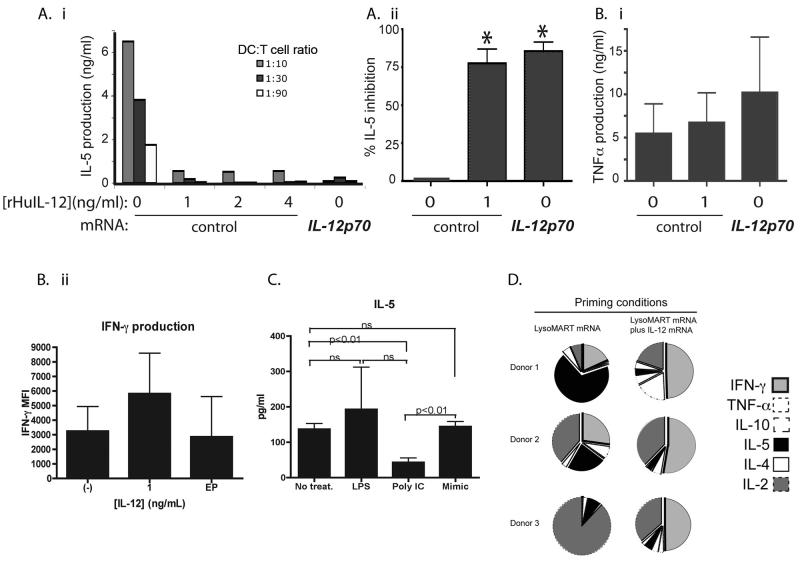
Figure 3. IL-12p70 transfection and recombinant IL-12p70 both inhibit IL-5 production in a primary allogeneic response.
Immature MDDC were transfected with IL-12p70 or control GFP mRNA and co-cultured with naïve allogeneic CD4+ T cells in the absence or presence of recombinant IL-12 for 7 days. At the end of the culture period, cells were stimulated with CD3/CD28 beads for 14-24 hrs, and cytokine production was determined by CBA.
A i. IL-5 production by T cells induced in the presence or absence of IL-12p70 as indicated.
A ii. Percent inhibition of IL-5 expression by IL-12p70 transfected DC or by addition of 1 ng/ml rHu-IL-12. T cell: DC ratio was 30:1. Inhibition was calculated as a percentage: ([IL-5]no IL-12 condition−[IL-5]with IL-12)/[IL-5]no IL-12 condition. Inhibition was significant for both recombinant and transfected IL-12 (P=0.014 and 0.005, respectively).
B i. In contrast to IL-5, TNF-α production by allogeneic T cells is not inhibited by IL-12p70 transfection of MDDC. Results are representative of 3 experiments.
B ii . IFN-γ was measured from the primed T cells elicited in Figure 3. IFN-γ was measured using CBA assay (BD). Results are representative of 3 separate experiments.
C. Differentially matured MDDC were incubated with allogeneic CD4+CD45RA+ T cells at a ratio of 1:90 for 12 days. Subsequently T cells were stimulated with anti-CD3/CD28 beads overnight, and the presence of IL-5 was detected using CBA assay. MDDC were left immature or matured with LPS, Poly IC or Mimic. Differentially treated MDDC secreted varying amounts of IL-12p70. Immature and mimic matured MDDC failed to secrete IL-12p70 whereas LPS matured MDDC secreted from 0-100 pg/ml of IL-12. Poly IC induced IL-12p70 in the amounts of 250pg-20ng/ml (data from over 5 experiments).
