Abstract
Non-steroidal anti-inflammatory agents (NSAIDs) are associated with a marked reduction in the risk of developing Alzheimer’s disease, a form of dementia characterized by the accumulation of amyloid plaques containing the amyloid-β protein (Aβ). Studies of the effects of NSAIDs upon the inflammatory response surrounding amyloid plaques and upon the generation of Aβ from the amyloid precursor protein (APP) have led to two proposed mechanisms by which NSAIDs may protect against Alzheimer’s disease: one, the selective lowering of Aβ42 by a subset of NSAIDs; and two, the reduction of inflammation. Although Alzheimer’s disease is a disorder of brain and synaptic function, the effects of NSAIDs on Aβ-mediated suppression of synaptic plasticity and memory function have never been reported. We therefore investigated how three different NSAIDs, chosen for their distinct effects on Aβ42 production and the inhibition of the cyclooxygenase (COX) isoenzymes, COX-1 and COX-2, affect memory function and synaptic plasticity. By focusing upon brain and synapse function, we made novel observations about the effects of NSAIDs on Aβ-mediated neural processes. Here we report that the selective inhibition of COX-2, but not COX-1, acutely prevented the suppression of hippocampal long-term plasticity (LTP) by Aβ. The non-selective NSAIDs, ibuprofen and naproxen, and a selective COX-2 inhibitor, MF-tricyclic, each restored memory function in Tg2576 mice over-expressing APP, and also blocked Aβ-mediated inhibition of LTP. There was no advantage of ibuprofen, a selective Aβ42-lowering agent (SALA), over the non-SALAs, naproxen and MF-tricyclic. The beneficial effects on memory did not depend upon lowered levels of Aβ42 or the inflammatory cytokines, tumour necrosis factor α (TNF-α) and interleukin 1β (IL-1β). Intriguingly, improved memory function was inversely related to prostaglandin E2 (PGE2) levels. Conversely, exogenous PGE2 prevented the restorative effects of COX-2 inhibitors on LTP. The data indicate that the inhibition of COX-2 blocks Aβ-mediated suppression of LTP and memory function, and that this block occurs independently of reductions in Aβ42 or decreases in inflammation. The results lead us to propose a third possible mechanism by which NSAIDs may protect against Alzheimer’s disease, involving the blockade of a COX-2-mediated PGE2 response at synapses.
Keywords: NSAIDs, inflammation, transgenic, memory, synaptic plasticity
Introduction
Non-steroidal anti-inflammatory agents (NSAIDs) have been associated with up to an 80% reduction in the incidence of Alzheimer’s disease (in t’ Veld et al., 2001), but the underlying protective mechanism is unknown. Given the potential benefits of NSAIDs in individuals at risk for Alzheimer’s disease, it is important to understand the mechanism by which this class of drugs may protect memory and synaptic plasticity using in vitro and in vivo models.
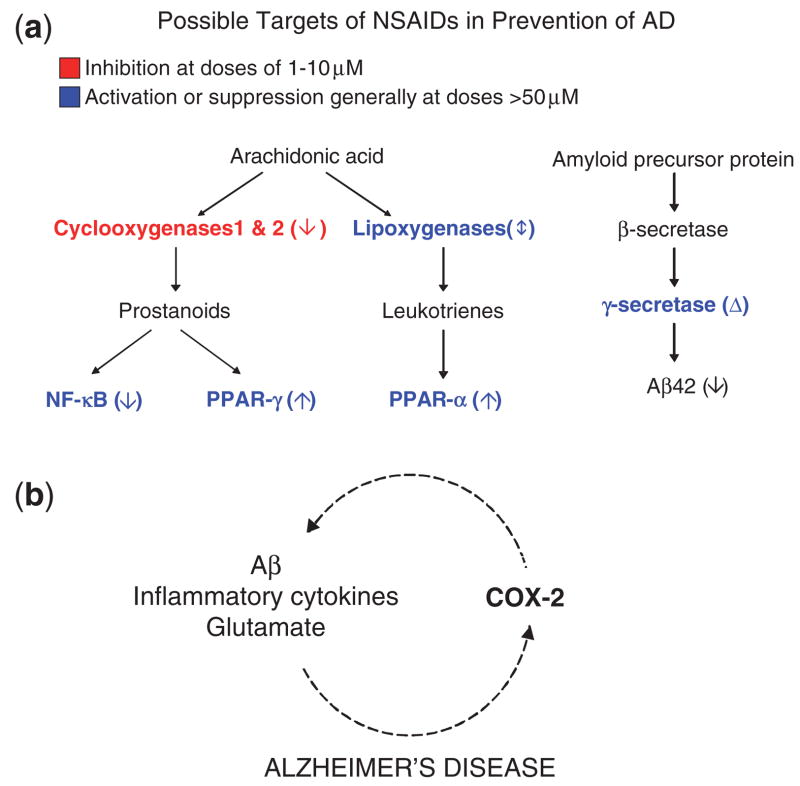
More than 20 targets of NSAIDs have been identified (Tegeder et al., 2001; Weggen et al., 2001). The major NSAID targets involved in inflammatory cascades and amyloid precursor protein (APP) processing are shown in Fig. 1a. COX-1 and COX-2 are the principal targets of NSAIDS at low doses between 1 and 10 μM (Tegeder et al., 2001). COX-1 and COX-2 catalyse the formation of prostaglandins, including prostaglandin E2 (PGE2), from arachadonic acid. The activation or suppression of other targets, including peroxisome proliferator-activated receptors PPARs and nuclear factor-κB, generally requires higher concentrations (>50 μM) of NSAIDs (Tegeder et al., 2001). Non-selective NSAIDs inhibit both COX-1 and COX-2. Selective inhibitors of COX-2 are called coxibs.
Fig. 1.
Possible targets of NSAIDS in the prevention of Alzheimer’s disease and proposed pathophysiology of memory loss in the Tg2576 mouse model of Alzheimer’s disease. (a) The activities of enzymes and factors (blue and red lettering) are affected by NSAIDs in the direction indicated by the arrow in parentheses or by the delta sign signifying a qualitative alteration in activity. The cyclooxygenases COX-1 and COX-2 are major targets of NSAIDs because their inhibition can be achieved at doses between 1 and 10 μM. The activation or suppression of other targets, including peroxisome proliferators-activated receptors (PPARs) and nuclear factor-kB (NF-kB), generally requires higher concentrations (>50 μM) of NSAIDs. Non-selective NSAIDs inhibit both COX-1 and COX-2. The coxibs selectively inhibit COX-2. A subset of NSAIDs, including ibuprofen but not naproxen or the coxibs, lowers production of Aβ42, the highly amyloidogenic 42-residue form of Aβ, by modulating γ-secretase activity. (b) In Alzheimer’s disease COX-2 is stimulated by Aβ, glutamate and inflammatory cytokines, which in turn are modulated by COX-2.
In Alzheimer’s disease, microglial cells expressing COX-1 surround Aβ deposits (Hoozemans et al., 2001), while COX-2 accumulates in neurons (Pasinetti and Aisen, 1998; Ho et al., 2001; Hoozemans et al., 2001). The protective effects of NSAIDs have been ascribed to their anti-inflammatory properties (McGeer, 2000; van Gool et al., 2003), but epidemiological data supporting the efficacy of low-dose (below anti-inflammatory doses) NSAIDs argue against the anti-inflammation effect (Broe et al., 2000). A subset of NSAIDs, including ibuprofen but not naproxen or the coxibs, lowers production of the highly amyloidogenic 42-residue form of amyloid-β protein (Aβ), Aβ42, by modulating γ-secretase activity (Weggen et al., 2001; Eriksen et al., 2003; Zhou et al., 2003). This COX-independent activity has been proposed as an alternative explanation to anti-inflammation as the mechanism by which NSAIDs exert their neuroprotective effects (Weggen et al., 2001).
In most tissues, COX-1 is constitutively expressed, while COX-2 is induced in response to injury. However, neurons differ because they express COX-2 during both physiological and pathological processes. The evidence for a physiological role of COX-2 is derived from the observations that there are high basal levels of COX-2 in the hippocampus and cerebral cortex, that activation of NMDA receptors transiently increases COX-2 expression, which is concentrated in dendritic spines (Yamagata et al., 1993; Kaufmann et al., 1996), and that high-dose coxibs interfere with memory consolidation (Teather et al., 2002). Support for a pathological role of COX-2 comes from data showing that COX-2 expression in neurons is induced by Aβ, glutamate and inflammatory cytokines (Fig. 1b) (Tocco et al., 1997; Pasinetti and Aisen, 1998; Bazan, 2001), and that PGE2 levels are increased in Alzheimer’s disease (Montine et al., 1999).
