Abstract
The objective of the present research was to stabilize a novel hemiglutarate ester prodrug of Δ9-tetrahydrocannabinol (THC), in polyethylene oxide (PEO) polymeric matrices produced by hot-melt fabrication, for systemic delivery of THC through the oral transmucosal route. For this purpose, the influence of pH modifiers and antioxidants employed as stabilizing agents in these matrices was investigated. Based on the stability studies, two final formulations were made, and the stability of the active was assessed in these systems. In addition, the bioadhesive properties of PEO matrices were studied as a function of bioadhesive polymer type and concentration, contact time, drug loading and wetting time. Of all of the polymers investigated, bioadhesion was highest with Carbopol® 971p. Bioadhesion increased with bioadhesive polymer concentration and wetting time to a certain level beyond which there was no further contribution. Both the contact time and drug loading influenced the bioadhesion. Severe degradation of the prodrug was observed during storage, even at room temperature (75% at the end of 3 months). Incorporation of the stabilizing agents in the PEO matrices reduced the degradation of the prodrug considerably. Citric acid was the most effective of all of the pH modifiers studied. Among the various antioxidants utilized, degradation was observed least in presence of BHT and ascorbic acid. Only 7.6% and 8.2% of prodrug degraded in these matrices, respectively, as compared to the PEO only matrices (59.4%) at the end of 3 months at 25 °C/60% RH. The prodrug was very stable in both of the final formulations at the end of the 3 months at 40 °C/75% RH.
Keywords: Bioadhesion, THC, Poly (ethylene oxide), Stability, Prodrug, Antioxidants
1. Introduction
Δ9-Tetrahydrocannabinol (THC), the major pharmacologically active constituent of Cannabis sativa exhibits therapeutic potential in the treatment of nausea and vomiting during cancer chemotherapy, anorexia associated with weight loss in AIDS patients, glaucoma, analgesia, anxiety as well as other potential indications (Voth et al., 1997). Despite the promising clinical potential of THC, an effective dosage form has not been developed to date. The only commercially available dosage form with a constant THC content is the soft gelatin capsule for oral administration, marketed in the US as Marinol®. In this formulation, however, the drug has limited stability and therefore has to be stored at low temperatures (4 °C). Moreover, the oral bioavailability of the drug is low (~ 6 %) and inconsistent, which is mainly due to its high first-pass metabolism and poor solubility (Ohlsson et al., 1980). In addition to the pharmacokinetic limitations, the physicochemical properties of THC present a major challenge in the development of a suitable dosage form. THC is a poorly water-soluble, amorphous substance which is sticky, resin-like and highly viscous, which makes it difficult to handle and process. Furthermore, the instability of THC, especially in acidic solutions, and when exposed to heat, air and light has been reported by various researchers (Mechoulam, 1970; Fairbairn et al., 1976).
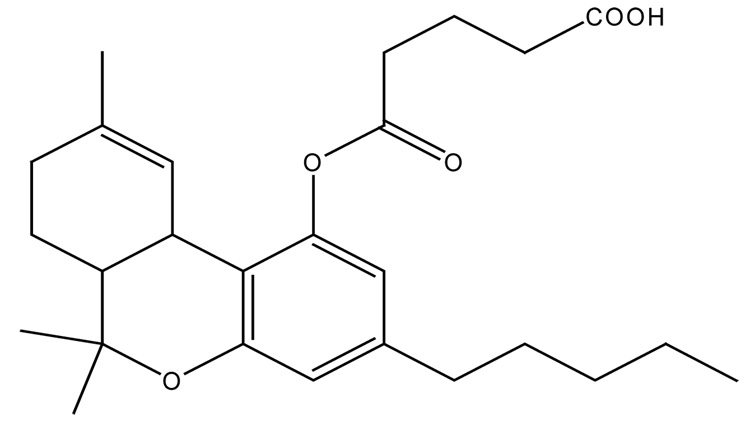
THC-hemiglutarate (THC-HG), a prodrug of THC, has been developed in an attempt to overcome the pharmacokinetic limitations and improve the physicochemical properties of the parent drug. The structure of THC-HG is depicted in Figure 1. Due to the significant limitations associated with traditional routes of administration, several non-parenteral routes have also been explored for systemic delivery of THC. These include sublingual (Guy et al., 2000), rectal (ElSohly et al., 1991), nasal (Harris et al., 1988) and transdermal (Challapalli et al., 2002). With each of these routes having their own disadvantages, an attempt has been made to systemically deliver THC in the form of its novel prodrug, THC-HG through the oral transmucosal route, since it offers distinct advantages including avoidance of first-pass effect, easy accessibility and enhanced patient compliance. The oral mucosa is relatively permeable with a rich blood supply, robust and shows short recovery times after stress or damage. These factors make the oral mucosal cavity a very attractive and feasible site for systemic drug delivery (Shojaei, 1998).
