Abstract
Background & Aims
Nonalcoholic fatty liver disease (NAFLD) is the most prevalent liver disease in American children. Noninvasive means to discriminate between NAFLD and nonalcoholic steatohepatitis (NASH) might diminish requirement for liver biopsy or predict those at increased risk for progression.
Methods
Data obtained prospectively from children (aged 6–17 yrs) enrolled in the NASH Clinical Research Network (NASH CRN) were analyzed to identify clinical-pathological correlates of pediatric NAFLD. All participants underwent liver biopsy within 6 months of clinical data that was reviewed by a central pathology committee.
Results
176 children (mean age 12.4 years, 77% male) were eligible for inclusion. Using ordinal logistic regression analysis, increasing AST (OR 1.017 per U/L, 95% CI 1.004–1.031) and GGT (OR 1.016 per U/L, 95% CI 1.000–1.033) were independently associated with increasing severity of NASH. Increasing AST (OR 1.015 per U/L, 95% CI 1.006–1.024), increasing white blood cell count (OR 1.22 per 1000/mm3, 95% CI 1.07–1.38), and decreasing hematocrit (OR 0.87 per %, 95% CI 0.79–0.96) were independently associated with increasing severity of fibrosis. Area under the ROC for a model with AST and ALT was 0.75 (95% CI=0.66–0.84) and 0.74 (95% CI 0.63–0.85) for distinguishing steatosis from more advanced forms of NASH and bridging fibrosis from lesser degrees of fibrosis, respectively.
Conclusions
Certain components of routine laboratory tests are predictive of NAFLD pattern and fibrosis severity, but do not have adequate discriminate power to replace liver biopsy in evaluating pediatric NAFLD.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease in the preadolescent and adolescent age groups in the United States1. The term NAFLD includes a spectrum of histological features, including simple steatosis, steatosis with inflammation, and steatosis with inflammation, ballooning degeneration, and pericellular fibrosis or Mallory’s hyaline (nonalcoholic steatohepatitis or NASH)2. This distinction is important, as the prognosis of these conditions may differ. While the natural history of NAFLD remains incompletely characterized, simple steatosis is thought to be predominantly non-progressive while NASH has potential to lead to cirrhosis and hepatocellular carcinoma (though these natural histories require validation from longitudinal cohort studies) 3–5. No clinical features, biochemical parameters, or imaging studies have been identified that allow for reliable distinction between various forms of NAFLD. Thus, accurate diagnosis and staging of NAFLD requires liver biopsy.
Despite the prevalence of pediatric NAFLD, overweight, obesity and related conditions remain under-diagnosed by health care providers6, 7. Reliance on liver biopsy may be one of the deterrents to the evaluation of overweight children for NAFLD. Because of the risk and expense of this procedure, it would be advantageous to identify clinical predictors of histological severity so that children at greatest risk for progression could be identified. Previous histology-based studies of pediatric NAFLD have been retrospective, single-center case series and none have resulted in validation studies to verify the accuracy of reported clinical-histological correlates in large, diverse populations8–10.
The National Institute of Diabetes and Digestive and Kidney Diseases with additional support from the National Institute of Childhood Health and Human Development funded a Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) beginning in 2002. The objective of this network, composed of eight clinical centers and a data coordinating center, is the conduct of research designed to progress understanding of the pathogenesis, natural history, prognostic features, and treatment of adult and pediatric NAFLD11, 12. Data collected as part of the NASH CRN provide a unique resource in that the liver histology undergoes systematic review by an expert panel of pathologists. The aim of this study is to evaluate whether simple, readily available clinical and laboratory measures have predictive power with respect to the histological pattern or severity of NAFLD among children enrolled in studies conducted at multiple centers within the NASH CRN.
Methods
Patient Selection Criteria
The Treatment of NAFLD in Children (TONIC) and NAFLD Database studies have institutional review board approval at each of the 8 clinical centers participating in the NASH CRN (appendix). Written consent was obtained from a parent or guardian and written assent obtained from all children 8 years and older prior to participation. The NAFLD Database is an observational study of patients 2 years and older and TONIC is a phase III, masked, randomized, placebo-controlled trial of metformin and vitamin E in children ages 8–17 years with NAFLD. Exclusion criteria for both studies include alcohol intake, other liver diseases, history of parenteral nutrition, bariatric or hepatobiliary surgery, HIV infection, or short bowel syndrome. Participants in TONIC were required to have a baseline ALT value ≥60 U/L. Additional exclusion criteria for TONIC include diagnosis of diabetes, cirrhosis, use of drugs associated with NAFLD, anti-diabetic or anti-NAFLD drugs, metabolic acidosis, and renal dysfunction. Enrollment for NAFLD database began in September 2004 and TONIC in August 2005. Participants from both studies age 17 years and younger were eligible for inclusion if they had baseline clinical data within 6 months of liver biopsy. Liver biopsy specimens must have undergone review by the Pathology Committee of the NASH CRN.
Clinical and Laboratory Assessments
Demographic data were obtained via structured interview and questionnaires. Height, weight, waist and hip measurements were taken in duplicate while standing and wearing light clothing. Height and weight were measured without shoes to the nearest 0.1cm and 0.1kg, respectively. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters) squared. BMI percentile was determined according to age and gender based on data from the Centers for Disease Control and Prevention.13 Tanner staging was performed by study physicians on all participants. Study physicians determined the presence of acanthosis nigricans and documented severity according to the extent of pigmentation (inches).
Fasting whole blood samples were obtained via venipuncture following overnight fast of ≥8 hours and processed for plasma and serum within 2 hours. Laboratory assays were performed at individual clinical centers and included: white blood cell count (103 cells/mL), hematocrit (%), platelet count (cells/mL), bilirubin (mg/dL), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), alkaline phosphatase (U/L), gamma glutamyl transferase (GGT, U/L), albumin (g/dL), prothrombin time (sec), fasting triglycerides (mg/dL), fasting glucose (mg/dL), fasting insulin (mU/mL), antinuclear antibody (ANA) titer, anti-smooth muscle antibody (ASMA) titer, and anti-mitochondrial antibody (AMA) titer. Titers of ≥1:40 were considered positive for autoantibodies. Homeostasis model assessment (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were calculated from fasting insulin and glucose values. 14, 15 Dual energy x-ray absorptiometry (DEXA) scans were used to determine body composition.