APP transgenic mice develop little or no loss of neurons or synapses (Irizarry et al., 1997a, b), yet nevertheless exhibit age-related memory loss (Hsiao et al., 1996; Chen et al., 2000; Westerman et al., 2002), which is rapidly reversible (Dodart et al., 2002; Kotilinek et al., 2002). A mechanistic explanation for these observations has been provided by the observation that oligomeric assemblies of Aβ are both necessary and sufficient for Aβ to disrupt cognition without inducing permanent neurological deficits (Cleary et al., 2005), and that a specific 56-kDa soluble assembly of Aβ, called Aβ*56, appears to impair memory in the well-characterized Tg2576 model of Alzheimer’s disease lacking neurodegeneration (Lesné et al., 2006). Soluble oligomers of Aβ also inhibit hippocampal long-lasting synaptic plasticity (Walsh et al., 2002; Wang et al., 2002, 2004). These observations suggest that APP transgenic mice represent a prodromal or latent stage of Alzheimer’s disease rather than the actual disease itself (Zandi and Breitner, 2001; Ashe, 2005; Lesné et al., 2006). Tg2576 mice may therefore be a good model in which to examine prophylactic mechanisms.
More than 10 studies of NSAIDs in transgenic APP mice have documented their effects on amyloid load and inflammation (reviewed in McGeer and McGeer, 2007), but none to date have reported the effects of NSAIDs on Aβ-mediated disruption of synaptic plasticity and memory. Therefore, we used well-established in vitro and in vivo model systems to examine the effects of NSAIDs on Aβ-mediated inhibition of synaptic plasticity and memory, measuring spatial reference memory in Tg2576 mice and LTP in rat hippocampal slices exposed to soluble Aβ oligomers (Wang et al., 2004). Our observations indicate that NSAIDs sharing the common property of blocking COX-2 ameliorate Aβ-induced neuronal dysfunction, independently of an anti-inflammatory effect or a reduction in the levels of Aβ, including Aβ42 and Aβ*56. Importantly, improved memory function correlated with decreased PGE2 levels. Moreover, the ability of COX-2 inhibitors to rescue Aβ-mediated inhibition of LTP was blocked by the addition of exogenous PGE2. Because COX-2 in rodent brains is concentrated in dendritic spines (Yamagata et al., 1993; Kaufmann et al., 1996), our results suggest a role for PGE2 signalling in dendritic spines in Aβ-mediated synaptic and memory dysfunction.
Material and Methods
Long-term potentiation studies
Transverse slices (350 μm) of the rat (males, age 3–4 weeks, weight 40–80 g) hippocampus were placed in oxygenated medium at room temperature for 1 h, then continuously superfused in the recording chamber at 30–32°C. The control medium contained: (mM) NaCl, 120; KCl 2.5, NaH2P04, 1.25; NaHC03 26; MgS04, 2.0; CaCl2, 2.0; d-glucose 10. All solutions contained 100 μM picrotoxin (Sigma, St Louis, MO) to block GABA-mediated activity. Drugs used were NS-398, piroxicam (both from Tocris Cookson Ltd, Bristol, UK), MF tricyclic (Merck, Whitehouse Station, NJ), ibuprofen, naproxen and PGE2 (from Cayman Chemical, Ann Arbor, MI). NS-398 and piroxicam were dissolved in DMSO, with a maximum final concentration of 0.1% DMSO. MF tricyclic was dissolved in acetone, with a final concentration of acetone of 0.0375%. Ibuprofen, naproxen and PGE2 were dissolved in artificial CSF media. Synthetic Aβ1–42 (Bachem UK Ltd, St Helens, UK) was prepared as a stock solution of 50 μM in ammonium hydroxide (0.1%), stored at −20°C and added immediately prior to each experiment. Presynaptic stimulation was applied to the medial perforant pathway of the dentate gyrus using a bipolar insulated tungsten wire electrode, and field excitatory postsynaptic potentials (EPSPs) were recorded with a glass microelectrode at a control test frequency of 0.033 Hz from the middle one-third of the molecular layer of the dentate gyrus. An input–output curve (afferent stimulus intensity versus EPSP amplitude) was plotted at the test frequency. The amplitude of the test EPSP was adjusted to one-third of maximum (~1.2 mV). Long-term plasticity (LTP) was evoked by high-frequency stimulation (HFS) consisting of eight trains, each of eight stimuli at 200 Hz, intertrain interval 200 ms, with the stimulation voltage increased during the HFS so as to elicit an initial EPSP of the train of double the normal test EPSP amplitude. The measurements of LTP are at 60 min post-HFS.
NSAID treatment
Tg2576 mice were in a hybrid C57B6/SJL (B6SJL) (Hsiao et al., 1996) or congenic 129S6 × FVB F1 (129FVBF1) background. We used B6SJL in the prophylactic study and 129FVBF1 in the restoration study. Groups were counterbalanced by gender and litter. Drugs were administered orally by supplementing basal feeding chow. Ibuprofen (375 p.p.m.; Sigma, St Louis, MO) and naproxen (375 p.p.m.; BIOMOL, Plymouth Meeting, Pennsylvania) were prepared in basal chow NIH-31 by Harlan Teklad (Madison, WI) and MF tricyclic (13 p.p.m.; Merck, Whitehouse Station, NJ) was prepared in LabDiet 5010 by Merck. The pharmacokinetics and selectivity of MF tricyclic for COX-2 over COX-1 is almost identical to rofecoxib (Oshima et al., 1996).
Behavioural tests
Spatial reference memory was measured using the Morris water-maze (Westerman et al., 2002). Since 129FVBF1 mice learn more rapidly than B6SJL mice, testing was tailored for each background strain (Westerman et al., 2002). B6SJL mice received visible platform training for 3 days, eight trials per day, followed by hidden platform training for 9 days, four trials per day. Three probe trials were performed 20 h after 12, 24 and 36 training trials, and the average target quadrant occupancy for the three probe trials (mean probe score), was calculated. Visible platform training of 11.6-month and 12.5-month 129FVBF1 mice was done for 2 days, six trials per day or 3 days, 6 trials per day respectively, followed by hidden platform training for 6 days, four trials per day. In 11.6-month 129FVBF1 mice, four probe trials were performed 20 h after 4, 8, 16 and 24 training trials. In 12.5-month 129FVBF1 mice, five probe trials were performed 20 h after 4, 8, 12, 16 and 24 training trials. The mean probe score (MPS) was the mean target quadrant occupancy of probes obtained between the 8th and 17th trials. Probe trials lasted 60 s, but MPSs in 129FVBF1 mice were calculated using the first 30 s because they exhibited extinction.
Spatial working memory was tested using forced-choice alternation in a T-maze, described previously (Chapman et al., 1999). Reward was 25% sucrose in tap water. The test consisted of 4 days of adaptation to the T-maze (5 min/day) and 10 consecutive days of acquisition with six pairs of trials per day with forced choices pseudo-randomly assigned to left and right. Mice with neophobia manifested by immobility were excluded.
IL-1β and tumour necrosis factor-α (TNF-α)
Interleukin 1β (IL-1β) was measured by ELISA (Lim et al., 2000). (TNF-α) was measured by ELISA using monoclonal anti-mouse TNF-α capture antibody (MM-350C; Endogen, Woburn, MA).
COX-2
In situ hybridization was carried out as described for COX-2 mRNA in rat brain tissue (Simonyi et al., 2002), except that sagittal instead of coronal sections were used.
PGE2
Forebrain (minus hippocampus) tissue was weighed, homogenized and sonicated, and acetone added. Samples were centrifuged at 1500 g for 10 min and the supernatant dried by vacuum centrifugation. The dried samples were reconstituted in buffer, and PGE-2 levels were determined by EIA (Cayman Chemical) according to manufacturer’s methods. Each sample was tested in duplicate and levels represented as pg PGE-2/mg tissue.
Ibuprofen, naproxen and MF tricyclic
Plasma samples were mixed with two volumes of acetonitrile and mouse brains were homogenized in five equivalents of water and centrifuged. Quantitation of the compounds in extracted supernatants was carried out by HPLC and tandem mass spectrometric (LC/MS/MS) detection. Tandem mass spectrometric (MS/MS) detection was carried out in negative ion mode by multiple reaction monitoring (ibuprofen at m/z 205 →161.0, naproxen at m/z 229.0 → 170.0 and MF tricyclic at m/z 349.0 → 320.7).
Aβ
Aβ40 and Aβ42 were measured by ELISA in SDS and formic acid extracts of forebrain (minus hippocampus) tissue, using the 3160 polyclonal capture antibody (Kawarabayashi et al., 2001).
Aβ*56
Aβ*56 was measured in membrane-enriched extracts of hemi-brains following a four-step extraction procedure, by immunoblotting using the 6E10 monoclonal antibody (Lesné et al., 2006).
Statistical analysis
ANOVA and Student’s t-tests were used.