Figure 1.
Chemical structure of THC-HG.
A major disadvantage of oral mucosal delivery, however, is the lack of retention of the dosage form at the intended site for a desirable duration and hence drug loss due to salivary wash out, involuntary tongue movements and swallowing. Consequently, oral mucosal delivery requires the use of mucoadhesive polymers to overcome these limitations. The mucoadhesives increase residence time at the absorption site, improve contact between the delivery system and the absorption site, and provide localization to specified oral mucosa regions to enhance bioavailability. The interaction between the mucus and mucoadhesive polymers has been thought to be a two stage process (Wu, 1982). In the first stage known as the contact stage, the wetting of the mucoadhesive polymer upon contact with a mucosal membrane leads to swelling of the polymer followed by disentanglement and interpenetration of polymer and mucus chains. The second stage known as the consolidation stage, then involves the formation of interfacial bonds between the interpenetrated chains leading to prolonged adhesion. These bonds are of secondary type, such as electrostatic forces, Van der Waal’s forces, hydrogen bonds and hydrophobic interactions and are relatively weak (Solomonidou et al., 2001). Physical properties of the mucoadhesive such as chemical structure, molecular weight, rate of hydration and polymer concentration can have a major impact on their mucoadhesion and consequently their eventual duration of retention (Smart, 1991). Hence, for successful delivery of THC-HG across the oral mucosa, a bioadhesion assessment in the presence of potential bioadhesive polymers is imperative.
A survey of the scientific literature indicates that there are only few references on prodrugs of THC (all of them on THC-hemisuccinate) that have focused on studying the stability and bioavailability of the drug in various dosage forms (ElSohly et al., 1991; Munjal et al., 2006). However, no research has been focused to investigate the physicochemical properties or stability of the novel hemiglutarate prodrug in any of the dosage forms to date. Preformulation studies conducted by our research group revealed that THC-HG is highly unstable even when stored at 4° C due to a low glass transition temperature (0.586 °C). pH stability studies revealed the instability of the prodrug in an aqueous environment. Furthermore, THC-HG was found to be sensitive in the presence of oxygen and exhibited significant degradation under elevated relative humidity conditions (Thumma et al.). In the present study, an attempt has therefore been made to stabilize the prodrug via incorporation into PEO polymeric matrices produced by a hot-melt method. The influence of pH modifiers and antioxidants employed as stabilizing agents in these matrices was investigated. In addition, since optimal bioadhesion is essential for the successful application of an oral transmucosal matrix system, the bioadhesive performance of the active-incorporated PEO matrices in the presence of various potential mucoadhesive polymers was also assessed.