Histological Evaluation
Biopsies were evaluated for the following according to the validated histological scoring system by Kleiner et al for the NASH CRN16: Steatosis [grade 0 (<5% macrovesicular fat), grade 1 (5–33%), grade 2 (34–66%), grade 3(>66%)], portal inflammation (0–2), lobular inflammation (0–3), ballooning degeneration (0–2), and fibrosis [stage 0, stage 1a (mild perisinusoidal), stage 1b (moderate perisinusoidal), stage 1c (portal/periportal fibrosis only), stage 2 (zone 3 and periportal), stage 3 (bridging fibrosis), stage 4 (cirrhosis)]. For analysis, fibrosis stage was grouped into four categories: none (stage 0); mild zone 3 only, moderate zone 3 only, or periportal only (stage 1); mild or moderate zone 3 and periportal (stage 2); and bridging (stage 3). A NAFLD activity score (NAS) was tabulated by summing scores for steatosis, lobular inflammation, and ballooning degeneration (1–7). For analysis, cases with NAS 1–3 were compared to those with scores of 4–5 and 6–7. A diagnostic categorization was determined for each case: “Not NASH”, “Borderline zone 3”, “Borderline zone 1”, or “Definite NASH” (Figure 2, online).17 “Definite NASH” unequivocally fulfills previously defined criteria for steatohepatitis2, while the category of “Not NASH” encompasses cases of NAFLD in which the changes are so mild or non-specific that more specific classification cannot be made. The intermediate category of “Borderline zone 3” was created for cases that had some, but not all, histologic features of steatohepatitis, so that an unequivocal diagnosis could not be made. “Borderline zone 1” was used for cases that fit the zone 1 pattern of injury previously described in children.9 Cases placed in this category, typically had periportal fibrosis and steatosis in zone 1 while lacking distinctive zone 3 injury. The following NAFLD patterns were compared: Not NASH vs. Borderline zone 3 vs. definite NASH, as these were felt to represent different stages along a spectrum, and Borderline zone 1 vs. definite NASH, as these were felt to represent two distinct categories of NAFLD (“pediatric type” and “adult type”). Biopsy specimen length was evaluated to determine whether this is associated with histological pattern or staging in NAFLD.
Statistical Analysis
P-values for bivariate relationships were based on either the chi-square test for trend for categorical predictors or ordered logistic regression of the outcome on the rank of continuous predictors.18, 19 Adjusted cumulative relative odds were estimated from models using ordered multiple logistic regression for ordinal outcomes (NAFLD pattern, fibrosis stage, NAS) whereas adjusted relative odds were estimated from models using binary multiple logistic regression for binary outcomes (i.e. borderline zone 1 NASH vs. definite NASH). Significant predictors from multiple regression models were chosen using forward selection (p<0.05 for entry) of all predictors except histologic variables.20 For multiple regression, missing values were imputed with the median for continuous predictors or the most frequent category for categorical predictors.21 Area under the ROC curves were derived using binary logistic regression.22 Statistical analyses used both SAS version 9 and Stata version 10.20, 23 P-values are two-sided and nominal.
Results
At the time of analysis, 177 children were eligible for inclusion. One participant with stage 4 fibrosis was excluded, leaving 176 subjects for analysis. Study sample characteristics are summarized in Table 1. Distribution of children by recruitment site was: Case Western Reserve University 6%, Johns Hopkins University 8%, Indiana University 13%, Baylor University 6%, University of California San Diego 49%, University of Washington 10%, and Virginia Commonwealth University 3%. Most children (97%) were obese (BMI ≥95th percentile). Patients recruited from UCSD were predominantly Hispanic, 76% male, mean age (SD) 12.1 years (2.6). When compared to patients recruited from other sites, those from UCSD were more likely to be Hispanic (84% vs. 35%, p<0.001) and to have acanthosis nigricans (89% vs. 63%, p<0.001). No other significant differences existed in the demographics, anthropometrics, or histology findings by recruitment site.
Table 1.
Sample characteristics for children enrolled in NASH CRN eligible for study inclusion.
| Characteristic | N (%) |
|---|---|
| Study | |
| TONIC | 136 (77) |
| Database | 40 (23) |
|
| |
| Demographics | |
| Male | 136 (77) |
| Age (yrs), mean ± SD (range) | 12.4 ± 2.6 (6–17) |
| White race | 128 (73) |
| Hispanic ethnicity | 104 (59) |
|
| |
| Histology | |
| Biopsy length (mm), median ± pseudoSD (range) | 15 ± 6.7 (5–53) |
| NAFLD pattern | |
| Not NASH | 36 (20) |
| Borderline Zone 3 pattern | 26 (15) |
| Borderline Zone 1 pattern | 50 (28) |
| Definite NASH | 64 (36) |
| Fibrosis Stage | |
| None (score=0) | 45 (26) |
| Mild zone 3 only (score=1) | 12 (7) |
| Moderate zone 3 only (score=1) | 8 (5) |
| Periportal only (score=1) | 57 (32) |
| Mild/moderate zone 3 and periportal (score=2) | 29 (16) |
| Bridging (score=3) | 24 (14) |
| Fibrosis score, mean ± SD | 1.2 ± 1.0 |
| NAFLD Activity Score (NAS) | |
| 1 | 1 (1) |
| 2 | 15 (9) |
| 3 | 33 (19) |
| 4 | 43 (24) |
| 5 | 41 (23) |
| 6 | 27 (15) |
| 7 | 16 (9) |
| NAS, mean ± SD | 4.4 ± 1.4 |
|
| |
| Anthropometrics | |
| BMI (kg/m2), median ± pseudoSD (range) | 33 ± 5.2 (18.2–57.9) |
| BMI (age-sex percentile), median ± pseudoSD (range) | 99.1 ± 0.8 (89.6–100.0) |
| Body fat (%), median ± pseudoSD (range) | 44 ± 7 (28–59) |
|
| |
| Clinical | |
| Tanner Stage, mean ± SD (range) | 2.5 ± 1.4 (1–5) |
| Acanthosis nigricans | 133 (76) |
TONIC = Treatment of NAFLD in Children; BMI = body mass index
Histological Pattern: Not NASH vs. Borderline zone 3 vs. Definite NASH
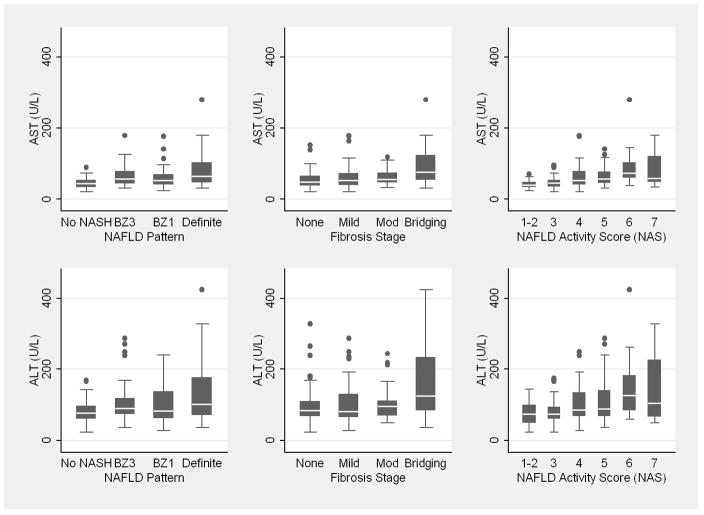
Comparing subjects with definite NASH to those without NASH and those with intermediate findings, ALT, AST, alkaline phosphatase and GGT increased among these categories (Table 2). Box plots of AST and ALT by NAFLD pattern are shown in Figure 1. Hematological parameters and fasting lipids did not vary by histological pattern. Fasting glucose did not vary by histological category, but markers of insulin resistance were increased among subjects with definite NASH. Anthropometric measures and developmental stage did not vary according to NAFLD pattern. Acanthosis nigricans was prevalent but did not vary by NAFLD pattern. None of the subjects had positive titers of AMA. ASMA titer was positive in 32% but was not associated with NAFLD pattern. Results did not vary by UCSD versus other clinical sites.