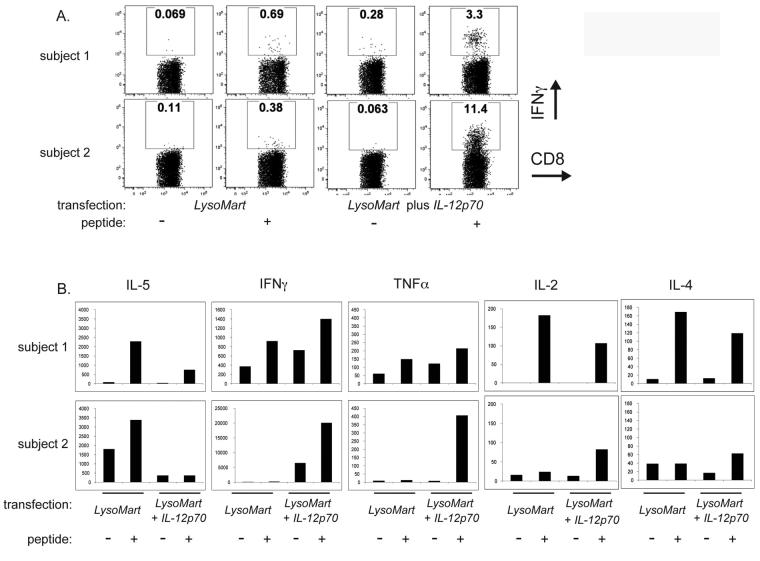
D. PBMC from healthy donors were used to obtain MDDC. DC were matured using cytokine cocktail and used to prime autologous CD25+-depleted T cells at a T cell : DC ratio of 10:1. DC were transfected with LysoMART-1 mRNA or LysoMART-1 mRNA plus IL-12p70 mRNA. T cell cultures were restimulated 2 times and tested for specificity using LysoMART-1 transfected autologous DC. Supernatants of co-cultures were evaluated for cytokine secretion. T cells primed in the presence of IL-12p70 secreted more IFN-γ and less IL-5. Results illustrated are proportions of concentrations of cytokine secreted based on proportion of absolute values.
We next evaluated the effect of IL-12p70 transfection on the induction of tumor antigen-specific T cell responses by DC. The melanoma associated antigen MART-1 was used as a model antigen. In order to enhance antigen presentation to CD4+ T cells, we expressed MART-1 as a chimeric fusion construct (LysoMART-1) with the lysosomal targeting sequence of LAMP-1, as described for other antigens (26). MDDC prepared from normal donors were transfected with LysoMART-1 mRNA only, or LysoMART-1 plus IL-12p70 mRNA and co-cultured with autologous T cells for two cycles of stimulation at 1-week intervals. At the end of this period, the resulting T cell lines were stimulated with autologous LysoMART-1-transfected MDDC, and cytokine production was analyzed by CBA. Priming with IL-12p70-transfected MDDC led to a significant increase in IFNγ production. A mean enhancement of 54-fold in the IFN-γ:IL-5 ratio was observed, compared with control MDDC which did not produce IL-12 (Figure 3D).
MDDC derived from melanoma patients prime and expand IFN-γ producing cells following transfection with mRNA encoding IL-12p70
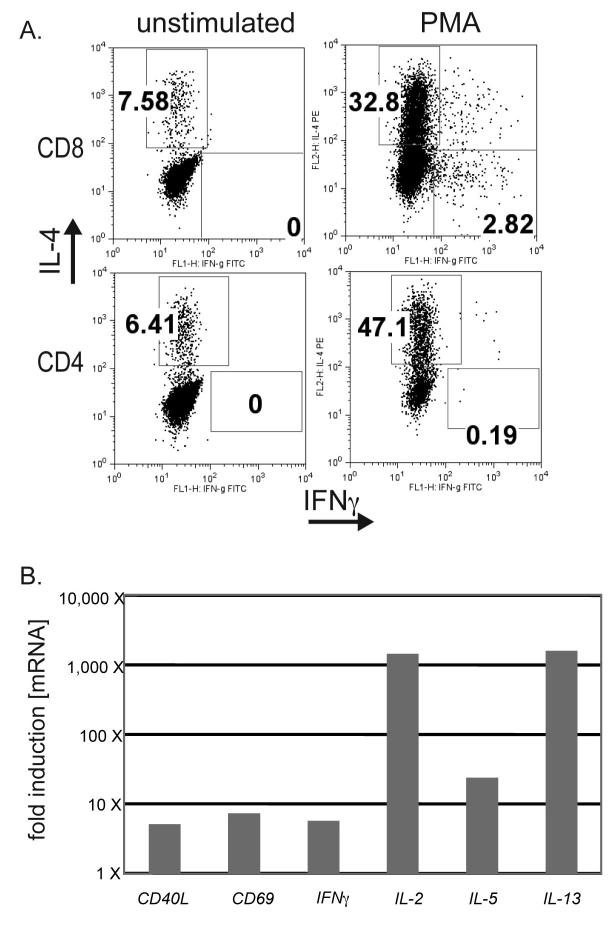
TH2 skewed lymphocytes have been documented in circulating blood cells and TIL of melanoma patients (5, 8, 9). We grew TIL from resected melanoma patients and chose to focus our studies on one TIL cell line. TIL obtained by isolating and culturing T cells from a resected melanoma tumor in the presence of IL-2 had properties consistent with previous observations. The line contained both CD4+ and CD8+ T cells and produced large amounts of IL-4, but not IFN-γ in response to PMA/Ionomycin stimulation (Figure 4A). qRT-PCR analysis to examine expression and/or production of CD40L, CD69, IFN-γ, IL-2, IL-5, IL-4 and IL-13 was also undertaken. Following stimulation, mRNA for IL-2, IL-5, and IL-13 was significantly upregulated (Figure 4B). The overall levels of these cytokines are also shown in (Table S2). IL-4 expression was constitutively high and no detectable upregulation was detected following stimulation of the cells (data not shown). These results indicate that the line exhibited a TH2-like phenotype, inclusive of both CD4+ (TH2) and CD8+ (TC2) T cells.