2. Materials and Methods
2.1. Materials
PEO [PolyOx® WSR N-80 (PEO N-80), MW 200,000 Daltons] and hydroxypropylmethyl cellulose (Methocel® K4M) (HPMC) were kindly donated by Dow Chemical Company (Midland, MI) and hydroxylpropyl cellulose (HPC) (Klucel® LF) by Aqualon Division, Hercules Inc (Wilmington, DE). Vitamin E succinate (VES), sodium carboxymethylcellulose (SCMC), fumaric acid, citric acid anhydrous, monobasic sodium phosphate, tartaric acid, succinic acid, sodium citrate dihydrate, sodium tartarate, BHT, BHA, ascorbic acid, propyl gallate, EDTA and sodium dodecyl sulfate (SLS) were purchased from Spectrum Chemical, Inc., (Gardena, CA). Methanol and acetonitrile (both HPLC grade) were obtained from Fischer Chemicals (Fair Lawn, NJ). Carbopol® 971p and polycarbophil (Noveon® AA-1) were purchased from Noveon, Inc., (Cleveland, OH). Chitosan was procured from Sigma-Aldrich (St. Louis, MO) and glacial acetic acid from J.T. Baker (Phillipsburg, NJ).
2.2. Preparation of Polymeric Matrices by Hot-Melt Method
Polymeric matrices incorporating THC-HG at 5% w/w were made utilizing a hot-melt method. Briefly, a die containing a 13 mm diameter opening was placed on top of a brass sheet and heated at 110 ° C. Approximately 200 mg of the physical mixture of drug, polymer and other excipients was positioned in the orifice of the die, and compressed using a punch. This compressed mixture was heated for 5–10 min to form a melt, followed by cooling under room conditions to form a thin polymeric patch. Patch thickness ranged from 1.1 mm to 1.3 mm. The diameter of the patches produced was approximately 12.9 ± 0.2 mm. PEO N-80 grade (molecular weight, 200,000) was used as the matrix polymer for all of the studies, unless otherwise stated.
2.3. Bioadhesion Studies
Bioadhesive measurements were performed on the PEO polymeric matrices utilizing a TA.XT2i Texture Analyzer (Texture Technologies Corp., Scarsdale, NY/Stable Micro Systems, Godalming, Surrey, UK) equipped with Texture Expert™ software. Porcine buccal mucosa was used as a biological substrate. The samples (n = 5) were wetted with artificial saliva (Prodduturi et al., 2005) (adjusted to a pH of 6.8 ± 0.05) for approximately 60 seconds and placed on the lower base of the instrument. The mucosal substrate was attached to the probe with a cyanoacrylate adhesive and equilibrated with the artificial saliva before the bioadhesion testing. The probe lined with mucosa was set to approach the sample with a predetermined speed of 0.5 mm/s and applied a force of 3.5N. The test speed was 0.1 mm/s. The probe was then withdrawn at a speed of 1 mm/s following the application of force. These parameters were chosen based on previous studies (Repka et al., 2000; Repka et al., 2001). The Texture Expert™ software was utilized to record and process the data. During the withdrawal phase of the probe, the software recorded the force deflection profiles. The maximum force required to detach the film on the lower base die from the upper probe, known as the peak adhesive force (PAF), and the area under the curve (AUC) representing the work of adhesion of the films were calculated. The influence of the following variables on bioadhesion was studied.
2.3.1. Wetting time
the influence of wetting time was assessed at 15, 30, 60 and 120 sec on drug incorporated PEO matrices.
2.3.2. Bioadhesive polymer type
The bioadhesive polymers investigated included anionic (Carbopol® 971p, Noveon® AA-1 and SCMC), cationic (chitosan) and non-ionic (HPMC and HPC) polymers.
2.3.3. Bioadhesive polymers concentration
The influence of bioadhesive polymer concentration (Carbopol® 971p and Noveon® AA-1) at 5% w/w drug load was investigated at 0, 2, 4, 7 and 10 % w/w.
2.3.4. Drug loading and contact time
The polymeric matrices incorporated with prodrug at 0, 2.5, 5, 7.5 and 10 % w/w were all studied at contact times of 15, 30, 60 and 120 sec.