Results
Effects of COX inhibition on Aβ-induced inhibition of LTP
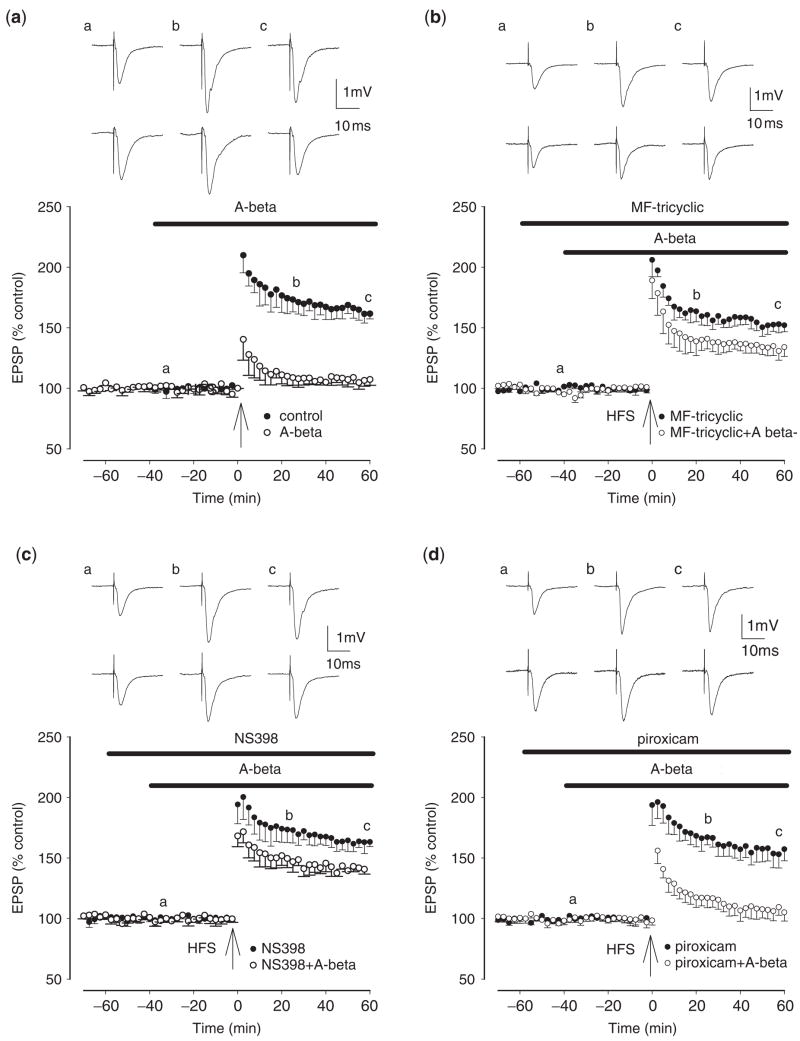
To address the importance of synaptic COX-2 in mediating the beneficial effects of NSAIDS on memory, we examined the involvement of COX-2 in the induction of LTP in rat hippocampal slices exposed to soluble, synthetic Aβ42, as described previously (Wang et al., 2004). Aβ42 prevented the induction of LTP at the medial perforant path to dentate gyrus granule cell, LTP measuring 161 ± 4% in control and 107 ± 5% in the presence of Aβ42 (n = 5, P<0.01) (Fig. 2a). The selective COX-2 inhibitors MF tricyclic and NS398 partially prevented the inhibition of LTP by Aβ42, LTP measuring 134 ± 8% (n = 5, P<0.01) and 143 ± 3% (n = 5, P<0.01) in the presence of Aβ plus MF tricyclic and NS-398, respectively (Fig. 2b and c). In contrast, the selective COX-1 inhibitor piroxicam failed to restore LTP disrupted by Aβ, LTP measuring 105 ± 7% in the presence of Aβ plus piroxicam (n = 5, P>0.05) (Fig. 2d). Neither the COX-2 inhibitors MF tricyclic and NS398 nor the COX-1 inhibitor piroxicam altered LTP induction in the absence of Aβ, LTP measuring 152 ± 5%, 163 ± 4% and 157 ± 7% in the presence of MF tricyclic, NS398 and piroxicam, respectively (n = 5, P>0.05) (Fig. 2b–d). Non-selective NSAIDs also largely prevented the inhibition of LTP by Aβ42, LTP measuring 152 ± 6% (n = 6, P<0.01) and 153 ± 6% (n = 6, P<0.01) in the presence of Aβ plus ibuprofen and naproxen, respectively (Supplementary Fig. 1), but neither drug altered LTP induction in the absence of Aβ, LTP measuring 163 ± 7% and 167 ± 6%, respectively. Thus the inhibition of synaptic COX-2 activity was sufficient to restore long-lasting potentiation which had been disrupted by soluble, synthetic Aβ42.
Fig. 2.
Selective COX-2, but not COX-1, inhibitors prevent the inhibition of LTP induction by synthetic, soluble Aβ42. (a) Control LTP induced by a single brief high-frequency stimulation (HFS) (closed circles) and LTP induction in the presence of synthetic, soluble Aβ42 (500 nM), applied 40 min prior to HFS (open circles). LTP in the presence of synthetic, soluble Aβ42 was significantly reduced from control. (b) The COX-2 inhibitor MF tricyclic (3 μM), applied 60 min prior to HFS, does not inhibit LTP induction (closed circles) but prevents the Aβ-mediated inhibition of LTP induction (open circles). (c) The COX-2 inhibitor NS-398 (20 μM), applied 60 min prior to HFS, does not inhibit LTP induction (closed circles) but prevents the Aβ-mediated inhibition of LTP induction (open circles). (d) The COX-1 inhibitor piroxicam (10 μM), applied 60 min prior to HFS, does not inhibit LTP induction (closed circles) and also does not prevent the Aβ-mediated inhibition of LTP induction (open circles). The traces a, b and c are field EPSPs at the times indicated by a, b and c on the graphs, with the top set of traces corresponding to the closed circles and the lower set of traces to the open circles.
Effects of COX inhibition on spatial memory inTg2576 mice
To ascertain the effects of COX inhibition on the restoration of memory in Tg2576 mice (Hsiao et al., 1996), we tested two non-selective COX inhibitors, ibuprofen and naproxen, and one selective COX-2 inhibitor, MF tricyclic (Oshima et al., 1996), and administered dosages equivalent to low to moderate doses in humans (e.g. 400–1200 mg ibuprofen per day), based upon serum level comparisons (Table 1). Brain levels of these drugs were 1–3 μM (Table 1). At these concentrations, their principle common activity is to inhibit COX-2, thus excluding most other potential targets of NSAIDs (Tegeder et al., 2001).
Table 1.
Drug levels in NSAID-treated mice
| Drug | 3 months old
|
13 months old | ||
|---|---|---|---|---|
| Brain (μM) | Serum (μM) | Brain/Serum Ratio (%) | Serum (μM) | |
| Ibuprofen | 1.73 ± 0.21 (12) | 63.00 ± 8.83 (12) | 2.87% ± 0.17 (12) | 28.81 ± 7.18 (10) |
| Naproxen | 1.72 ± 0.46 (6) | 82.51 ± 23.94 (6) | 2.35% ± 0.36 (6) | 51.65 ± 15.34 (10) |
| MF tricyclic | 1.99 ± 0.4 (6) | 4.81 ± 0.74 (6) | 40.14% ± 2.39 (6) | 3.72 ± 0.62 (10) |
Ibuprofen, naproxen and MF tricyclic are present in the brain and serum of g2576 mice following oral NSAID treatment for 30 days (3 months old) and 40 days (13 months old) respectively. Data are presented as mean±SD (no. of mice).
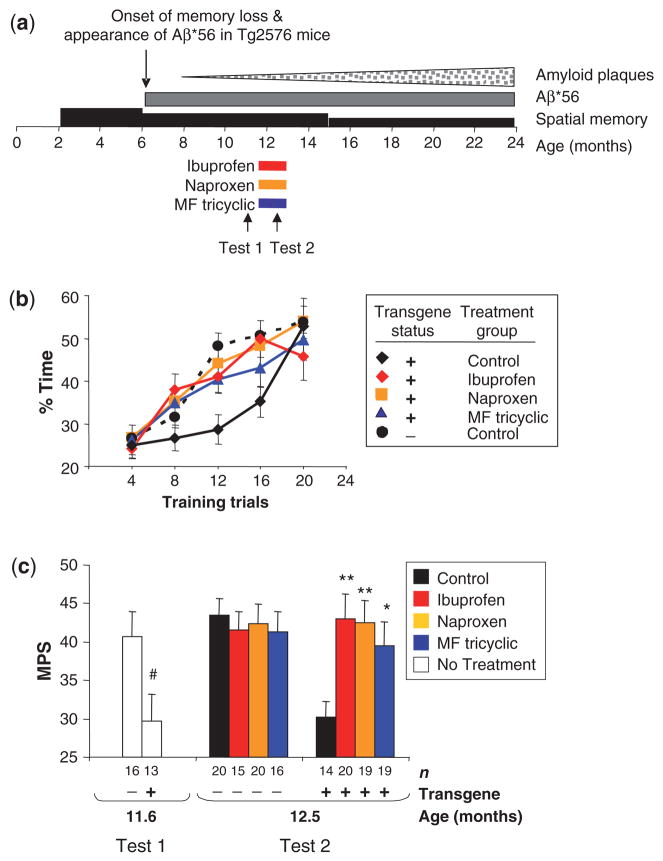
Memory loss in Tg2576 mice begins at ~6 months of age (Fig. 3a) (Westerman et al., 2002). NSAIDs were started at 11.5-months and mice were treated for 4 weeks before behavioural testing was begun (Fig. 3a). Using the Morris water-maze tailored to Tg2576 mice, we defined an index of spatial reference memory called the mean probe score (MPS) (Westerman et al., 2002). Analysis of MPSs in treated mice revealed a significant treatment group by transgene status interaction [F(3,135) = 3.1, P = 0.03], indicating that treatment significantly altered behavioural outcome only in transgene positive (Tg+) mice (Fig. 3b and c), in which there was a significant main effect of treatment [F(3,68) = 3.6, P = 0.02]. Tg+ mice fed ibuprofen, naproxen or MF tricyclic showed significantly higher MPSs than mice fed control chow (P = 0.004, P = 0.006 and P = 0.036, respectively). NSAIDs did not impair memory significantly in non-transgenic (Tg−) mice (Fig. 3c). In mice fed control-chow, but not NSAID-chows, there was a significant main effect of transgene status (P<0.0001), showing that NSAIDs eliminated the distinction between Tg+ and Tg− littermates. To determine if improved performance in the water maze could be attributed to potential analgesic effects of NSAIDs, we compared swim speeds and found no significant main effect of treatment (data not shown). These results indicate that blocking COX-2 was sufficient to restore memory in Tg2576 mice.