Figure 4. TIL isolated from a patient with metastatic melanoma secrete predominantly TH2-type cytokines.
A. Tumor infiltrating lymphocytes (TIL) were isolated from a resected melanoma, expanded in the presence of IL-2, and frozen for storage in liquid nitrogen. Thawed TIL were rested for 24 hrs in the absence of any cytokines. Upon stimulation with PMA plus Ionomycin for 6 hours, both CD8+ and CD4+ T cells secreted high levels of the IL-4 and little or no IFN-γ. T cells were tested by ICS. Results are representative of 3 separate experiments.
B. TIL were stimulated as above with PMA plus Ionomycin and lysed. mRNA was extracted and subjected to RT-PCR. cDNA for 49 different T cell products was measured by qPCR, and values were normalized to the quantity of actin mRNA. Values shown represent those factors for which PMA treatment resulted in >5 X induction of specific message.
We next tested whether circulating blood T cells of melanoma patients are similarly skewed towards a TH2-like phenotype. We co-cultured CD25+ depleted T cells isolated from the blood of 4 melanoma patients with autologous MDDC transfected with LysoMART-1-1 alone, or LysoMART-1 plus IL-12p70 mRNA. The T cells were stimulated every seven days with MDDC identical to those used in the induction step. One week after the induction step or the first restimulation, T cell responses were tested for specificity by CBA assay using a pool of overlapping peptides (OLP) spanning the entire MART-1 antigen sequence. Transfection of MDDC with IL-12p70 mRNA skewed the phenotype of the resulting T cell lines by enhancing production of IFN-γ and inhibiting production of IL-5 and IL-4 (data not shown). The resulting MART-1-primed T cell lines were restimulated for an additional 7 days with autologous LysoMART-1- or LysoMART-1 + IL-12p70- mRNA transfected MDDC. They were evaluated for TH1/ TH2 type skewing after non-specific, polyclonal stimulation with PMA/ Ionomycin, and results for 1 representative patient are shown in (Figure 5). Priming by IL-12p70-transfected MDDC strongly skewed T cells toward a TH1-type phenotype, as indicated by a 50-fold enhancement in the ratio of IFN-γ/IL-13-producingCD8+ T cells (Figure 5A) and CD4+ T cells (Figure 5C). Notably, in the absence of IL-12p70 the majority of primed IL-2+ CD8+ and CD4+ T cells did not produce IFN-γ, whereas IL-12p70 transfection caused most IL-2+ CD8+ and CD4+ T cells to acquire the ability to produce IFN-γ (Figure 5B and Figure 5D, compare internal panels Bi with iii and Di with iii). In the absence of IL-12p70, the majority of IL-2+, IFN-γ - T cells instead produced IL-13 and IL-5, whereas IL-12p70 strongly curtailed IL-5 production in these cells (Figure 5Biiv, 5Di-iv). Interestingly, the amount of IL-5 secreted by T cells primed in the absence of IL-12 was increased after each round of DC stimulation (data not shown). Of note the IFN-γ producing cells were both CD8+ and CD4+ T cells, although the vast majority of antigen-specific T cells were found to be CD8+.