2.4. Stability Studies
The hot-melt method aforementioned was utilized to fabricate the PEO polymeric matrices (n = 3) incorporated with various excipients. The prodrug was incorporated at 5% w/w in all of these matrices. The processed matrices were stored in stability chambers (Caron 6030 Environmental Test Chamber, Caron Products and Services, Marietta, OH) at 25°C/60% RH or 40 °C/75% RH in an unpackaged condition and analyzed at pre-determined time intervals for THC-HG and THC content utilizing a high performance liquid chromatography (HPLC) method. Furthermore, the stability of the prodrug in each of these matrices was compared with a THC-HG-PEO only matrix without any additive, processed and stored at the same conditions. The influence of the following excipients on prodrug stability was studied.
2.4.1. pH Modifiers
Citric acid, fumaric acid, monobasic sodium phosphate, tartaric acid, succinic acid, sodium citrate and sodium tartarate were tested.
2.4.2. Anti-oxidants
Three classes of antioxidants were utilized: (i) free radical scavengers (BHT, BHA, propyl gallate), (ii) reducing agents or oxygen scavengers (ascorbic acid) and (iii) chelating agents (EDTA).
2.5. Microenvironmental pH Measurements
Approximately 200 mg of the polymeric matrix was caused to swell and dissolve to form a gel by sonicating it with 1mL of water for 30 minutes. The pH was recorded by immersing the electrode into the gel matrix and allowing it to equilibrate for 1 minute.
2.6. Chromatographic Analysis
The chromatographic system consisted of a Waters 600 pump and a dual wavelength Waters 2487 UV detector (Waters Corp, Milford, MA). A Luna 5µ C-18 (2), 150 × 4.60 mm column (Phenomenex, Torrance, CA), were used for the detection of the drug. The mobile phase consisted of 52% methanol, 30% acetonitrile and 18% water with 0.75 mL acetic acid added per 1000 mL solvent. The flow rate was maintained at 1.8 mL/min, with THC-HG and THC eluting within 15 minutes. The injection volume was 20 µL, and the column effluent was monitored by UV absorption at 228 nm. The temperature of the column was maintained at 25 °C.
2.7. Sample Preparation
A weighed portion of the THC-HG or drug-incorporated polymeric matrix was dissolved in a known volume of methanol by sonicating it for 10–15 min. The resulting solution was filtered, transferred into vials and 20 µL was injected into the HPLC column for drug analysis.
2.8. Statistical Analysis
Statistical analysis was performed using Microsoft Excel® and a Students t-test was used to analyze the results. A p < 0.05 was considered statistically significant.
3. Results and Discussion
Bioadhesion Studies
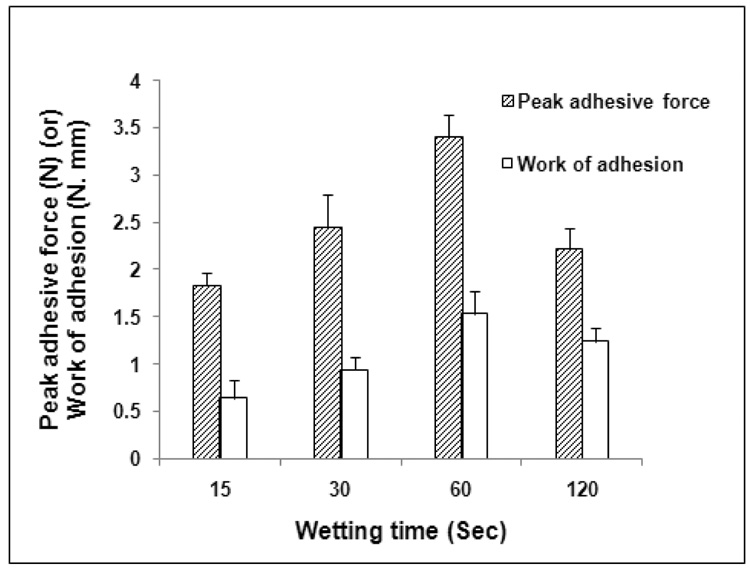
3.1. Influence of Wetting Time