Table 2.
Predictors of NAFLD pattern (excluding borderline zone 1)
| Not NASH (n=36) | BZ3 (n=26) | Definite NASH (n=64) | P | |
|---|---|---|---|---|
| Demographics | ||||
| Male (%) | 67 | 73 | 78 | 0.21 |
| Age (years), mean | 13.1 | 12.4 | 13.1 | 0.91 |
| Race: White (%) | 72 | 69 | 77 | 0.58 |
| Ethnicity: Hispanic (%) | 58 | 62 | 50 | 0.37 |
| Clinic Site: UCSD (%) | 50 | 42 | 44 | 0.58 |
|
| ||||
| Laboratory Data (median values) | ||||
| ALT (U/L) | 76 | 90 | 102 | 0.002 |
| AST (U/L) | 44 | 57 | 64 | <0.0001 |
| Alkaline Phosphatase (U/L) | 188 | 225 | 214 | 0.40 |
| GGT (U/L) | 32 | 36 | 49 | <0.0001 |
| Fasting glucose (mg/dL) | 89 | 89 | 89 | 0.46 |
| Fasting insulin (mU/mL) | 27 | 27 | 36 | 0.06 |
| HOMA-IR | 5.5 | 6.1 | 8.0 | 0.04 |
| QUICKI | 0.299 | 0.295 | 0.285 | 0.04 |
| ANA (% positive) | 14 | 12 | 22 | 0.26 |
| ASMA (% positive) | 28 | 24 | 38 | 0.28 |
|
| ||||
| Anthropometric (median values) | ||||
| BMI (kg/m2) | 33 | 33 | 33 | 0.54 |
| BMI percentile | 98.9 | 99.2 | 99.0 | 0.41 |
| %Body Fat | 41 | 42 | 44 | 0.33 |
|
| ||||
| Clinical | ||||
| Tanner Stage (mean) | 3.3 | 2.5 | 2.8 | 0.32 |
|
| ||||
| Histologic | ||||
| Biopsy length (median, in mm) | 14 | 13 | 15 | 0.24 |
| <10mm (%) | 16 | 27 | 9 | 0.12 |
| Fibrosis score (mean) | 0.4 | 1.0 | 1.6 | <0.0001 |
BZ3 = Borderline Zone 3 pattern (“adult type”)
Figure 1.
Box plot of AST and ALT value according to NAFLD pattern, fibrosis stage, and NAFLD Activity Score (NAS)
Histological Pattern: Borderline zone 1 vs. Definite NASH
Subjects with borderline zone 1, a pattern observed predominantly in children9, 17, were compared to those with definite NASH (Table 3). Subjects without fibrosis were excluded from this analysis to control for differences in fibrosis distribution. Subjects with borderline zone 1 pattern were younger than those with definite NASH and Tanner stage was lowest among those with borderline zone 1 pattern. Children with borderline zone 1 pattern were more likely to be of Hispanic ethnicity and there was a larger percentage of children with this pattern recruited from UCSD versus other clinical sites. ALT, AST, and GGT were lower and alkaline phosphatase higher among those with borderline zone 1 pattern. Hematological parameters and fasting lipids did not vary according to histological pattern. Fasting glucose did not differ between groups, but insulin resistance was increased among subjects with definite NASH. Absolute BMI was higher among those with definite NASH, though BMI percentile and percent body fat were no different between groups. Positive titers for autoantibodies did not vary according to histological pattern.
Table 3.
Predictors of borderline zone 1 versus definite NASH pattern in children with some fibrosis present.
| BZ1 (n=49) | Definite NASH (n=54) | P | |
|---|---|---|---|
| Demographics | |||
| Male (%)4 | 86 | 76 | 0.21 |
| Age (years), mean | 11.1 | 13.0 | 0.0002 |
| Race: White (%) | 73 | 74 | 0.89 |
| Ethnicity: Hispanic (%) | 69 | 46 | 0.02 |
| Clinic Site: UCSD (%) | 61 | 41 | 0.04 |
|
| |||
| Laboratory Data (median values) | |||
| ALT (U/L) | 84 | 101 | 0.08 |
| AST (U/L) | 53 | 64 | 0.05 |
| Alkaline Phosphatase (U/L) | 267 | 216 | 0.002 |
| GGT (U/L) | 39 | 47 | 0.04 |
| Triglycerides (mg/dL) | 109 | 142 | 0.02 |
| Fasting glucose (mg/dL) | 88 | 88 | 0.43 |
| Fasting insulin (mU/mL) | 23 | 36 | 0.01 |
| HOMA-IR | 4.8 | 7.7 | 0.006 |
| QUICKI | 0.304 | 0.286 | 0.006 |
| ANA (% positive) | 18 | 22 | 0.63 |
| ASMA (% positive) | 31 | 37 | 0.53 |
|
| |||
| Anthropometric (median values) | |||
| BMI (kg/m2) | 31 | 33 | 0.05 |
| BMI percentile | 99.3 | 99.1 | 0.94 |
| %Body Fat | 46 | 44 | 0.13 |
|
| |||
| Clinical | |||
| Tanner Stage (mean) | 1.8 | 2.7 | 0.001 |
|
| |||
| Histologic | |||
| Biopsy length (median, in mm) | 16 | 15 | 0.51 |
| <10mm (%) | 4 | 11 | 0.18 |
| Fibrosis score (mean) | 1.3 | 1.9 | 0.0002 |
BZ1 = Borderline Zone 1 pattern (“pediatric type”)
Fibrosis Stage: None vs. Mild zone 3 or periportal vs. Moderate zone 3 and periportal vs. Bridging
Mean age of subjects with bridging fibrosis was lower compared to those with lesser degrees of fibrosis (Table 4). Hispanic ethnicity was predictive of mild zone 3 or periportal (69%) versus moderate zone 3 and periportal (31%) fibrosis (P=0.0004). Fibrosis stage did not vary by UCSD versus other clinical sites. ALT, AST, and GGT were increased among subjects with advanced fibrosis, though only GGT distinguished those with mild fibrosis (median 32 U/L) from those with moderate fibrosis (median 52 U/L, P=0.0003). Box plots of AST and ALT by fibrosis stage are shown in Figure 1. Subjects with moderate zone 3 and periportal fibrosis had lower median INR (P=0.005) compared to those with mild fibrosis. Fasting lipids and glucose did not vary according to fibrosis severity. Insulin resistance was increased among subjects with moderate versus mild fibrosis (P=0.03). BMI did not vary according to fibrosis severity, but percent body fat was lower among subjects without fibrosis. Tanner stage was higher among subjects with no fibrosis compared to those with bridging fibrosis. Definite NASH and higher mean NAS were seen among those with more advanced fibrosis. Adequacy of biopsy sample did not differ according to fibrosis stage.
Table 4.