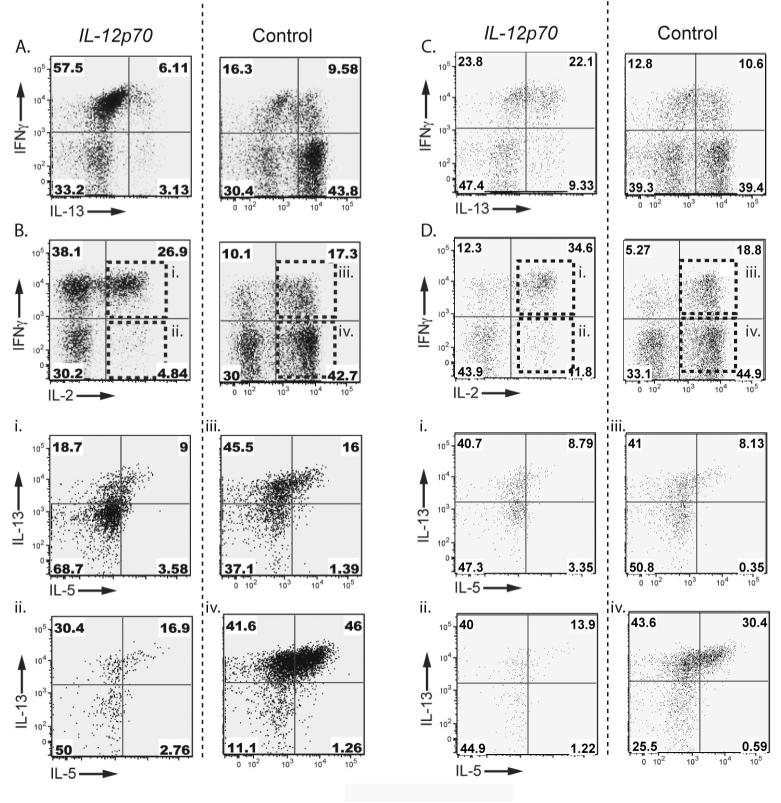
Figure 5. IL-12p70-transfected DC preferentially induce Tc1 responses from PBMC of melanoma patients.
MDDC from a melanoma patient were transfected with LysoMART-1 mRNA plus IL-12p70 mRNA (left column) or LysoMART-1 mRNA only (right column) and used to prime autologous T cells. T cells were restimulated once or twice with MDDC at 7-10 day intervals. Thirty days after induction, T cell lines were stimulated by PMA/ Ionomycin and cytokine production was determined by ICS. Results shown are gated on CD8+ T cells (panels A, Bi-iv) or CD4+ T cells (panels C, Di-iv).
A,C. IL-12p70 transfection enhanced IFN-γ and suppressed IL-13 production by induced T cells.
B,D. IL-12p70 transfection reduced the frequency of IL-2+IFNneg cells and enhanced the frequency of IL-2+IFN-γ+ cells. For C-F and I-L (internal panels i-iv), cells within the upper-right and lower-right quadrants in B and D respectively as shown were further analyzed for production of IL-5 and IL-13.
Bi,iii, Di,iii. A minority of IL-2+IFN-γ+ cells from B and D produced IL-5.
Bii,iv, Dii,iv. IL-2+IFN-γneg cells shown in B and D produce TH2 type cytokines (IL-13 and IL-5), and IL-5 production by these cells was strongly suppressed by IL-12p70 transfection. Results are representative of 4 patients tested.
Finally, the tumor antigen-specific responses in these induced T cell lines were also measured. The MART-1 specific responses for two of the patients are shown in Figure 6. Induction with IL-12p70-transfected MDDC produced an enhancement of 5-30-fold in the frequency of CD8+ T cells that produced IFN-γ in response to the immunodominant MART-1 peptide (Figure 6A). IL-12p70 also skewed responses to MART-1 peptide toward TH1-type cytokine production as measured by CBA, specifically by enhancing production of IFN-γ and reducing production of IL-5 (Figure 6B).
Figure 6. IL-12-transfected DC enhance priming of MART-1-specific TH1 like cells.
A. MDDC from HLA A*0201 melanoma patients were transfected with LysoMART-1 mRNA only or with LysoMART-1 plus IL-12p70 mRNA and used to prime autologous T cells. T cells lines from two patients shown were stimulated using 2 μM of the HLA A*0201 restricted MART-1(21-35) peptide in the presence of BFA for 6 hours and IFN-γ production was determined by ICS.
B. Culture supernatants of T cell lines were evaluated for cytokine secretion following overnight stimulation in the absence or presence of 2 μM MART-1(21-35) peptide.