The drug incorporated PEO matrices (n = 5) were wetted with artificial saliva (adjusted to a pH of 6.8 ± 0.05) for various time intervals (15, 30, 60 and 120 sec) before testing for their bioadhesion in vitro. The contact time was maintained at 60 sec and the drug load was 5 wt %. Increasing the wetting time from 15 sec to 60 sec resulted in a significant increase in both the PAF and WA values (Figure 2). A further increase in wetting time however, decreased the bioadhesive strength of these matrices. These results may be explained as follows. The formation of an adhesive bond requires swelling of the polymer chains followed by their interpenetration and entanglement with mucus chains. Swelling however, depends on the presence of water besides polymer concentration and ionic strength. During the process of bioadhesion, maximum bioadhesion in vitro occurs with optimum water content (Lee et al., 2000). Excessive hydration of the polymeric matrices (120 sec in this case) resulted in a reduction in bioadhesion probably due to over-expansion of hydrogen bonds and other forces. In addition, binding of the water molecules may result in dilution of the polymer functional groups available for adhesive interaction at the interface between the biodhesive and the mucus (Nafee et al., 2004).
Figure 2.
Influence of wetting time on the bioadhesion of THC-HG-PEO polymeric matrices. The matrices (n = 5) were fabricated at 110 ° C.
3.2. Bioadhesion of Various Polymers
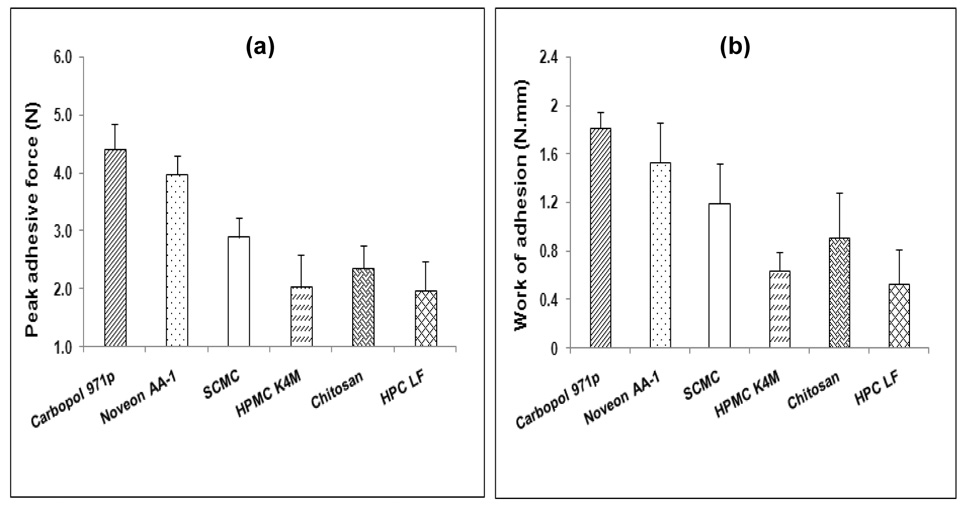
Various types of bioadhesive polymers were incorporated into the PEO polymeric matrices and their bioadhesive performance was assessed at a contact time of 60 sec (time based on previous results). THC-HG was incorporated in these matrices at 5% w/w. The peak adhesive force and work of adhesion values of the various polymers are depicted in Figures 3a and 3b, respectively. Because of the difference in their chemical nature, molecular structure as well as their hydration status, these polymers exhibited different bioadhesion properties. Of all of the polymers investigated, Carbopol® 971p matrices exhibited the highest bioadhesion. This may be attributed to the fact that Carbopols® contain a large number of carboxylic acid groups that provide the ability to form hydrogen bonds with mucus (Mortazavi et al., 1994). The rank order of the bioadhesive strength of the polymers was Carbopol® 971p> Noveon® AA-1> SCMC> chitosan> HPMC> HPC. Noveon® AA-1 exhibited a lower PAF and WA values as compared to Carbopol® 971p which may be explained based on its cross-linking. The extent of cross-linking is higher in Noveon® AA-1, which results in certain groups being unavailable to interact in the consolidation of the adhesive bond with the mucus. The bioadhesion force of the cationic polymer, chitosan was lower than that of the anionic polymers, Carbopol® 971p, Noveon® AA-1 and SCMC which may be