Predictors of fibrosis stage
| None (n=46) | Mild (n=77) | Moderate (n=29) | Bridging (n=24) | P Trend | |
|---|---|---|---|---|---|
| Demographics | |||||
| Male (%) | 72 | 79 | 72 | 88 | 0.26 |
| Age (years), mean | 13.1 | 12.1 | 13.0 | 11.5 | 0.05 |
| Race: White (%) | 74 | 71 | 76 | 74 | 0.89 |
| Ethnicity: Hispanic (%) | 63 | 69 | 31 | 54 | 0.05 |
| Clinic Site: UCSD (%) | 52 | 53 | 38 | 46 | 0.33 |
|
| |||||
| Laboratory Data (median values) | |||||
| ALT (U/L) | 84 | 82 | 96 | 126 | 0.01 |
| AST (U/L) | 48 | 52 | 56 | 76 | 0.003 |
| Alkaline Phosphatase (U/L) | 202 | 250 | 218 | 263 | 0.02 |
| GGT (U/L) | 38 | 32 | 52 | 54 | 0.001 |
| Albumin (g/dL) | 4.5 | 4.3 | 4.2 | 4.4 | 0.01 |
| White Blood Cells (1K/mm3) | 7.2 | 7.6 | 7.8 | 8.3 | 0.004 |
| Hematocrit (%) | 42 | 41 | 40 | 40 | 0.002 |
| Fasting Insulin (mU/mL) | 30 | 24 | 33 | 33 | 0.17 |
| HOMA-IR | 6.7 | 5.2 | 7.5 | 7.6 | 0.14 |
| QUICKI | 0.291 | 0.301 | 0.287 | 0.287 | 0.14 |
| ANA (% positive) | 20 | 19 | 24 | 4 | 0.26 |
| ASMA (% positive) | 27 | 32 | 37 | 32 | 0.54 |
|
| |||||
| Anthropometric (median values) | |||||
| BMI (kg/m2) | 34 | 32 | 34 | 32 | 0.99 |
| BMI percentile | 99.0 | 99.1 | 99.0 | 99.2 | 0.31 |
| %Body Fat | 41 | 44 | 45 | 44 | 0.02 |
|
| |||||
| Clinical | |||||
| Tanner Stage (mean) | 3.0 | 2.3 | 2.7 | 2.0 | 0.03 |
|
| |||||
| Histologic | |||||
| Biopsy length (median, in mm) | 14 | 15 | 13 | 15 | 0.75 |
| <10mm (%) | 17 | 6 | 17 | 17 | 0.79 |
| Definite NASH (%) | 22 | 26 | 72 | 54 | <0.0001 |
| NAFLD Activity Score (mean) | 4.1 | 4.2 | 5.0 | 5.1 | 0.0006 |
NAFLD Activity Score (1–3, 4–5, 6–7)
Demographic variables did not differ according to NAS (Table 5). ALT, AST, GGT, and markers of insulin resistance increased in a stepwise fashion according to increasing NAS, while alkaline phosphatase and glucose did not differ according to NAS. Box plots of AST and ALT by NAS are shown in Figure 1. Prevalence of ASMA positivity increased with increasing NAS. Anthropometric measures, Tanner stage, acanthosis nigricans and adequacy of liver biopsy specimen did not vary according to NAS. A diagnosis of definite NASH was more common with increasing NAS. Fibrosis score increased in conjunction with increasing NAS.
Table 5.
Predictors of NAFLD Activity Score (NAS)
| NAS 1–3 (n=49) | NAS 4–5 (n=84) | NAS 6–7 (n=43) | Trend P | |
|---|---|---|---|---|
| Demographics | ||||
| Male (%) | 73 | 75 | 86 | 0.16 |
| Age (years), mean | 12.4 | 12.4 | 12.5 | 0.96 |
| Race: White (%) | 67 | 75 | 77 | 0.30 |
| Ethnicity: Hispanic (%) | 55 | 62 | 58 | 0.74 |
| Clinic Site: UCSD (%) | 45 | 49 | 56 | 0.30 |
|
| ||||
| Laboratory Data (median values) | ||||
| ALT (U/L) | 74 | 87 | 114 | <0.0001 |
| AST (U/L) | 42 | 56 | 66 | <0.0001 |
| Alkaline Phosphatase (U/L) | 239 | 236 | 257 | 0.32 |
| GGT (U/L) | 29 | 36 | 53 | <0.0001 |
| Fasting glucose (mg/dL) | 87 | 88 | 89 | 0.12 |
| Fasting insulin (mU/mL) | 23 | 30 | 37 | 0.0008 |
| HOMA-IR | 5.0 | 6.1 | 8.5 | 0.0005 |
| QUICKI | 0.303 | 0.295 | 0.293 | 0.0005 |
| ANA (% positive) | 16 | 17 | 23 | 0.40 |
| ASMA (% positive) | 20 | 31 | 45 | 0.01 |
|
| ||||
| Histologic | ||||
| Biopsy length (median, in mm) | 14 | 15 | 15 | 0.29 |
| <10mm (%) | 14 | 14 | 7 | 0.30 |
| Definite NASH (%) | 6 | 31 | 81 | <0.0001 |
| Fibrosis score (mean) | 0.8 | 1.2 | 1.4 | 0.002 |
Area under the ROC curve for AST and ALT according to histologic pattern and severity
Area under the ROC curve results demonstrate AST to be a better predictor of histological variables than ALT (Table 6). AST and ALT together did not perform significantly better than AST alone. AST appears to be best able to distinguish those without NASH from those with borderline zone 3 pattern or definite NASH, bridging fibrosis from those with lesser degrees of fibrosis, and NAS scores of 1–3 from scores of 4–7.
Table 6.
Area under the ROC curve results for AST and/or ALT according to histological variables
| AST | ALT | P-value AST vs. ALT | AST + ALT | P-value AST vs. AST + ALT | |
|---|---|---|---|---|---|
| NAFLD Pattern* | |||||
| None vs. BZ3/Definite | 0.75 | 0.69 | 0.04 | 0.75 | 0.42 |
| None/BZ3 vs. Definite | 0.69 | 0.63 | 0.04 | 0.71 | 0.21 |
|
| |||||
| Fibrosis | |||||
| None vs. Mild/Mod/Bridging | 0.61 | 0.55 | 0.02 | 0.66 | 0.14 |
| None/Mild vs. Mod/Bridging | 0.66 | 0.62 | 0.24 | 0.65 | 0.67 |
| None/Mild/Mod vs. Bridging | 0.74 | 0.69 | 0.13 | 0.74 | 0.41 |
|
| |||||
| NAFLD Activity Score | |||||
| 1–2 vs. 3–7 | 0.76 | 0.68 | 0.14 | 0.76 | 0.71 |
| 1–3 vs. 4–7 | 0.75 | 0.70 | 0.06 | 0.75 | 0.78 |
| 1–4 vs. 5–7 | 0.70 | 0.66 | 0.06 | 0.68 | 0.13 |
| 1–5 vs. 6–7 | 0.70 | 0.68 | 0.40 | 0.69 | 0.56 |
| 1–6 vs. 7 | 0.60 | 0.59 | 0.61 | 0.61 | 0.57 |
Excluding patients with borderline zone 1 pattern
Significant predictors of histological variables from multiple regression analysis
On multiple ordinal logistic regression analysis of increasing severity of NAFLD pattern, increasing AST, inclusion in TONIC (versus database), and increasing GGT were independent predictors when excluding those with borderline zone 1 pattern (Table 7). Increasing age and prothrombin time and decreasing insulin resistance were independent predictors of definite NASH versus borderline zone 1 pattern (excluding those without fibrosis). Increasing AST and white blood cell count and decreasing hematocrit were significant predictors of increasing fibrosis severity. Hispanic ethnicity, increasing insulin level, and decreasing INR were predictive of moderate versus mild degrees of fibrosis. Significant predictors of increasing NAS were increasing AST and GGT, ASMA positivity, and inclusion in TONIC (versus database). Prediction equations based on regression coefficients from ordered (or binary) logistic regression and the area under the ROC (AUROC) for these equations are shown below Table 7.