In summary, the expression of IL-12p70 by cytokine cocktail matured DC shifts the underlying type 2 balance in melanoma patients' T cells towards type 1. The ability of IL-12 to skew CD8+ and CD4+ T cell responses by enhancing IFN-γ secretion, reducing the production of IL-5 and IL-13 and priming tumor-antigen specific T cells emphasizes the potential advantage of IL-12 mRNA transfected-DC as adjuvants in tumor immunotherapy.
Discussion
Therapeutic induction of effective anti-tumor T cell responses in human cancer patients will require one to overcome several hurdles. These include a relative paucity of tumor-specific antigens (compared to vaccination against infectious diseases), tumor antigen loss variants, and tumor-associated immune deviation and dysfunction. Various lines of evidence suggest that in vivo skewing of T cell responses toward a TH2 type is an important mechanism of immune evasion in cancer patients (5, 6, 8, 9, 32), (33) and animal models (34). This skewing may limit the efficacy of immunotherapeutic approaches. Several reports indicate that, compared to TH1 skewing, TH2 skewing of the vaccine-induced tumor-specific response correlates with worse disease outcome (6, 9, 34). Along those lines, we found that a TIL line isolated from resected melanoma exhibit a strong TH2 type phenotype, producing relatively large amounts of IL-4 and IL-13 compared to IFN-γ or TNF-α (Figure 4, above). Taken together these results suggest that both spontaneous and vaccine-induced tumor-specific T cell responses in human subjects are often deviated toward a non-protective type 2 phenotype. Ensuring induction of a strong type 1 response may be critical to the development of effective cancer vaccines.
The events that lead to induction of type 1 or type 2 immune responses are complex and multifactorial. One of the most important determinants of type 1 skewing is production of IL-12p70 during the induction phase of the response (35). However, the production of clinical grade DC that are competent to produce IL-12p70 has proven to be extremely difficult, because MDDC matured in vitro lose the ability to migrate to the lymph node upon clinical infusion unless they are matured in the presence of PGE-2 (15, 36). PGE-2, however, blocks IL-12p70 expression by MDDC (17). Thus most MDDC presently used in clinical trials do not make IL-12p70, and this defect may impair the efficacy of such vaccines.
Several promising methods exist to load antigen-presenting cells with tumor-derived antigens in order to maximize the breadth of antigen-specific responses. In particular, transfection of MDDC with synthetic mRNA encoding tumor-derived antigens offers several advantages. Transfected MDDC present endogenously processed antigenic peptides. Secondly, mRNA transfection is far more efficient than DNA transfection of MDDC, with nearly 100% of MDDC expressing antigen of interest (29). Third, mRNA lacks the theoretical risks associated with chromosomal integration as occurs in DNA transfection or retroviral transduction. Finally, RNA transfection offers a relatively straightforward method to alter expression of immunomodulatory factors to enhance DC function. Thus mRNA transfection has been used to produce expression of pro-inflammatory factors [including IL-12 (37), OX-40L (38) and constitutively active TLR4 (39, 40)] and siRNA has been used to prevent expression of inhibitory factors [eg. SOCS (41)]. RNA transfection may present a general tool to derive DC capable of overcoming the various challenges associated with anti-tumor vaccination. Recently Bontkes et al demonstrated that transfecting MDDC with IL-12p70 mRNA could cause MDDC to produce clinically relevant amounts of IL-12p70 without compromising the ability of MDDC to migrate in response to CCR7 stimulation in vitro (37). These investigators reported that MDDC transfected with IL-12p70 induced an antigen-specific response of larger magnitude than those transfected without IL-12p70; however, they did not examine the cytokine profiles of the induced T cell lines.
In the experiments we present here, we investigated the effect that IL-12p70 transfection had on activating the innate immune system, and on the phenotype of induced T cell responses. IL-12p70 transfected MDDC were shown to produce significant quantities of IL-12p70 (1-4 ng/million cells/ day; Figure 1). The majority of IL-12 was secreted within the first six hours following transfection, and production of IL-12 was retained even after MDDC were immediately frozen and thawed. Several studies have indicated that the migration of DC into tumor draining lymph nodes occurs as early as 0.5-6 hrs following injection intradermally (42-44). Our studies suggest that IL-12p70-transfected DCs that are trafficking to draining lymph nodes would still be secreting sufficient amounts of IL-12 to skew naïve T cells to a helper type phenotype. Moreover, various strategies, such as employing ARCA capped mRNA and devising mRNA stability configurations that increase the half-life and translation efficiency of the transfected mRNA would be likely to improve the production and possibly duration of IL-12 production.