attributed to its hydrophobicity and poor wetting properties, resulting in the formation of a weaker bond with mucus chains. Non-ionic polymers, HPMC and HPC exhibited the lowest PAF and WA values of all the bioadhesives tested possibly due to the absence of carboxyl groups in their structures, which reduces their ability to form hydrogen bonds with mucus. In general, these findings were in good agreement with those reported by other researchers (Wong et al., 1999; Nafee et al., 2003). Based on these results, Carbopol® 971p and Noveon® AA-1 were chosen as the potential bioadhesive polymers for further incorporation into the hot-melt PEO matrices for delivery of THC-HG though the oral mucosa.
Figure 3.
Influence of various bioadhesive polymer on the (a) peak adhesive force and (b) work of adhesion of THC-HG-PEO matrices (n = 5).
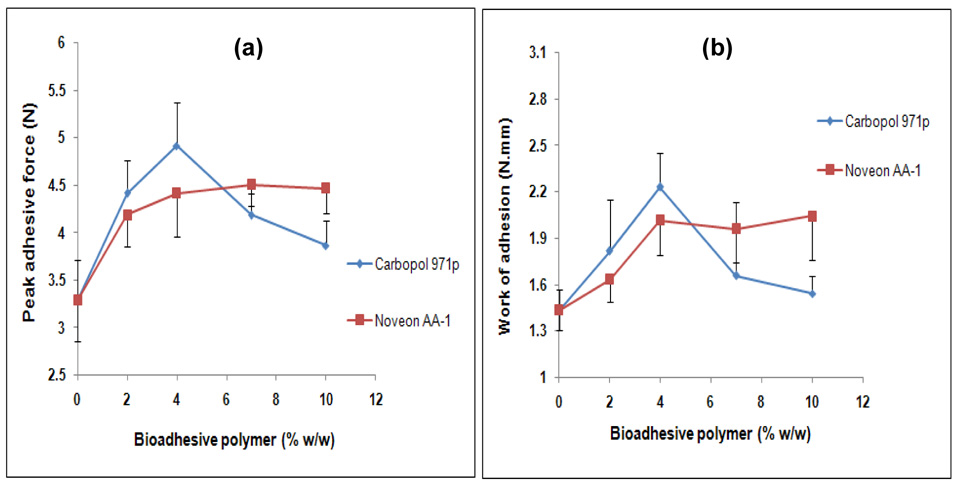
3.3. Bioadhesive Polymer Concentration
Carbopol® 971p and Noveon® AA-1 were incorporated into the PEO matrices at 0, 2, 4, 7 and 10 % w/w to investigate their influence on bioadhesion. The drug was incorporated at 5% w/w in these matrices. The results of peak adhesive force and work of adhesion as a function of polymer concentration are presented in Figures 4a and 4b, respectively. Increasing the concentration of Carbopol® 971p from 0 to 4 wt % resulted in a significant increase in both the PAF and WA values. A further increase in Carbopol® 971p concentration however, decreased the bioadhesive strength of these matrices. Similar results were obtained with Noveon® AA-1 incorporated matrices, wherein the bioadhesion (both PAF and WA) increased with concentration for up to 4 wt %. The bioadhesion however remained unchanged beyond 4 wt % in these matrices. The observed results in case of these polymers may be explained as follows. The development of a strong bond with mucus requires the interpenetration of polymeric chains into the mucus layer which further depends on the chain length available. As the polymer concentration was increased (from 0 to 4 wt % in this case), the number of penetrating polymer chains per unit volume of mucus increased, which resulted in the formation of a stronger adhesive bond (Salamat-Miller et al., 2005). However, as the concentration was further increased, an optimum (4 wt % in this case) was reached beyond which the polymers assumed a coiled structure. The number of carboxylic acid groups available for adhesive bond formation were shielded inside the coils due to the formation of intra-molecular hydrogen bonding and thus became unavailable for the adhesion process. The intra-molecular hydrogen bonding became more pronounced with increasing concentration of these polyacrylic acid derivatives thereby weakening the interaction with the mucus glycoproteins (Solomonidou et al., 2001).