Fig. 3.
Reversal of memory deficits and restoration of spatial memory by administration of the conventional NSAIDs, ibuprofen and naproxen, and the selective COX-2 inhibitor, MF tricyclic, in Tg2576 mice. (a) Experimental design. Wedges and rectangles above the timeline qualitatively represent amyloid plaques (stippled), Aβ*56 (dark grey) and memory ability (black) inTg2576 mice as a function of age. Mice<2 months were too young to test. Bars underneath the timeline indicate the duration of treatment. Arrows indicate the initiation of behavioural tests. To avoid possible retest effects related to the short inter-test interval, Tests 1 and 2 were performed on different groups of mice. (b) Acquisition of memory in NSAID-treated and control Tg2576 mice and non-transgenic littermates. Data are the mean target quadrant occupancy during probe trials as a function of number of training trials. (c) The mean probe score (MPS) in NSAID-treated and control Tg2576 mice and non-transgenic littermates. MPS is the mean target quadrant occupancy in probes performed between the eighth and 17th trials. Random swimming in all three probe trials would yield an MPS of 25. Ages at initiation of testing are indicated. Data are the mean±SEM of the MPS for each mouse (*P<0.05; **P<0.01, compared to age-matched Tg2576 mice on control diet; #P< during the final three training trials were equivalent for 11.6-month and 12.5-monthTg2576 and non-transgenic littermates (data not shown).
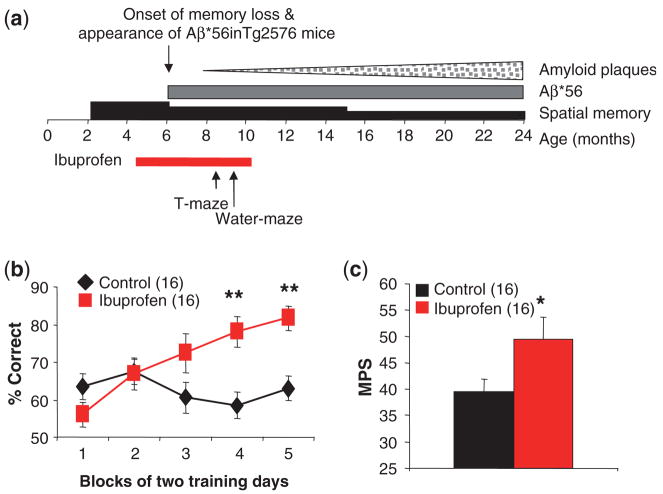
We also examined the effects of prophylactic ibuprofen administration. We initiated ibuprofen at 4.5 months of age, and tested spatial working memory using forced choice alternation in a T-maze, described previously (Chapman et al., 1999), beginning at 8.5 months and spatial reference memory starting at 9.5 months (Fig. 4a). Ibuprofen-treated Tg+ mice made a significantly higher percentage of correct choices during the choice trial in the T-maze compared to Tg+ mice on control diet Similarly, ibuprofen-treated Tg+ mice had significantly higher MPSs in the water maze than Tg+ mice on control diet (Fig. 4c), performing at levels similar to Tg− littermates shown previously (Westerman et al., 2002). These results show that ibuprofen prevented loss of spatial working and reference memory (Fig. 4b).
Fig. 4.
Preservation of spatial memory by chronic administration of ibuprofen inTg2576 mice. (a) Experimental design. (b) Spatial working memory in ibuprofen-treated and control Tg2576 mice, assessed using the T-maze. Data are the mean±SEM of the percentage of correct responses averaged over two days for each mouse (F = 5.6, P = 0.02, for treatment effect by multiple-measures ANOVA, **P<0.01 by t-test). Numbers of mice are in parentheses. (c) Spatial reference memory in ibuprofen-treated and control Tg2576 mice, assessed using the Morris water-maze. Data are the mean±SEM of the MPS for each mouse (F = 5.13, *P = 0.03, for treatment effect by ANOVA).
Effects of NSAIDs on inflammatory cytokines
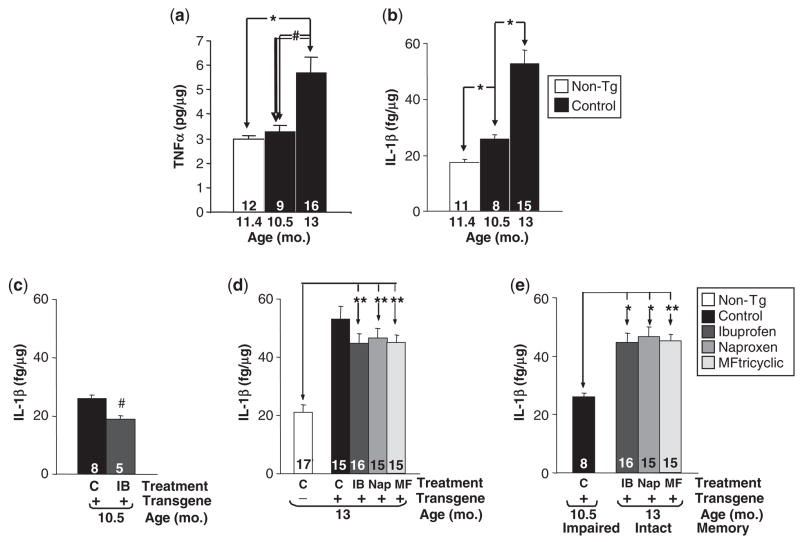
Because NSAIDs have anti-inflammatory properties, we examined the correspondence between memory and neurotoxic inflammation. At 8.5 to 9 months, the earliest age in which we found beneficial effects of NSAIDs, the hippocampus of Tg2576 mice is virtually devoid of plaques and consequently there is no plaque-associated microglial response (Frautschy et al., 1998). Aβ-stimulated microglia have been shown to produce TNF-α both in cultured cells and in Tg2576 mice (Tan et al., 1999). We therefore measured hippocampal TNF-α and found no significant elevations in 10.5-month Tg+ mice (Fig. 5a), consistent with the minimal amount of amyloid deposition at this age. In contrast, TNF-α levels were elevated by a factor of ~2-fold in 13-month-old Tg+ mice, consistent with previous studies (Tan et al., 1999), but TNF- expression was not reduced by NSAIDs in 13-month-old Tg+ mice (data not shown).
Fig. 5.
TNF-α and IL-1β levels inTg2576 mice. (a) TNF-α is significantly elevated in 13-monthTg2576 mice. Data are the mean ± SEM of hippocampal TNF-α measured by ELISA (#P<0.01; *P<0.001). TNF-α is not significantly reduced in 13-monthTg2576 mice treated with NSAIDs (data not shown). (b) Age-related increase in IL-1β inTg2576 mice. Data are the mean±SEM of hippocampal IL-1β measured by ELISA (*P<0.001). (c) IL-1β is suppressed in 10.5-month Tg2576 mice given ibuprofen prophylactically (#P<0.01). (d) IL-1β remains significantly elevated in13-monthTg2576 mice treated with NSAIDs (**P<0.0001). Control (C), Ibuprofen (IB), Naproxen (Nap), MF tricyclic (MF). (e) Memory-intact 13-monthTg2576 mice treated with NSAIDs showed significantly higher levels of IL-1β than memory-impaired 10.5-month Tg2576 mice on control diet. (*P<0.001; **P<0.0001). A similar relationship was observed for TNF-α (data not shown). Numbers of mice denoted inside bars.
To extend our investigations, we measured levels of another pro-inflammatory cytokine, IL-1β, which has been shown to be elevated in Alzheimer’s disease and to inhibit long-lasting synaptic plasticity in the hippocampus, and has therefore been implicated in memory loss in Alzheimer’s disease (Cacabelos et al., 1994; Murray and Lynch, 1998). In 16-month Tg2576 mice, we previously demonstrated an elevation in hippocampal IL-1β associated with the presence of abundant plaques (Lim et al., 2000). In the current study, we found significant increases in hippocampal IL-1β in 10.5-month Tg+ mice relative to Tg− littermates (P<0.001) (Fig. 5b). In addition, we compared levels of hippocampal IL-1β in 10.5-month and 13-month Tg+ mice to determine whether IL-1β expression was upregulated in an age-related fashion. As expected, we observed that IL-1β levels were increased with ageing in Tg+ mice (P<0.001) (Fig. 5b). Treating mice with Ibuprofen suppressed the rise in hippocampal IL-1β in 10.5-month-old Tg+ mice (Fig. 5c), as reported previously (Lim et al., 2000). These results indicate that elevations in IL-1β preceded significant plaque deposition, and suggested that IL-1β may contribute to memory loss in Tg2576 mice.