IL-12p70 transfected MDDC enhanced natural killer activation in overnight assays as well as NK cell cytolytic activity, specifically against K562 target cells, but not autologous MDDCs (Figure 2). Transfection with IL-12p70 also altered the cytokine profiles of allogeneic antigen-specific T cell lines that expanded in response to transfected MDDC. Whereas immature or cytokine cocktail-matured DC reproducibly induced TH2-skewed T cells, this phenotype was reversed when the DC were transfected with IL-12p70, resulting in a significant increase in the ratio of TNF-α : IL-5 produced in response to allogeneic targets (Figure 3). Additionally, we compared LPS and Poly IC matured MDDC to MDDC matured with cytokine cocktail in their ability to skew CD4+ T cell responses to TH1. Only MDDC matured with Poly I:C were capable of secreting IL-12p70. As anticipated they, but not LPS-matured MDDC inhibited the priming of TH2 type T cells in the allogeneic MLR (Figure 3D). Poly I:C has not as yet been proven to be an effective DC maturation stimulus for use in vivo, whereas cytokine cocktail containing PGE-2 is a widely used and FDA-approved method for preparing vaccine grade MDDC. IL-12p70 mRNA transfection of cytokine cocktail matured MDDC represents a new approach to improve the quality of cells delivered in vivo.
Our studies revealed that IL-12p70 transfection enhanced the antigen specificity and the TH1 type skewing of T cell lines induced from PBMC of healthy donors and of melanoma patients by autologous MART-1 transfected MDDC (Figs 3, 5, 6). Consistent with several studies examining the induction of TH2 responses of vaccine-induced immune responses in melanoma (9, 10), we found that repeated rounds of stimulation with autologous cytokine cocktail matured MDDC led to an overall increase in IL-5 production of the T cells that were primed. However, the transfection of the MDDC with IL-12 significantly inhibited the IL-5 production, and conversely upregulated the production of IFN-γ by the T cells. Although a large emphasis has been placed on the role of CD4+ T helper cells in establishing the TH1/TH2 balance, the role of CD8+ T cells in balancing the dichotomy of the Type 1/ Type 2 (Tc1/Tc2) immune response has been described to a lesser extent (45-47). Several studies have illustrated the capability of CD8+ T cells to develop into Type 2, Tc2 cells, capable of secreting “Type 2” cytokines, including IL-4 and IL-5, with decreased anti-tumor capacity (48-50). Our study demonstrated that both CD4+ and CD8+ T cells were shifted to a “Type 1” phenotype by IL-12 transfection of DC. The specificity of the immune response generated in the T cell priming cultures of the melanoma patients was predominantly towards the immunodominant region of MART-1, namely MART21-35. Consistently, these responses were mostly of CD8+ T cells. The in vitro priming conditions may allow for favored priming of the CD8+ T cells due to the high frequency of precursor CD8+ T cells towards this epitope in HLA A*0201 individuals. Nonetheless, a highly Type 1 skewed immune response was elicited in CD8+ T cells by priming with IL-12 transfected DC. Taken together, these results demonstrate that transfection with IL-12p70 mRNA can enhance anti-tumor T cell responses not only quantitatively, in terms of the magnitude, but qualitatively, in terms of phenotype.
Many previous studies have established the promise of DC vaccines as a potentially effective mode of therapy for cancer patients. IL-12p70 could play an important role in the development of beneficial anti-tumor immunity. However, for recombinant IL-12p70 to be delivered by injection, there is a risk that the minimal effective systemic dose would be accompanied by severe toxicity. MDDC that are capable of migrating to the lymph node and secreting IL-12p70 may provide a very potent vehicle for delivery of IL-12p70 to naive T cells in the lymph node without a risk of severe systemic side effects. Our results demonstrate the potential usefulness of IL-12p70–transfected MDDC derived from the blood of melanoma patients for the induction of innate and adaptive immune responses that exhibit a strong pro-inflammatory phenotype and can aid in reversal of Type 2 skewing of the anti-tumor immune response evoked in the melanoma patients.
Supplementary Material
Primer pairs used for quantitative PCR (Figure 4B).
Overall levels of individual factors normalized for the house keeping genes Actin and HPRT.