Figure 4.
Influence of bioadhesive polymers concentration on the (a) peak adhesive force and (b) work of adhesion of THC-HG-PEO matrices (n = 5).
3.4. Effect of Drug Loading and Contact Time
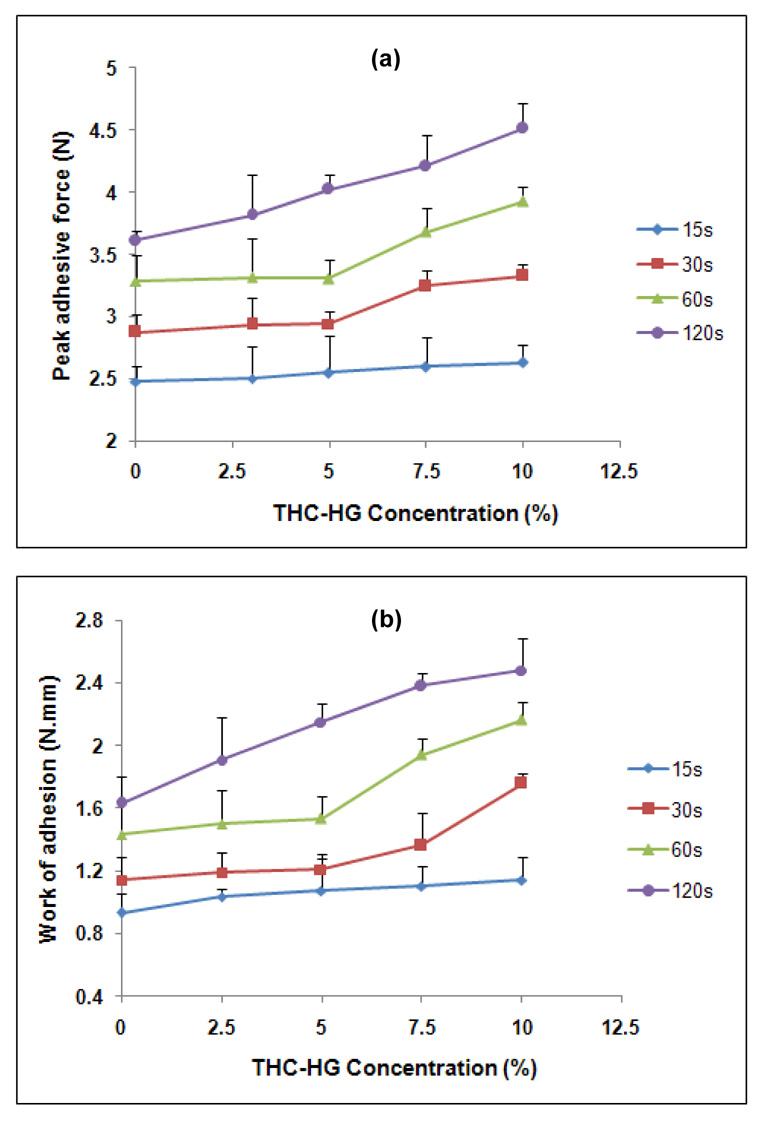
Incorporation of a hydrophobic drug into polymeric matrices can either increase or decrease the mucoadhesive strength by altering the chain mobility and swelling rate of the bioadhesive polymer, thus retarding or enhancing the formation of intimate contact between the mucosal and the adhesive surfaces (Shojaei et al., 1998). For example, a decrease in mucoadhesive strength of hydroxypropylmethyl cellulose-Carbopol buccoadhesive tablets was observed with an increase in drug content of morphine sulfate (Anlar et al., 1994). On the other hand, Ponchel et al has reported that there was no significant reduction in bioadhesive bond strength due to drug content of metronidazole in poly (acrylic acid)-hydroxypropyl methylcellulose tablets (Ponchel et al., 1987). Figures 5a and 5b present the effect of contact time and drug loading on the PAF and WA, respectively of THC-HG incorporated PEO matrices. The bioadhesive parameters (PAF and WA) demonstrated a statistical increase (p< 0.05) with an increase in contact time, irrespective of the drug load. For example, the matrices containing THC-HG at 5 wt% when tested at 120 sec exhibited a 1.6-fold higher PAF value as compared to those tested at 15 sec, while the corresponding work of adhesion was 2.0 fold higher at the same conditions. These results may be explained since contact time between the bioadhesive and mucus layer determines the extent of swelling and interpenetration of the bioadhesive polymer chains, a longer contact time permits sufficient time for the polymers to uncoil and penetrate into the mucus network to a greater extent thus resulting in the formation of a stronger bond between the two. These findings are consistent with the results of Wong and co-workers (Wong et al., 1999). In addition, bioadhesive strength of the matrices was found to be a function of drug loading. The matrices tested at a contact time of 120 sec demonstrated a statistical increase (p< 0.05) in both the PAF and WA values with an increase in drug load. In the case of the matrices tested at contact times of 