To investigate whether the recovery of cognitive function in NSAID-treated Tg2576 mice was related to the modulation of IL-1β levels, we examined 13-month Tg2576 mice treated with NSAIDs for 6 weeks. If elevated levels of IL-1β were necessary for impairing cognitive function, then we would expect the restoration of memory to be accompanied by reductions of IL-1β to levels close to those found in non-transgenic mice. Despite a modest lowering of IL-1β in NSAID-treated Tg+ mice, IL-1β levels remained >2.5-fold higher than in non-transgenic mice (P<0.0001) (Fig. 5d). Importantly, IL-1β levels in 13-month, NSAID-treated Tg+ mice with intact memory were >1.7 times higher than in untreated 10.5-month Tg+ mice with impaired memory (P<0.001) (Fig. 5e). A similar relationship was observed for TNF-α (data not shown). These studies dissociate IL-1β and TNF-α from memory loss, and suggest that the mechanism by which NSAIDs are able to restore memory function in Tg2576 mice is not related to their anti-inflammatory properties targeting TNF-α or IL-1β.
Effects of NSAIDs on Aβ
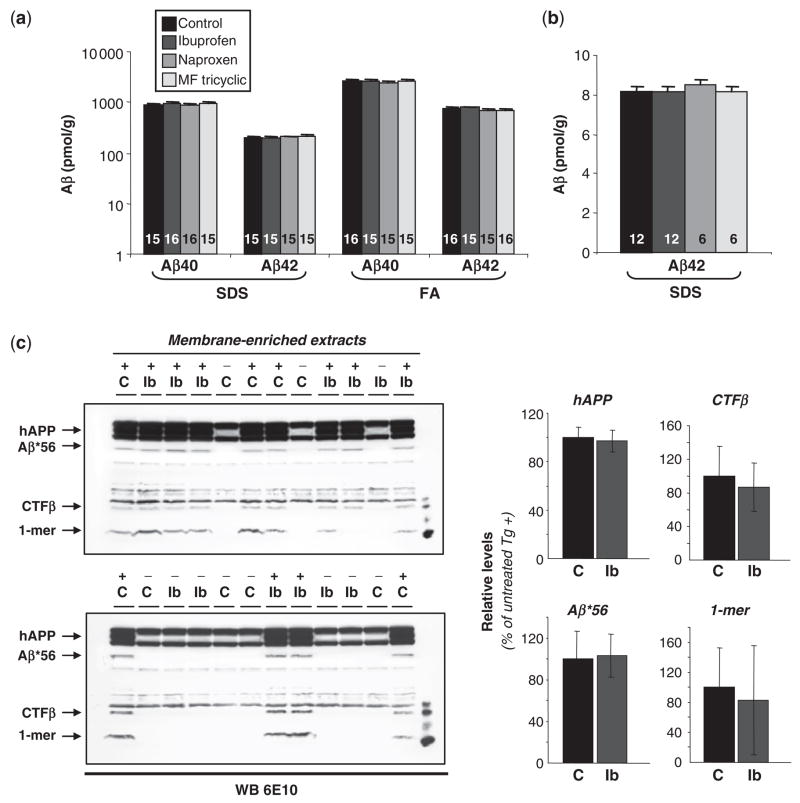
We then asked whether the beneficial effects of NSAIDs were related to reductions in Aβ. NSAIDs can stimulate Aβ clearance by activating microglia (Jantzen et al., 2002), and a subset of NSAIDs can reduce Aβ42 production (Weggen et al., 2001). We found that the levels of soluble (SDS) and fibrillar (FA) Aβ40 and Aβ42 in Tg+ mice were not modified by treatment with NSAIDs (Fig. 6a). In addition, both soluble and fibrillar Aβ levels remained >100 times higher than those evaluated in Tg+ mice <6 months of age, prior to the onset of memory loss (Kawarabayashi et al., 2001). This result indicates that although NSAIDs fully restored cognitive function, they failed to reduce Aβ levels to those found in young, cognitively intact mice.
Fig. 6.
Aβ levels inTg2576 mice. (a) No significant effects of treatment on Aβ levels in mice treated with NSAIDs from 11.5 to 13 months of age. Forebrain Aβ40 and Aβ42 levels were measured by ELISA in SDS and formic acid (FA) fractions. Numbers of mice denoted inside bars. (b) No significant effects of treatment on Aβ42 levels in 2-month-old mice treated with NSAIDs for 30 days. Brains were extracted in SDS and Aβ42 levels were measured by ELISA. (c) Aβ*56 levels are not modulated by ibuprofen treatment. Tg2576 mice (+) and non-transgenic littermates (−) were treated with ibuprofen (Ib) or control (C) diets from 11.5 to 13 months of age. Forebrain Aβ*56 was measured in membrane-enriched extracts by Western blotting (WB; 100 μg protein/lane) using 6E10 monoclonal antibodies (1:10,000). CTFβ and monomeric Aβ (1-mer) levels also showed no significant alterations.
The absence of effects on Aβ levels associated with naproxen and MF tricyclic was consistent with previous reports that naproxen and rofecoxib are incapable of lowering Aβ42 in vivo or in vitro (Weggen et al., 2001; Eriksen et al., 2003; Kukar et al., 2005). The failure of ibuprofen to reduce Aβ was unexpected initially, given previous findings that ibuprofen can selectively reduce brain Aβ42 in 3-month Tg2576 mice (Weggen et al., 2001; Eriksen et al., 2003), but is consistent with more recent findings by an independent group showing ibuprofen does not reduce Aβ levels in plaque-free, young Tg2576 mice (Lanz et al., 2005). To investigate this issue further, we analysed the effect of our dosage regimen on brain Aβ42 in plaque-free 2-month Tg2576 mice treated for 30 days and found no effects (Fig. 6b). Brain ibuprofen levels (Table 1) were within the range of concentrations reported to reduce Aβ42 in Tg2576 mice (Eriksen et al., 2003), which received the same total amount of ibuprofen per day as the mice in our experiments. The reduction of plaque burden previously observed with the same oral regimen in chow (Lim et al., 2000; Yan et al., 2003) may involve effects of ibuprofen that depend upon the plaque load and inflammatory state in the brain (Heneka et al., 2000; Morihara et al., 2005). Importantly, our results show that memory function was restored following NSAIDs regardless of their Aβ42 lowering capabilities, since improved memory was observed with naproxen which has no predicted or observed effects on lowering Aβ42 (Weggen et al., 2001; Kukar et al., 2005).
Because Aβ*56 appears to impair memory in Tg2576 mice (Lesné et al., 2006), we asked whether NSAIDs altered its concentration in the brain. We found no effect of ibuprofen treatment on Aβ*56 levels (Fig. 6c). The data indicate that the beneficial effects of NSAIDs on memory occurred independently of inhibiting the production of Aβ42 through γ-secretase modulation, reducing the accumulation of Aβ40, Aβ42 or Aβ*56 or increasing the clearance of Aβ in the brain.