Table 7.
Significant predictors of histological variables from multiple regression analysis
| Outcome (Categorization) Significant Predictors | Cumulative OR | 95% CI | P |
|---|---|---|---|
| 1NAFLD Pattern (Not NASH vs. Borderline zone 3 vs. Definite) | |||
| AST (U/L) | 1.017 | 1.004–1.031 | 0.014 |
| TONIC (yes vs. no) | 2.7 | 1.1–6.7 | 0.03 |
| GGT (U/L) | 1.017 | 1.000–1.033 | 0.04 |
|
| |||
| 2NAFLD Pattern (Borderline zone 1 vs. Definite NASH)* | |||
| Age (years) | 1.5 | 1.2–1.9 | <0.001 |
| Prothrombin time (seconds) | 1.5 | 1.1–2.0 | 0.005 |
| QUICKI (x100) | 0.75 | 0.61–0.83 | 0.009 |
|
| |||
| 3Fibrosis (None vs. Mild vs. Moderate vs. Bridging) | |||
| AST (U/L) | 1.015 | 1.006–1.024 | 0.001 |
| White Blood Cell Count (1000 cells/mm3) | 1.22 | 1.07–1.38 | 0.003 |
| Hematocrit (%) | 0.87 | 0.79–0.96 | 0.004 |
|
| |||
| 4Fibrosis (Mild vs. Moderate)** | |||
| Hispanic Ethnicity (yes vs. no) | 0.18 | 0.06–0.49 | 0.001 |
| Insulin (mU/mL) | 1.03 | 1.01–1.05 | 0.01 |
| International Normalized Ratio (x 100) | 0.91 | 0.84–0.99 | 0.04 |
|
| |||
| 5NAFLD Activity Score (NAS 1–3 vs. NAS 4–5 vs. NAS 6–7) | |||
| AST (U/L) | 1.020 | 1.010–1.031 | <0.001 |
| ASMA (positive vs. negative) | 2.8 | 1.4–5.4 | 0.002 |
| GGT (U/L) | 1.013 | 1.003–1.023 | 0.01 |
| TONIC (Yes vs. No) | 2.5 | 1.2–5.4 | 0.02 |
Excluding patients with no fibrosis
Excluding patients with none or bridging fibrosis
Prediction equations [regression coefficients from ordered (or binary) logistic regression]:
1a) Borderline zone 3 or Definite NASH vs. Not NASH = −1.55 + 0.017 AST (U/L) + 0.017 GGT (U/L) + 1.01 (if in TONIC study); AUROC = 0.82
1b) Definite NASH vs. Borderline zone 3 or Not NASH = −2.64+ 0.017 AST (U/L) + 0.017 GGT (U/L) + 1.01 (if in TONIC study); AUROC = 0.74
Prediction equation [regression coefficients from ordered (or binary) logistic regression]: 2) Definite NASH vs. Borderline zone 1 = −1.43 + 0.44 AGE (yrs) + 0.42 Prothrombin time (secs) − 0.28 QUICKI (x 100); AUROC = 0.80
Prediction equations [regression coefficients from ordered (or binary) logistic regression]:
3a) Mild/Moderate/Bridging Fibrosis vs. No Fibrosis = 4.32 + 0.015 AST (U/L) + 0.20 WBC (1000 cells/mm3) − 0.14 Hematocrit (%); AUROC = 0.68
3b) Moderate or Bridging Fibrosis vs. Mild or No Fibrosis = 2.18 + 0.015 AST (U/L) + 0.20 WBC (1000 cells/mm3) − 0.14 Hematocrit (%); AUROC = 0.73
3c) Bridging Fibrosis vs. Mild/Moderate/No Fibrosis = 1.04 + 0.015 AST (U/L) + 0.20 WBC (1000 cells/mm3) − 0.14 Hematocrit (%); AUROC = 0.77
Prediction equation [regression coefficients from ordered (or binary) logistic regression]: 4) Moderate vs. Mild Fibrosis = 8.11 − 1.74 (if Hispanic) + 0.030 insulin (□U/mL) − 0.091 INR(x 100); AUROC = 0.81
Prediction equations [regression coefficients from ordered (or binary) logistic regression]:
5a) NAS 4–7 vs. NAS 1–3 = −1.68 + 0.020 AST (U/L) + 1.03 (if positive ASMA) + 0.013 GGT (U/L) + 0.98 (if in TONIC study); AUROC = 0.80
5b) NAS 6–7 vs. NAS 1–5 = −4.31 + 0.020 AST (U/L) + 1.03 (if positive ASMA) + 0.013 GGT (U/L) + 0.98 (if in TONIC study); AUROC = 0.78
Discussion
Recent data from the US National Health and Nutrition Examination Survey (NHANES) reported elevated ALT (>30 U/L), a marker of potential NAFLD, is prevalent in 8% of adolescents 12–19 years of age.24 Histology-based autopsy data suggest that 8% of the population aged 2 to 19 years may be affected by NAFLD1. Progressive disease in even a small fraction represents a significant potential for disease burden. In a retrospective evaluation of screening practices at academic centers, 2% of general pediatricians and 23% of pediatric gastroenterologists performed screening for NAFLD among obese children, suggesting that most cases of pediatric NAFLD are currently undiagnosed7. Given the potential for NAFLD to progress to cirrhosis, it is imperative that screening tools be developed; ideally, with non-invasive means to identify children at greatest risk for progressive disease. Serum aminotransferase levels would represent the simplest screening test for this purpose, but have not been proven to accurately reflect histologic activity. We analyzed data collected for the NASH CRN, a large, multi-center study, to identify clinical indicators of histological activity.
Assuming that Not NASH, borderline zone 3 pattern and definite NASH represent a range of conditions along a spectrum, we sought to identify clinical features that would distinguish these patterns. AST and GGT were significant predictors of histology pattern in this analysis. While statistically significant, the cumulative odds ratios for AST (OR 1.017 per U/L = 1.18 per 10 U/L = 5.40 per 100 U/L) and GGT (OR 1.017 per U/L = 1.18 per 10 U/L = 5.40 per 100 U/L), demonstrate that these laboratory measures are likely of limited clinical utility in distinguishing children with these histological patterns.