Acknowledgments
This work was supported by NIH grants F32 AI058457-03 (DGK), R01 AI066992-01A1 (RTG), R01 044628 (NB), R01 1061684 (NB); by grants from the Doris Duke, Emerald and Burroughs Wellcome Foundations and the Elizabeth Glaser Pediatric AIDS Foundation (NB), and by the Howard Hughes Medical Institute (MAB and BDW).
Footnotes
Disclosures
One author (N.B) is co-inventor on patents relating to human DC production.
REFERENCES
- 1.Boon T, Cerottini J-C, Van Der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 2.Dhodapkar M,YJ, Chapman P, Cox W, Fonteneau J, Amigorena S, Houghton A, Steinman R, Bhardwaj N. Paucity of functional T-cell memory to melanoma antigens in healthy donors and melanoma patients. Clinical Cancer Research. 2000:4831–4838. [PubMed] [Google Scholar]
- 3.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 4.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother (1997) 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 5.Botella-Estrada R, Escudero M, O'Connor JE, et al. Cytokine production by peripheral lymphocytes in melanoma. Eur Cytokine Netw. 2005;16:47–55. [PubMed] [Google Scholar]
- 6.Lauerova L, Dusek L, Simickova M, et al. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma. 2002;49:159–166. [PubMed] [Google Scholar]
- 7.McCarter M, Clarke J, Richter D, Wilson C. Melanoma skews dendritic cells to facilitate a T helper 2 profile. Surgery. 2005;138:321–328. doi: 10.1016/j.surg.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Slager EH, Borghi M, van der Minne CE, et al. CD4+ Th2 cell recognition of HLA-DR-restricted epitopes derived from CAMEL: a tumor antigen translated in an alternative open reading frame. J Immunol. 2003;170:1490–1497. doi: 10.4049/jimmunol.170.3.1490. [DOI] [PubMed] [Google Scholar]
- 9.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–628. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyte JA, Kvalheim G, Lislerud K, et al. T cell responses in melanoma patients after vaccination with tumor-mRNA transfected dendritic cells. Cancer Immunol Immunother. 2007;56:659–675. doi: 10.1007/s00262-006-0222-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130–1136. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams S, O'Neill D, Bhardwaj N. Maturation matters: importance of maturation for antitumor immunity of dendritic cell vaccines. J Clin Oncol. 2004;22:3834–3835. doi: 10.1200/JCO.2004.99.019. author reply 3835. [DOI] [PubMed] [Google Scholar]
- 14.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AW, Truong T, Bickham K, et al. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(Suppl 4):A8–A22. doi: 10.1016/s0264-410x(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 16.Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 17.Kalinski P, Vieira PL, Schuitemaker JH, de Jong EC, Kapsenberg ML. Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood. 2001;97:3466–3469. doi: 10.1182/blood.v97.11.3466. [DOI] [PubMed] [Google Scholar]
- 18.Lee JJ, Takei M, Hori S, et al. The role of PGE(2) in the differentiation of dendritic cells: how do dendritic cells influence T-cell polarization and chemokine receptor expression? Stem Cells. 2002;20:448–459. doi: 10.1634/stemcells.20-5-448. [DOI] [PubMed] [Google Scholar]
- 19.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 20.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 22.Bhardwaj N, Seder RA, Reddy A, Feldman MV. IL-12 in conjunction with dendritic cells enhances antiviral CD8+ CTL responses in vitro. J Clin Invest. 1996;98:715–722. doi: 10.1172/JCI118843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golab J, Zagozdzon R. Antitumor effects of interleukin-12 in pre-clinical and early clinical studies (Review) Int J Mol Med. 1999;3:537–544. doi: 10.3892/ijmm.3.5.537. [DOI] [PubMed] [Google Scholar]
- 24.Bajetta E, Del Vecchio M, Mortarini R, et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res. 1998;4:75–85. [PubMed] [Google Scholar]
- 25.Gollob JA, Mier JW, Veenstra K, et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-gamma induction is associated with clinical response. Clin Cancer Res. 2000;6:1678–1692. [PubMed] [Google Scholar]
- 26.Robertson MJ, Cameron C, Atkins MB, et al. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin Cancer Res. 1999;5:9–16. [PubMed] [Google Scholar]
- 27.Atkins MB, Robertson MJ, Gordon M, et al. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res. 1997;3:409–417. [PubMed] [Google Scholar]