COX-2 levels inTg2576 mice
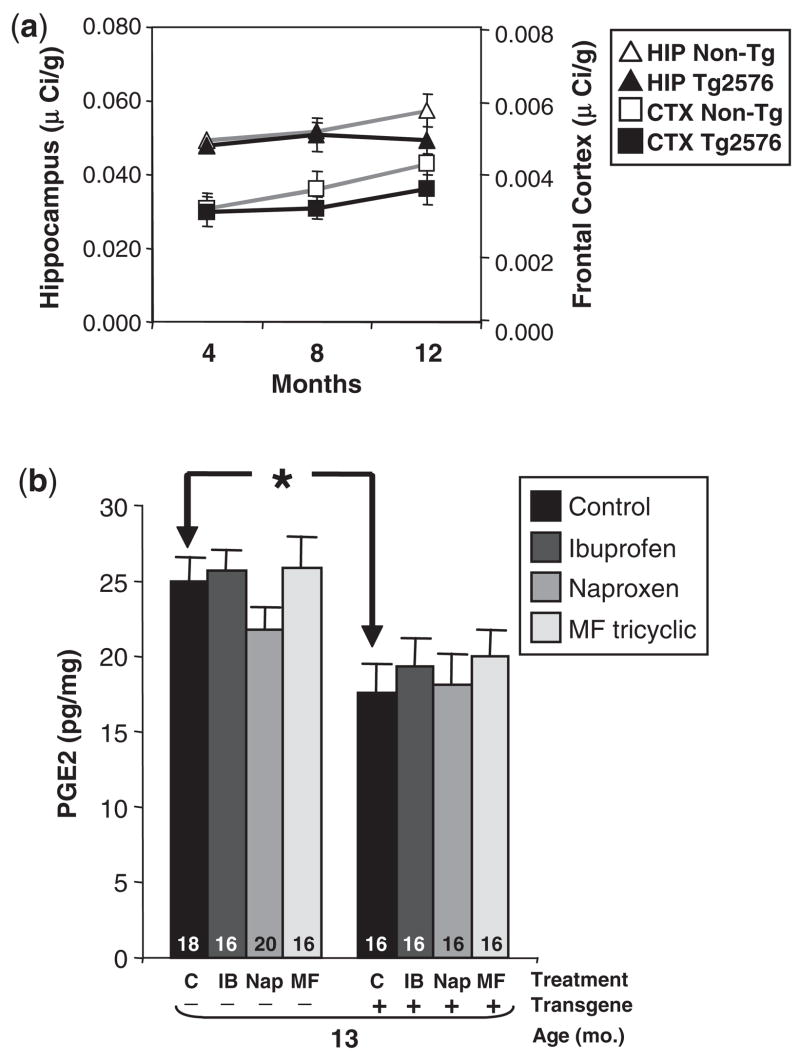
Since the electrophysiological and behavioural data supported the involvement of COX-2 in impairing neuronal function, we asked whether the beneficial effects of COX-2 inhibition depended upon the elevation of COX-2 in Tg2576 mice. We compared COX-2 messenger RNA (mRNA) by in situ hybridization in 4, 8 and 12-month Tg+ and Tg− littermates, which permitted semi-quantitative studies in different brain regions. Consistent with measurements using an mRNA protection assay (Sung et al., 2004), we found no increase in COX-2 expression in the hippocampus or the cerebral cortex (Fig. 7a), observing instead a non-significant decrease in COX-2 mRNA in 8 and 12-month Tg+ mice compared to Tg− littermates. The lack of change in COX-2 is consistent with other reports in aged Tg2576 mice showing no increase in prostaglandin E2 (PGE2), the major oxidation product of arachidonic acid and COX-2, and no increase in COX-2 mRNA in microarray analyses (Dickey et al., 2003; Quinn et al., 2003). The low levels of COX-2 in Tg2576 further support our assumption that the mice represent a prodromal or latent phase of Alzheimer’s disease, rather than clinically apparent Alzheimer’s disease.
Fig. 7.
COX-2 mRNA and PGE2 levels inTg2576 mice. (a) COX-2 expression is not elevated inTg2576 mice. COX-2 mRNA was measured by in situ hybridization in 4-, 8- and 12-monthTg2576 mice (n = 10) and non-transgenic littermates (n = 9). Data are the mean±SEM of COX-2 mRNA in the CA3 region of the hippocampus (HIP) and frontal cortex (CTX). (b) PGE2 levels are significantly lower in 13-monthTg2576 mice. No significant effect of NSAID treatment on PGE2 levels was observed. Data are mean±SEM of forebrain (minus hippocampus) PGE2 measured by EIA (*P<0.01).
Disruption of spatial memory and synaptic plasticity by Aβ is dependent upon COX-2-mediated PGE2 signalling
Because NSAIDs block the ability of COX-2 to catalyse the formation of PGE2 from arachadonic acid, we also measured the levels of PGE2, and found that they were lower in Tg+ mice compared to Tg− littermates (Fig. 7b). Next, we evaluated the effects of NSAIDs on PGE2 levels. While COX inhibitors consistently reduce elevated levels of PGE2 in pathological states, their effects on physiological PGE2 levels are inconsistent, with some reports showing no effects and others showing decreases (Baik et al., 1999; Klivenyi et al., 2004). We found no significant effect of NSAIDs on overall PGE2 levels in the forebrain tissue of Tg2576 mice (Fig. 7b). The PGE2 data was consistent with the COX-2 mRNA results, and indicate that the beneficial effects of NSAIDs on memory function do not require increases in COX-2 expression or PGE2 levels.
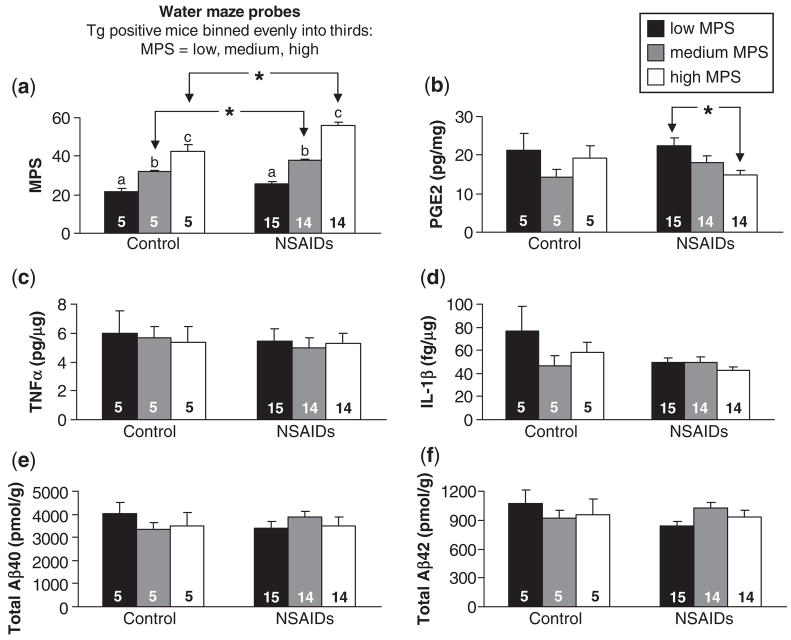
To more rigorously link the inhibition of COX-2 activity with the recovery of memory, we ascertained the relationship between memory and levels of PGE2, TNF-α, IL-1β, Aβ40 and Aβ42 in Tg2576 mice treated with NSAIDs. We divided Tg+ mice into three evenly sized bins based upon performance in the water maze. There were 5 Tg+ mice per bin on control diet, and 14–15 Tg+ mice per bin on NSAIDs (Fig. 8a). We found no relationship between PGE2 levels and water maze performance in Tg+ mice on control diet (Fig. 8b). However, in Tg+ mice given NSAIDs, we discovered an inverse relationship between performance in the water maze and PGE2 levels (Fig. 8b). Animals performing well in the water maze had significantly lower PGE2 levels than animals performing poorly. In contrast, there were no significant differences between high and low performers in the levels of TNF-α, IL-1β or total Aβ40 or Aβ42 (Fig. 8c–f).
Fig. 8.
Relationship between PGE-2, TNF-α, IL-1β, Aβ levels and spatial reference memory inTg2576 mice. (a) 13-month transgene positive Tg2576 mice (Tg+) on NSAID or control diets were divided into three evenly sized bins based upon performance in the water maze (low, medium and high MPS categories). There was a significant effect of MPS category in both control and NSAID-treated mice (P<0.001 and P<0.0001, respectively. a:b, b:c, a:c within control and NSAID treatment groups represent significant Fisher’s PLSD post hoc comparisons. *P<0.01 by unpaired t-test.) (b) There was an inverse relationship between water maze performance (MPS) and cortical PGE-2 levels in NSAID-treated mice (P<0.05). *P<0.01 by Fisher’s PLSD. No relationship between MPS and PGE-2 levels was observed in mice receiving Control diet. (c–f) No relationship was observed between MPS and hippocampal TNF-α, hippocampal IL-1β, cortical total Aβ40 or cortical total Aβ42 respectively in control or NSAID-treated mice. Data are means ± SEM. Numbers of mice denoted inside bars.
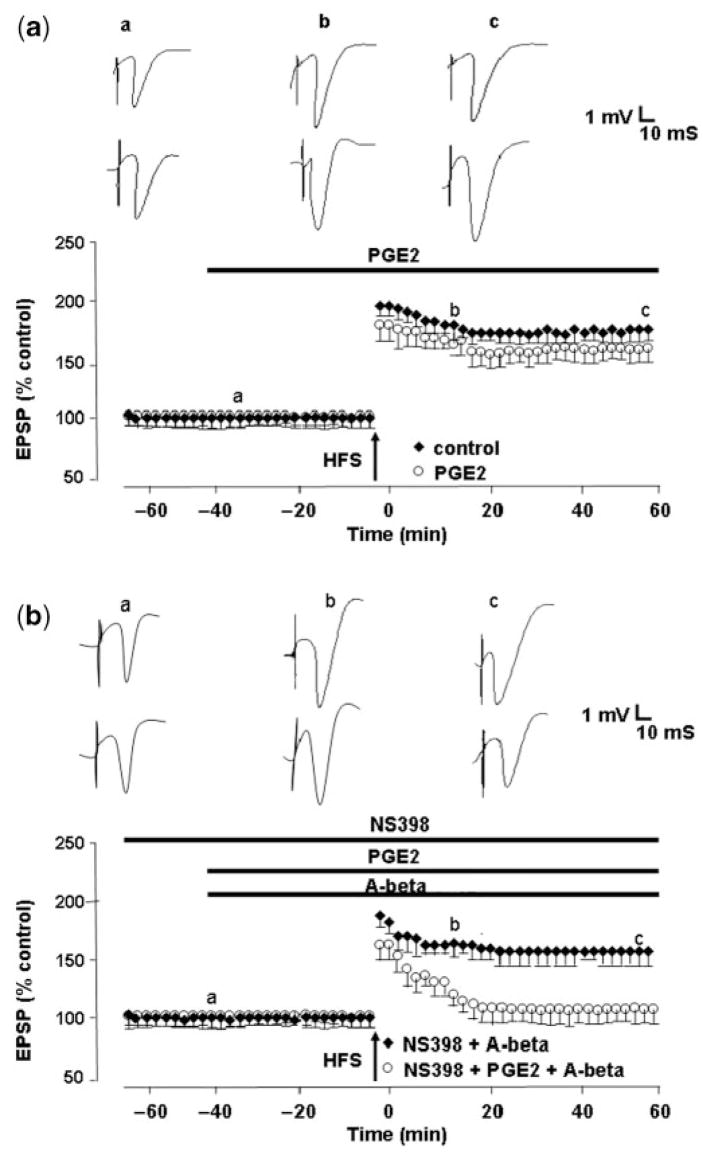
Since elevated PGE2 levels corresponded to poorer memory function, we sought to determine whether the application of PGE2 to hippocampal slices would reverse the beneficial effects of COX-2 inhibitors on Aβ-mediated LTP deficits. We found that 5 μM PGE2 had no effect on LTP per se, LTP measuring 154 ± 6% (n = 6), but blocked the ability of the selective COX-2 inhibitor NS398 to reverse Aβ-mediated LTP deficits, LTP measuring 106 ± 6% (n = 6, P<0.01) (Fig. 9). The data indicate that the effect of COX-2 inhibitors on preventing Aβ-mediated LTP deficits occurs through subsequent decrease in PGE2 production, and can be reversed by the addition of exogenous PGE2. Taken together, the results suggest that the disruption of memory function by Aβ is dependent upon COX-2-mediated PGE2 signalling at synapses, which is blocked by NSAIDs.