Serum antinuclear antibodies (ANA) and anti-smooth muscle antibodies (ASMA) have been reported in 6–15% of the population and 8–25% of patients with chronic liver disease in whom these antibodies often do not signify autoimmune liver disease.25–27 The largest study of autoantibodies in NAFLD was conducted in 225 patients with biopsy-proven NAFLD (age 13–73 years) from the Mayo Clinic.28 20% had positive titers for ANA, 3% for ASMA and a positive ANA and/or ASMA was associated with higher fibrosis stage and inflammation grade. An Italian study found ANA positive in 21.4%, ASMA positive in 4.7% and anti-mitochondrial antibody (AMA) positive in 2.4% of 84 adult patients with biopsy-proven NAFLD.29 3.6% of patients in this series had overlapping features of autoimmune hepatitis on biopsy. In a Japanese study of 212 adult patients with biopsy-proven NAFLD, 33% had positive ANA titers and 1.4% had both ANA and ASMA titers positive.30 An ANA titer of 1:80 or greater was associated with greater prevalence of severe necroinflammation (25%) compared to those with titers of 1:40 or less (11.3%, P=0.037).
Autoantibody positivity has been reported to occur in apparently healthy children with no personal or family history of autoimmune disease with rates of 3% for ANA, 2.6% for ASMA and 1.1% for AMA.31 The significance of autoantibody positivity in pediatric NAFLD is unknown. None of the children enrolled in our study had positive titers for AMA, but the prevalence of ANA positivity was 18% and ASMA positivity was 32%. Positive titer for ASMA was a significant predictor of increasing NAS (OR 2.8, 95% CI 1.4–5.4). The potential of ASMA as a non-invasive marker of histological severity in pediatric NAFLD is a novel finding that deserves further study.
Borderline zone 1 pattern of NAFLD is a unique histological pattern that appears to predominantly affect children.17 This pattern, previously referred to as type 2 or pediatric-type NASH, has been reported more frequently among boys, to affect younger children, be associated with more severe obesity, and to be the predominant pattern seen among those of Asian or Native American race and Hispanic ethnicity.9 A similar pattern emerged among children enrolled in the NASH CRN, though BMI percentile and percent body fat did not differ between those with borderline zone 1 versus definite NASH. On multiple regression analysis, age, prothrombin time, and insulin resistance were significant predictors of histological pattern. Our findings support the hypothesis that sex hormones and insulin resistance associated with pubertal development may be important variables in the predisposition to and determination of histological subtype of pediatric NAFLD.9, 32 Whether this borderline zone 1 form present in children represents an alternative or distinct pattern of NASH awaits demonstration of potential differences in etiopathogenesis, natural history or treatment response.
Markers of insulin resistance were consistently higher among children with definite NASH compared to other NAFLD patterns and with increasing NAS. In a multiple regression analysis of individual NAS scores, lower Tanner stage was predictive of higher NAS (OR 0.76 per Tanner stage, 95% CI 0.63–0.92) suggesting that hormonal changes associated with pubertal development may influence disease severity. Puberty is associated with a decrease in insulin sensitivity (25–30%) that is compensated for by an increase in insulin secretion. Insulin resistance occurs early in development, typically between Tanner stages I and II, with a nadir in insulin sensitivity at Tanner stage III and recovery by stage V.33, 34 Analysis of children with borderline zone 1 compared to definite NASH support the association of this pattern of developmental insulin resistance with NAFLD expression. Those with borderline zone 1 pattern were younger (median age 11.1 years) with mean Tanner stage 1.8 and had less severe insulin resistance (median HOMA-IR 4.8) compared to those with definite NASH (median age 13.0 years) with mean Tanner stage of 2.7 and greater insulin resistance (median HOMA-IR 7.7, P=0.006). Whether children with borderline zone 1 pattern may evolve into definite NASH and/or children with definite NASH regress to borderline zone 3 or simple steatosis with further developmental maturation is unknown and will require longitudinal data to determine.
Another potential mediator of pubertal development on disease expression in pediatric NAFLD are changes in sex steroid hormones. Sex hormones have been proposed to account for differences in prevalence rates and disease expression among males and females with NAFLD.32 The prevalence of elevated ALT among adolescents aged 12–19 years from NHANES was 12.4% in males compared to 3.5% in females.24 Most published series of pediatric NAFLD have reported males to be more commonly affected.10, 35–37 While our patient population was predominantly male (77%), gender was not predictive of borderline zone 1 pattern versus definite NASH.9 Similarly, while there was a larger percentage of males with definite NASH compared to borderline zone 3 and not NASH, this difference was not significant. We cannot exclude the possibility of type 2 error in determining the role of gender on NAFLD pattern due to the relatively smaller number (n=41) of female subjects.
AST was found to be a significant predictor of NAFLD pattern, fibrosis severity, and NAS. Area under the ROC curve analysis demonstrated AST to be superior to ALT in distinguishing NAFLD pattern and the addition of ALT to AST did not improve discriminate performance. However, AUROC demonstrate that AST value does not have sufficient discriminate power to reliably predict histology. While our results do not support the use of AST in place of liver biopsy, the strong association between AST and meaningful histological features in pediatric NAFLD support current recommendations to use serum aminotransferase levels in screening overweight children.38 It is important to note that the majority of participants in this study were required to have elevated ALT for study inclusion. Baseline ALT values were ≥60 U/L in 50% of Database participants and 99% of TONIC participants. Therefore, the associations that we have reported between serum aminotransferase levels and histology in NAFLD may not be applicable to other patient populations in whom serum transaminase levels may not be elevated to this extent.
Predictors of fibrosis varied depending upon whether all degrees of fibrosis were compared or only those with mild versus moderate fibrosis were considered. Overall predictors of increasing severity of fibrosis were increasing AST and white blood cell count and decreasing hematocrit. Platelet count, frequently included as a non-invasive marker of fibrosis in chronic hepatitis C virus infection, was not associated with fibrosis severity.39, 40 Hispanic ethnicity was predictive of fibrosis severity when comparing those with mild and moderate degrees of fibrosis, likely accounted for by the relatively small percentage (31%) of subjects with moderate zone 3 and periportal fibrosis of Hispanic ethnicity. Children with bridging fibrosis tended to be younger than those with lesser degrees of fibrosis, perhaps indicating that yet unidentified susceptibility genes predispose to a more aggressive course in these children. In contrast to previous studies, BMI was not associated with fibrosis severity, though percent body fat was lower among subjects without fibrosis. The study population may have been too skewed with respect to BMI (97% had BMI ≥95th percentile) and/or it may be that body fat distribution is a more important determinant of fibrosis than BMI. _Higher insulin levels also were predictive of moderate versus mild fibrosis. While in clinical practice insulin resistance is unlikely to be of use in distinguishing fibrosis stage, this finding supports insulin resistance as an important variable in disease progression.10, 41–43
The association of insulin resistance with portal fibrosis in NASH was recently investigated in context of a histological lesion called ductular reaction.44 In this study, insulin resistance was highly associated with replicative arrest of hepatocytes, an impaired response to necrotic and apoptotic hepatocytes. In the setting of impaired hepatic regeneration, a secondary replicative pathway of hepatic progenitor cells is induced.45 Ductular reaction is a lesion seen at the portal tract interface composed of small biliary ductules, stroma and inflammatory cells that occurs as a by-product of induction of this secondary pathway.46, 47 Ductular reaction was associated with the extent of replicative arrest and the extent of ductular reaction was strongly associated with fibrosis. Further studies are required to determine whether similar processes occur in pediatric NAFLD and whether this provides a mechanistic link between insulin resistance and portal fibrosis.