- 28.Nair S, Boczkowski D, Moeller B, et al. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood. 2003;102:964–971. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- 29.Kavanagh DG, Kaufmann DE, Sunderji S, et al. Expansion of HIV-specific CD4+ and CD8+ T cells by dendritic cells transfected with mRNA encoding cytoplasm- or lysosome-targeted Nef. Blood. 2006;107:1963–1969. doi: 10.1182/blood-2005-04-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nair SK, Boczkowski D, Morse M, et al. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 31.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 32.Wong R, Lau R, Chang J, et al. Immune responses to a class II helper peptide epitope in patients with stage III/IV resected melanoma. Clin Cancer Res. 2004;10:5004–5013. doi: 10.1158/1078-0432.CCR-04-0241. [DOI] [PubMed] [Google Scholar]
- 33.Becker Y. Molecular immunological approaches to biotherapy of human cancers--a review, hypothesis and implications. Anticancer Res. 2006;26:1113–1134. [PubMed] [Google Scholar]
- 34.Winter H, Hu HM, Poehlein CH, et al. Tumour-induced polarization of tumour vaccine-draining lymph node T cells to a type 1 cytokine profile predicts inherent strong immunogenicity of the tumour and correlates with therapeutic efficacy in adoptive transfer studies. Immunology. 2003;108:409–419. doi: 10.1046/j.1365-2567.2003.01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 36.Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood. 2002;100:1362–1372. doi: 10.1182/blood-2001-12-0360. [DOI] [PubMed] [Google Scholar]
- 37.Bontkes HJ, Kramer D, Ruizendaal JJ, et al. Dendritic cells transfected with interleukin-12 and tumor-associated antigen messenger RNA induce high avidity cytotoxic T cells. Gene Ther. 2007;14:366–375. doi: 10.1038/sj.gt.3302874. [DOI] [PubMed] [Google Scholar]
- 38.Dannull J, Nair S, Su Z, et al. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood. 2005;105:3206–3213. doi: 10.1182/blood-2004-10-3944. [DOI] [PubMed] [Google Scholar]
- 39.Abdel-Wahab Z, Cisco R, Dannull J, et al. Cotransfection of DC with TLR4 and MART-1 RNA induces MART-1-specific responses. J Surg Res. 2005;124:264–273. doi: 10.1016/j.jss.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Cisco RM, Abdel-Wahab Z, Dannull J, et al. Induction of human dendritic cell maturation using transfection with RNA encoding a dominant positive toll-like receptor 4. J Immunol. 2004;172:7162–7168. doi: 10.4049/jimmunol.172.11.7162. [DOI] [PubMed] [Google Scholar]
- 41.Shen L, Evel-Kabler K, Strube R, Chen SY. Silencing of SOCS1 enhances antigen presentation by dendritic cells and antigen-specific anti-tumor immunity. Nat Biotechnol. 2004;22:1546–1553. doi: 10.1038/nbt1035. [DOI] [PubMed] [Google Scholar]
- 42.Mackensen A, Krause T, Blum U, et al. Homing of intravenously and intralymphatically injected human dendritic cells generated in vitro from CD34+ hematopoietic progenitor cells. Cancer Immunol Immunother. 1999;48:118–122. doi: 10.1007/s002620050555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morse MA, Coleman RE, Akabani G, et al. Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res. 1999;59:56–58. [PubMed] [Google Scholar]
- 44.Ridolfi R, Riccobon A, Galassi R, et al. Evaluation of in vivo labelled dendritic cell migration in cancer patients. J Transl Med. 2004;2:27. doi: 10.1186/1479-5876-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996;8:336–342. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 46.Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodland DL, Dutton RW. Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol. 2003;15:336–342. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 48.Kemp RA, Ronchese F. Tumor-specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J Immunol. 2001;167:6497–6502. doi: 10.4049/jimmunol.167.11.6497. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Sad S, Kagi D, Mosmann TR. CD8Tc1 and Tc2 cells secrete distinct cytokine patterns in vitro and in vivo but induce similar inflammatory reactions. J Immunol. 1997;158:4152–4161. [PubMed] [Google Scholar]
- 50.Mosmann TR, Li L, Sad S. Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer pairs used for quantitative PCR (Figure 4B).
Overall levels of individual factors normalized for the house keeping genes Actin and HPRT.