Fig. 9.
Exogenous PGE2 blocks the ability of a selective COX-2 inhibitor to prevent the inhibition of LTP induction by synthetic, soluble Aβ42. (a) Control LTP induced by a single brief high-frequency stimulation (HFS) (closed circles) and LTP induction in the presence of PGE2 (5μM), applied 40 min prior to HFS (open circles). PGE2 at this concentration has no effect on LTP. (b) The COX-2 inhibitor NS398 (20 μM), applied 60 min prior to HFS prevents the Aβ-mediated inhibition of LTP induction (closed circles), but is unable to do so in the presence of 5 μM PGE2 (open circles).
Discussion
Our results indicate that blocking COX-2 prevents the inhibition of LTP by synthetic, soluble Aβ42 and therefore restores synaptic function. The administration of NSAIDs that inhibit COX-2 alone, or COX-1 and COX-2 together, improves memory function in Tg2576 mice, indicating that memory dysfunction is also dependent upon COX-2. There was no advantage of ibuprofen relative to naproxen and MF-tricyclic. The recovery of memory function was inversely related to PGE2 levels, but did not depend upon TNF-α, IL-1β or changes in Aβ. Thus, NSAIDs improved Aβ-induced deficits in memory and long-lasting plasticity independently of reducing inflammation or lowering Aβ42, and the inhibition of COX-2 was sufficient to improve synaptic plasticity and memory function.
That NSAIDs influence cytokines other than TNF-α and IL-1β is possible, but was not studied. The involvement of other cytokines or inflammatory processes is unlikely, however, because we demonstrated beneficial effects of NSAIDs at 8.5 to 9 months, an age prior to the appearance of significant plaques or inflammatory pathology in Tg2576 mice. We conclude that neurotoxic inflammation leading to microglial activation, TNF-α production and IL-1β activation plays little, if any, role in disrupting memory in Tg2576 mice at ages when the plaque load is still modest. Our results do not exclude the possibility that inflammation plays a role when the plaque burden is greater.
Changes in dendritic spines are believed to underlie synaptic plasticity and memory. COX-2 is a signalling molecule concentrated in dendritic spines (Yamagata et al., 1993; Kaufmann et al., 1996), and soluble Aβ oligomers bind specifically at dendritic spines (Lacor et al., 2004). It is intriguing that COX-2 and the presumed site of action of soluble Aβ oligomers converge on dendritic spines. This convergence places anatomical constraints upon possible mechanisms of action of COX-2 inhibitors on Aβ-mediated impairment of memory and synaptic plasticity. Taking into consideration this anatomical constraint, the rapidity of the effects of COX-2 inhibitors and their reversal by the addition of exogenous PGE2 on Aβ-mediated suppression of LTP in vitro, and the significant correlation between better memory function and lower PGE2 levels in vivo, led us to hypothesize a novel mechanism by which NSAIDs may protect against Alzheimer’s disease. We propose that NSAIDs block COX-2-mediated production of PGE2 within dendritic spines, and thereby prevent the detrimental effects of Aβ on synaptic plasticity and memory function. Our work complements and supports recent findings that COX-2 potentiates the deleterious effects of Aβ on learning and memory in transgenic mice without changes in the overall levels of Aβ (Melnikova et al., 2006). The modulation of Aβ-induced behavioural abnormalities independently of changes in the levels of Aβ is not unprecedented; blueberries contain substances, possibly anti-oxidants, that improve behavioural performance in the absence of changes in amyloid load but with attendant changes in several signalling molecules (Joseph et al., 2003).
The COX-2 inhibitor dose we administered to mice (~2 mg/kg/day) was lower than doses disrupting memory in rats (5–10 mg/kg/bolus) (Teather et al., 2002). The low doses of the COX-2 inhibitors appeared to block PGE2 production required for soluble Aβ oligomers to disrupt memory, but largely spared COX-2 activity involved in facilitating memory, since NSAID-treated non-transgenic mice showed only a minor trend towards impairment. These observations suggest that synthetic, soluble Aβ42-and Aβ*56-mediated inhibition of plasticity and memory are dependent upon basal COX-2, and that the beneficial effect of NSAIDs is not directed at suppressing the activity of induced COX-2, in contrast to other disease paradigms in which COX-2 mRNA is upregulated in response to injury (Iadecola et al., 2001). The explanation we favour for how this might occur is that COX-2, which is normally present in dendritic spines, plays a permissive role in the inhibition of plasticity and memory by synthetic, soluble Aβ42 and Aβ*56. Another possible explanation would involve a change in the synaptic compartmentalization of COX-2 in response to soluble Aβ, without an overall increase in COX-2 levels, or the existence of distinct pools of COX-2 in separate microdomains of dendritic spines—one pool that facilitates LTP and memory in response to glutamate binding at NMDA receptors, and another pool that disrupts LTP and memory in response to Aβ binding to dendritic spines. A third explanation would be the occurrence of an increase in COX-2 or PGE2 turnover in synapses or dendrites, without a dramatic change in the levels of COX-2 or PGE2.
It has been suggested that NSAIDs protect against Alzheimer’s disease by reducing pro-inflammatory cytokine production by inhibiting COX or activating PPAR-γ (Lehmann et al., 1997; Combs et al., 2000). It has also been proposed that modulation of γ-secretase may be important (Eriksen et al., 2003; Weggen et al., 2001). We found no evidence for the involvement of these mechanisms in improving Aβ-induced deficits in memory or synaptic plasticity, which has never been specifically examined before. Importantly, the NSAIDs that were not ‘selective Aβ42-lowering agents’ (SALAs) were as effective at rescuing memory and LTP as ibuprofen, a SALA (although Aβ42 levels were unaffected by ibuprofen under the conditions of our study). Our results are supported by a recent analysis of pooled data from six prospective epidemiological studies, which provided the statistical power required to address specifically the impact of SALAs on the prevention of Alzheimer’s disease, showing no advantage of SALAs in conferring protection against Alzheimer’s disease (Szekely et al., 2008).
Why have NSAIDs, specifically coxibs, been disappointing in the treatment of patients? In Alzheimer’s disease, COX-2 levels progressively increase (Ho et al., 2001), probably sustained by positive feedback loops involving Aβ, glutamate and inflammatory cytokines. Elderly people on high-dose NSAIDs are more likely to have memory deterioration, possibly indicative of their increased susceptibility to the toxic effects of inhibiting physiological COX-2 (Hanlon et al., 1997). Chronic COX-2 elevation might also lead to cellular changes that are unresponsive to NSAIDs (Dore et al., 2003). Furthermore, COX-2 inhibition may fail to block other pathological cascades downstream of Aβ, like those involving tau, which could play significant roles in the pathogenesis of later stages of Alzheimer’s disease (Lewis et al., 2001; Oddo et al., 2004; SantaCruz et al., 2005; Roberson et al., 2007). This would explain why NSAIDs have largely failed to slow Alzheimer’s disease progression in clinical trials (reviewed in McGeer and McGeer, 2007), why NSAIDs fail to prevent Alzheimer’s disease in elderly individuals >70 years of age (Group et al., 2007), and also why NSAIDs appear to exert more robust protection against Alzheimer’s disease in individuals who were exposed several years prior to the onset of symptoms in epidemiological studies (Stewart et al., 1997;in t’ Veld et al., 2001; Zandi et al., 2002). Our results predict that the predominant efficacy of NSAIDs will be found in individuals with normal levels of COX-2, prior to increases in COX-2 due to inflammation or glutamate. Broadening investigations on the effects of NSAIDs to involve synapse and brain function may renew interest in other potential protective mechanisms of NSAIDs, thus widening the possibilities for developing safe prophylactic medications for Alzheimer’s disease.