Because of the expense and risk of liver biopsy, there is enthusiasm for development of noninvasive markers of liver histology. Factors to consider in studies designed to identify such markers include adequacy of biopsy specimens and interpretation of histology by pathologists with appropriate expertise.48 We included biopsy specimen length in all of our analyses and central review by the Pathology Committee of the NASH CRN, composed of pathologists with expertise in the histopathology of NAFLD, was one of the inclusion criteria for our study. More difficult to account for is the possibility of sampling error in NAFLD.49, 50 As recently highlighted, increased size may minimize heterogeneity between biopsy specimens.48
In conclusion, AST, GGT, and positive ASMA titers were the clinical variables most consistently associated with the pattern and severity of NAFLD in this large prospective, multi-center, histology-based study of children. The association between ASMA and pediatric NAFLD is a novel finding deserving of further study. Our results support the hypothesis that insulin resistance associated with pubertal development may be an important determinant of disease expression in pediatric NAFLD. These results also support a role for insulin resistance in the development of fibrosis in pediatric NAFLD. Unfortunately, none of the clinical predictors of histology appear sufficiently powerful to replace liver biopsy as a non-invasive means of staging disease. However, these clinical markers may be employed by pediatric gastroenterologists in evaluating overweight children suspected to have NAFLD.
Supplementary Material
Comparison between a case of steatohepatitis with zone 3 injury pattern and the zone 1 steatosis and fibrosis pattern observed in some children. A. Steatohepatitis showing prominent ballooning injury with Mallory body formation in the center of the field. A small normal appearing portal area is present nearby. (H&E, 200x); B. A liver biopsy showing periportal, zone 1 macrovesicular steatosis adjacent to a fibrotic portal area. The zone 3 region and the central vein at the top of the photo are unremarkable. There was portal-portal bridging fibrosis elsewhere in the biopsy, but no perisinusoidal fibrosis.
Acknowledgments
Grant Support: The Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (grants U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, U01DK061713), and the National Institute of Child Health and Human Development (NICHD). Several clinical centers use support from General Clinical Research Centers or Clinical and Translational Science Awards in conduct of NASH CRN Studies (grants UL1RR024989, M01RR000750, M01RR00188, RR02413101, M01RR000827, UL1RR02501401, M01RR000065, M01RR020359). This study was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations
- ALT
Alanine aminotransferase
- AMA
anti-mitochondrial antibody
- ANA
antinuclear antibody
- ASMA
anti-smooth muscle antibody
- AST
aspartate aminotransferase
- BMI
body mass index
- DEXA
Dual energy x-ray absorptiometry
- GGT
gamma glutamyl transferase
- HOMA-IR
Homeostasis model assessment
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- QUICKI
Quantitative insulin sensitivity check index
Appendix: Members of the Nonalcoholic Steatohepatitis Clinical Research Network
Writing committee members
Heather Patton1, Joel Lavine2, Mark L. Van Natta3, Jeffrey Schwimmer2, David Kleiner4, Jean Molleston5
1Division of Gastroenterology, University of California, San Diego, San Diego, CA
2Division of Pediatric Gastroenterology, Hepatology, and Nutrition, University of California, San Diego, San Diego, CA
3Data Coordinating Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD
4Laboratory of Pathology, National Cancer Institute, Bethesda, MD
5Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Indiana University School of Medicine, Indianapolis, IN
Clinical Centers: Principal Investigators and *Pediatric Co-Investigators
Case Western Reserve University: Drs. Arthur McCullough, Margaret Stager* & Ariel Feldstein*
Duke University Medical Center: Drs. Anna Mae Diehl & Ann Scheimann (Johns Hopkins University)*
Indiana University: Drs. Naga Chalasani, Jean Molleston* & Girish Subbarao*
St. Louis University: Drs. Brent Tetri, Sarah Barlow* & Stephanie Abrams (Baylor University)*
University of California, San Diego: Drs. Joel Lavine & Jeffrey Schwimmer*
University of California, San Francisco: Drs. Nathan Bass & Philip Rosenthal*
University of Washington: Drs. Kris Kowdley & Karen Murray*
Virginia Commonwealth University: Drs. Arun Sanyal & Daphne Bryan*
Data Coordinating Center: Principal Investigator
Johns Hopkins Bloomberg School of Public Health: Dr. James Tonascia
NIH
NIDDK: Dr. Patricia Robuck, Project Scientist
NIDDK: Dr. Jay Hoofnagle, Scientific Advisor
NICHD: Dr. Terry Huang, Scientific Advisor
NCI: Dr. David Kleiner, Pathologist
Footnotes
Financial Disclosures: None of the authors have any conflicts of interest to disclose
Writing Assistance: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Brunt EM. Pathology of nonalcoholic steatohepatitis. Hepatol Res. 2005;33:68–71. doi: 10.1016/j.hepres.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, Musso A, De Paolis P, Capussotti L, Salizzoni M, Rizzetto M. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 4.Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–54. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 5.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 6.Children and teens told by doctors that they were overweight--United States, 1999–2002. MMWR Morb Mortal Wkly Rep. 2005;54:848–9. [PubMed] [Google Scholar]
- 7.Riley MR, Bass NM, Rosenthal P, Merriman RB. Underdiagnosis of pediatric obesity and underscreening for fatty liver disease and metabolic syndrome by pediatricians and pediatric subspecialists. J Pediatr. 2005;147:839–42. doi: 10.1016/j.jpeds.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol Hepatol. 2006;4:226–32. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 9.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, Lavine JE. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 10.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–5. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 11.Nonalcoholic steatohepatitis clinical research network. Hepatology. 2003;37:244. doi: 10.1002/hep.510370203. [DOI] [PubMed] [Google Scholar]
- 12.Lavine JE, Schwimmer JB. Pediatric initiatives within the Nonalcoholic Steatohepatitis-Clinical Research Network (NASH CNR) J Pediatr Gastroenterol Nutr. 2003;37:220–1. doi: 10.1097/00005176-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2000;11:1–190. [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner DE, Behling CB, Brunt EM, Lavine JE, McCullough AJ, Sanyal AJ, Schwimmer JB, Tonascia J. Comparison of adult and pediatric NAFLD- confirmation of a second pattern of progressive fatty liver disease in children. Hepatology. 2006;44:259A–260A. [Google Scholar]
- 18.Agresti A. Categorical Data Analysis. John Wiley and Sons; 1990. [Google Scholar]
- 19.McCullagh P. Regression models for ordinal data. JRSS (B) 1980;42:109–142. [Google Scholar]