Acknowledgments
We gratefully acknowledge Deirdre Cooper-Blacketer, Jen Paulson, Dina Nash, Jennifer Lang, Aaron Guimaraes, Stephen Casper, Brian Clark and Mathew Sherman for help with animal breeding, genotyping and behavioural testing, Daniel Fadale for technical assistance, Mike Kuskowski for help with statistical analysis and Kathleen Zahs, John Breitner, Paul Chapman and Riley McCarten for critical discussions. We thank Merck & Co. Inc. for providing M-F tricyclic chow and Peppi Prasit and Weichao Chen at Merck & Co. Inc. for measuring levels of brain and serum ibuprofen, naproxen and M-F tricyclic. We declare no competing interests. Supported by NIH grants R01-NS33249 (K.H.A.), R01-MH65465 (K.H.A.), P01-AG15453 (P.F.C., S.G.Y. and K.H.A.), and PO1-AG18357 (G.Y.S. and A.S.), the Tulloch Endowment (K.H.A.), R01-AG13471 (G.M.C.) and the Wellcome Trust (R.A.). Funding to pay the Open Access publication charges for this article was provided by the Minnesota Aging and Alzheimer’s Research Endowment.
Abbreviations
- APP
amyloid precursor protein
- Aβ
amyloid-β protein
- COX-1and COX-2
cyclooxygenase isoenzymes
- IL-1β
interleukin 1β
- LTP
long-term plasticity
- MPS
mean probe score
- NSAIDs
Non-steroidal anti-inflammatory agents
- PGE2
prostaglandin E2
- SALA
selective Aβ42-lowering agent
- TNF-α
tumour necrosis factor α
Footnotes
Supplementary material
Supplementary material is available at Brain online.
References
- Ashe KH. Mechanisms of memory loss in Abeta and tau mouse models. Biochem Soc Trans. 2005;33:591–4. doi: 10.1042/BST0330591. [DOI] [PubMed] [Google Scholar]
- Baik EJ, Kim EJ, Lee SH, Moon C. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999;843:118–29. doi: 10.1016/s0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]
- Bazan NG. COX-2 as a multifunctional neuronal modulator. Nat Med. 2001;7:414–5. doi: 10.1038/86477. [DOI] [PubMed] [Google Scholar]
- Broe GA, Grayson DA, Creasey HM, Waite LM, Casey BJ, Bennett HP, et al. Anti-inflammatory drugs protect against Alzheimer disease at low doses. Arch Neurol. 2000;57:1586–91. doi: 10.1001/archneur.57.11.1586. [DOI] [PubMed] [Google Scholar]
- Cacabelos R, Alvarez XA, Fernandez-Novoa L, Franco A, Mangues R, Pellicer A, et al. Brain interleukin-1 beta in Alzheimer’s disease and vascular dementia. Methods Find Exp Clin Pharmacol. 1994;16:141–51. [PubMed] [Google Scholar]
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–6. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–9. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer’s disease: inhibition of beta-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J Neurosci. 2000;20:558–67. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey CA, Loring JF, Montgomery J, Gordon MN, Eastman PS, Morgan D. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J Neurosci. 2003;23:5219–26. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, et al. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer’s disease model. Nat Neurosci. 2002;5:452–7. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Dore S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, et al. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003;54:155–62. doi: 10.1002/ana.10612. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–9. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irizarry M, Hyman B, Saido TC, Hsiao K, et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–17. [PMC free article] [PubMed] [Google Scholar]
- Group AR, Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- Hanlon JT, Schmader KE, Landerman LR, Horner RD, Fillenbaum GG, Pieper CF, et al. Relation of prescription nonsteroidal antiinflammatory drug use to cognitive function among community-dwelling elderly. Ann Epidemiol. 1997;7:87–94. doi: 10.1016/s1047-2797(96)00124-x. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Klockgether T, Feinstein DL. Peroxisome proliferator-activated receptor-gamma ligands reduce neuronal inducible nitric oxide synthase expression and cell death in vivo. J Neurosci. 2000;20:6862–7. doi: 10.1523/JNEUROSCI.20-18-06862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Purohit D, Haroutunian V, Luterman JD, Willis F, Naslund J, et al. Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch Neurol. 2001;58:487–92. doi: 10.1001/archneur.58.3.487. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Rozemuller AJ, Janssen I, De Groot CJ, Veerhuis R, Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol (Berl) 2001;101:2–8. doi: 10.1007/s004010000251. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Niwa K, Nogawa S, Zhao X, Nagayama M, Araki E, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci USA. 2001;98:1294–9. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001;345:1515–21. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997a;56:965–73. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, et al. Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997b;17:7053–9. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, et al. Microglial activation and beta-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22:2246–54. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, et al. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr Neurosci. 2003;6:153–62. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci USA. 1996;93:2317–21. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–81. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klivenyi P, Kiaei M, Gardian G, Calingasan NY, Beal MF. Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2004;88:576–82. doi: 10.1046/j.1471-4159.2003.02160.x. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, et al. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J Neurosci. 2002;22:6331–5. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, et al. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Abeta42 production. Nat Med. 2005;11:545–50. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TA, Fici GJ, Merchant KM. Lack of specific amyloid-beta(1–42) suppression by nonsteroidal anti-inflammatory drugs in young, plaque-free Tg2576 mice and in guinea pig neuronal cultures. J Pharmacol Exp Ther. 2005;312:399–406. doi: 10.1124/jpet.104.073965. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–10. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-beta protein assembly in the brain impairs memory. Nature. 2006;440:352–7. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–91. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–14. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL. Cyclo-oxygenase-2 inhibitors: rationale and therapeutic potential for Alzheimer’s disease. Drugs Aging. 2000;17:1–11. doi: 10.2165/00002512-200017010-00001. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–47. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Melnikova T, Savonenko A, Wang Q, Liang X, Hand T, Wu L, et al. Cycloxygenase-2 activity promotes cognitive deficits but not increased amyloid burden in a model of Alzheimer’s disease in a sex-dimorphic pattern. Neuroscience. 2006;141:1149–62. doi: 10.1016/j.neuroscience.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Sidell KR, Crews BC, Markesbery WR, Marnett LJ, Roberts LJ, II, et al. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53:1495–8. doi: 10.1212/wnl.53.7.1495. [DOI] [PubMed] [Google Scholar]
- Morihara T, Teter B, Yang F, Lim GP, Boudinot S, Boudinot FD, et al. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer’s models. Neuropsychopharmacology. 2005;30:1111–20. doi: 10.1038/sj.npp.1300668. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–81. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–32. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience. 1998;87:319–24. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- Quinn J, Montine T, Morrow J, Woodward WR, Kulhanek D, Eckenstein F. Inflammation and cerebral amyloidosis are disconnected in an animal model of Alzheimer’s disease. J Neuroimmunol. 2003;137:32–41. doi: 10.1016/s0165-5728(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–4. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyi A, Woods D, Sun AY, Sun GY. Grape polyphenols inhibit chronic ethanol-induced COX-2 mRNA expression in rat brain. Alcohol Clin Exp Res. 2002;26:352–7. [PubMed] [Google Scholar]
- Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–32. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- Sung S, Yang H, Uryu K, Lee EB, Zhao L, Shineman D, et al. Modulation of nuclear factor kappaB activity by indomethacin influnces Aβ levels but not Aβ precursor protein metabolism in a model of Alzheimer’s disease. Am J Pathol. 2004;165:2197–206. doi: 10.1016/s0002-9440(10)63269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely CA, Breitner JC, Fitzpatrick AL, Rea TD, Psaty BM, Kuller LH, et al. NSAID use and dementia risk in the Cardiovascular Health Study: role of APOE and NSAID type. Neurology. 2008;70:17–24. doi: 10.1212/01.wnl.0000284596.95156.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Town T, Paris D, Mori T, Suo Z, Crawford F, et al. Microglial activation resulting from CD40-CD40L interaction after beta- amyloid stimulation. Science. 1999;286:2352–5. doi: 10.1126/science.286.5448.2352. [DOI] [PubMed] [Google Scholar]
- Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002;9:41–7. doi: 10.1101/lm.43602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–72. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- Tocco G, Freire-Moar J, Schreiber SS, Sakhi SH, Aisen PS, Pasinetti GM. Maturational regulation and regional induction of cyclooxygenase-2 in rat brain: implications for Alzheimer’s disease. Exp Neurol. 1997;144:339–49. doi: 10.1006/exnr.1997.6429. [DOI] [PubMed] [Google Scholar]
- van Gool WA, Aisen PS, Eikelenboom P. Anti-inflammatory therapy in Alzheimer’s disease: is hope still alive? J Neurol. 2003;250:788–92. doi: 10.1007/s00415-003-1146-5. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, et al. Soluble oligomers of beta amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–40. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–8. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–6. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2002;22:1858–67. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–86. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23:7504–9. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59:880–6. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Breitner JC. Do NSAIDs prevent Alzheimer’s disease? And, if so, why? The epidemiological evidence Neurobiol Aging. 2001;22:811–7. doi: 10.1016/s0197-4580(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–7. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]