- 20.StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 21.Little R, Rubin D. Statistical Analysis with Missing Data. John Wiley & Sons, Inc; 2002. [Google Scholar]
- 22.Green D, Swets J. Signal detection theory and psychophysics. Wiley; 1966. [Google Scholar]
- 23.SAS. SAS, Version 9.1. Cary, NC: SAS Institute Inc.; 2007. [Google Scholar]
- 24.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–20. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homburger HA, Cahen YD, Griffiths J, Jacob GL. Detection of antinuclear antibodies: comparative evaluation of enzyme immunoassay and indirect immunofluorescence methods. Arch Pathol Lab Med. 1998;122:993–9. [PubMed] [Google Scholar]
- 26.Lenzi M, Bellentani S, Saccoccio G, Muratori P, Masutti F, Muratori L, Cassani F, Bianchi FB, Tiribelli C. Prevalence of non-organ-specific autoantibodies and chronic liver disease in the general population: a nested case-control study of the Dionysos cohort. Gut. 1999;45:435–41. doi: 10.1136/gut.45.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Genetic predispositions for immunological features in chronic liver diseases other than autoimmune hepatitis. J Hepatol. 1996;24:52–9. doi: 10.1016/s0168-8278(96)80186-3. [DOI] [PubMed] [Google Scholar]
- 28.Adams LA, Lindor KD, Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2004;99:1316–20. doi: 10.1111/j.1572-0241.2004.30444.x. [DOI] [PubMed] [Google Scholar]
- 29.Loria P, Lonardo A, Leonardi F, Fontana C, Carulli L, Verrone AM, Borsatti A, Bertolotti M, Cassani F, Bagni A, Muratori P, Ganazzi D, Bianchi FB, Carulli N. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci. 2003;48:2173–81. doi: 10.1023/b:ddas.0000004522.36120.08. [DOI] [PubMed] [Google Scholar]
- 30.Yatsuji S, Hashimoto E, Kaneda H, Taniai M, Tokushige K, Shiratori K. Diagnosing autoimmune hepatitis in nonalcoholic fatty liver disease: is the International Autoimmune Hepatitis Group scoring system useful? J Gastroenterol. 2005;40:1130–8. doi: 10.1007/s00535-005-1711-z. [DOI] [PubMed] [Google Scholar]
- 31.Martini A, Lorini R, Zanaboni D, Ravelli A, Burgio RG. Frequency of autoantibodies in normal children. Am J Dis Child. 1989;143:493–6. doi: 10.1001/archpedi.1989.02150160123025. [DOI] [PubMed] [Google Scholar]
- 32.Patton HM, Sirlin C, Behling C, Middleton M, Schwimmer JB, Lavine JE. Pediatric nonalcoholic fatty liver disease: a critical appraisal of current data and implications for future research. J Pediatr Gastroenterol Nutr. 2006;43:413–27. doi: 10.1097/01.mpg.0000239995.58388.56. [DOI] [PubMed] [Google Scholar]
- 33.Cook JS, Hoffman RP, Stene MA, Hansen JR. Effects of maturational stage on insulin sensitivity during puberty. J Clin Endocrinol Metab. 1993;77:725–30. doi: 10.1210/jcem.77.3.7690363. [DOI] [PubMed] [Google Scholar]
- 34.Roemmich JN, Clark PA, Lusk M, Friel A, Weltman A, Epstein LH, Rogol AD. Pubertal alterations in growth and body composition. VI. Pubertal insulin resistance: relation to adiposity, body fat distribution and hormone release. Int J Obes Relat Metab Disord. 2002;26:701–9. doi: 10.1038/sj.ijo.0801975. [DOI] [PubMed] [Google Scholar]
- 35.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–9. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 36.Guzzaloni G, Grugni G, Minocci A, Moro D, Morabito F. Liver steatosis in juvenile obesity: correlations with lipid profile, hepatic biochemical parameters and glycemic and insulinemic responses to an oral glucose tolerance test. Int J Obes Relat Metab Disord. 2000;24:772–6. doi: 10.1038/sj.ijo.0801224. [DOI] [PubMed] [Google Scholar]
- 37.Manton ND, Lipsett J, Moore DJ, Davidson GP, Bourne AJ, Couper RT. Non-alcoholic steatohepatitis in children and adolescents. Med J Aust. 2000;173:476–9. doi: 10.5694/j.1326-5377.2000.tb139299.x. [DOI] [PubMed] [Google Scholar]
- 38.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120 (Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 39.Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, Bauer B, Stauber RE. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376–82. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 40.Snyder N, Gajula L, Xiao SY, Grady J, Luxon B, Lau DT, Soloway R, Petersen J. APRI: an easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J Clin Gastroenterol. 2006;40:535–42. doi: 10.1097/00004836-200607000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Bugianesi E, Manzini P, D'Antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–87. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 42.Haukeland JW, Konopski Z, Linnestad P, Azimy S, Marit Loberg E, Haaland T, Birkeland K, Bjoro K. Abnormal glucose tolerance is a predictor of steatohepatitis and fibrosis in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2005;40:1469–77. doi: 10.1080/00365520500264953. [DOI] [PubMed] [Google Scholar]
- 43.Angulo P, Alba LM, Petrovic LM, Adams LA, Lindor KD, Jensen MD. Leptin, insulin resistance, and liver fibrosis in human nonalcoholic fatty liver disease. J Hepatol. 2004;41:943–9. doi: 10.1016/j.jhep.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, Dixon JB, Weltman MD, Tilg H, Moschen AR, Purdie DM, Demetris AJ, Clouston AD. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Roskams T, Yang SQ, Koteish A, Durnez A, DeVos R, Huang X, Achten R, Verslype C, Diehl AM. Oxidative stress and oval cell accumulation in mice and humans with alcoholic and nonalcoholic fatty liver disease. Am J Pathol. 2003;163:1301–11. doi: 10.1016/S0002-9440(10)63489-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roskams T. Progenitor cell involvement in cirrhotic human liver diseases: from controversy to consensus. J Hepatol. 2003;39:431–4. doi: 10.1016/s0168-8278(03)00333-7. [DOI] [PubMed] [Google Scholar]
- 47.Roskams TA, Theise ND, Balabaud C, Bhagat G, Bhathal PS, Bioulac-Sage P, Brunt EM, Crawford JM, Crosby HA, Desmet V, Finegold MJ, Geller SA, Gouw AS, Hytiroglou P, Knisely AS, Kojiro M, Lefkowitch JH, Nakanuma Y, Olynyk JK, Park YN, Portmann B, Saxena R, Scheuer PJ, Strain AJ, Thung SN, Wanless IR, West AB. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–45. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 48.Brunt EM. Do you see what I see? The role of quality histopathology in scientific study. Hepatology. 2008;47:771–4. doi: 10.1002/hep.22185. [DOI] [PubMed] [Google Scholar]
- 49.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 50.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, Bass NM. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–80. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison between a case of steatohepatitis with zone 3 injury pattern and the zone 1 steatosis and fibrosis pattern observed in some children. A. Steatohepatitis showing prominent ballooning injury with Mallory body formation in the center of the field. A small normal appearing portal area is present nearby. (H&E, 200x); B. A liver biopsy showing periportal, zone 1 macrovesicular steatosis adjacent to a fibrotic portal area. The zone 3 region and the central vein at the top of the photo are unremarkable. There was portal-portal bridging fibrosis elsewhere in the biopsy, but no perisinusoidal fibrosis.