30 and 60 sec, bioadhesion remained unchanged at lower drug loads (2.5 and 5.0 wt %), while increased at higher drug loads (7.5 and 10.0 wt %). For the films tested at 15 s contact time, drug loading had no significant effect on bioadhesion. “The increase in bioadhesion with increasing drug concentration may be attributed to the highly lipophilic and sticky resinous nature of the prodrug at room temperature and the presence of a relatively flexible carbon chain in its structure (no steric hindrance to the movement of polymeric chains as opposed to large size molecules which might hinder the movement of polymeric chains) (Seymour et al., 1984). However at lower drug loads (2.5 and 5.0 wt %), the drug concentration was insufficient to impact adhesion and therefore the bioadhesion remained unchanged. These results are consistent with those obtained by Repka and co-workers (Repka et al., 2006). These findings indicated that both the contact time and the drug loading can influence the bioadhesion of polymeric matrices.
Figure 5.
Influence of drug loading and contact time on the (a) peak adhesive force and (b) work of adhesion of THC-HG-PEO matrices (n = 5).
3.5. Stability Studies
Hot-melt processing has been demonstrated to be a viable method for the preparation of drug-incorporated polymeric films (Repka et al., 1999; Prodduturi et al., 2005). The hot-melt processing requires a pharmaceutical grade thermoplastic polymer that can be processed at relatively low temperatures (low melting or glass transition temperatures) due to thermal sensitivity of many drugs. In the present study, THC-HG was incorporated into hot-melt fabricated PEO matrices intended for systemic delivery of THC through the oral transmucosal route. For fabrication of polymeric matrices by a hot-melt method, a processing temperature of at least 20–50° C above the melting temperature of a semi-crystalline polymer or glass transition temperature of an amorphous polymer is desirable to lower the polymer melt viscosity (Crowley et al., 2007). The low melting temperature (melting range 57–73 °C, based on the molecular weight) of PEO facilitates thermal processing to be performed at 110–120 °C. Hence, PEO was chosen as base polymer for fabricating drug incorporated matrices.
3.5.1. Effect of pH Modifiers
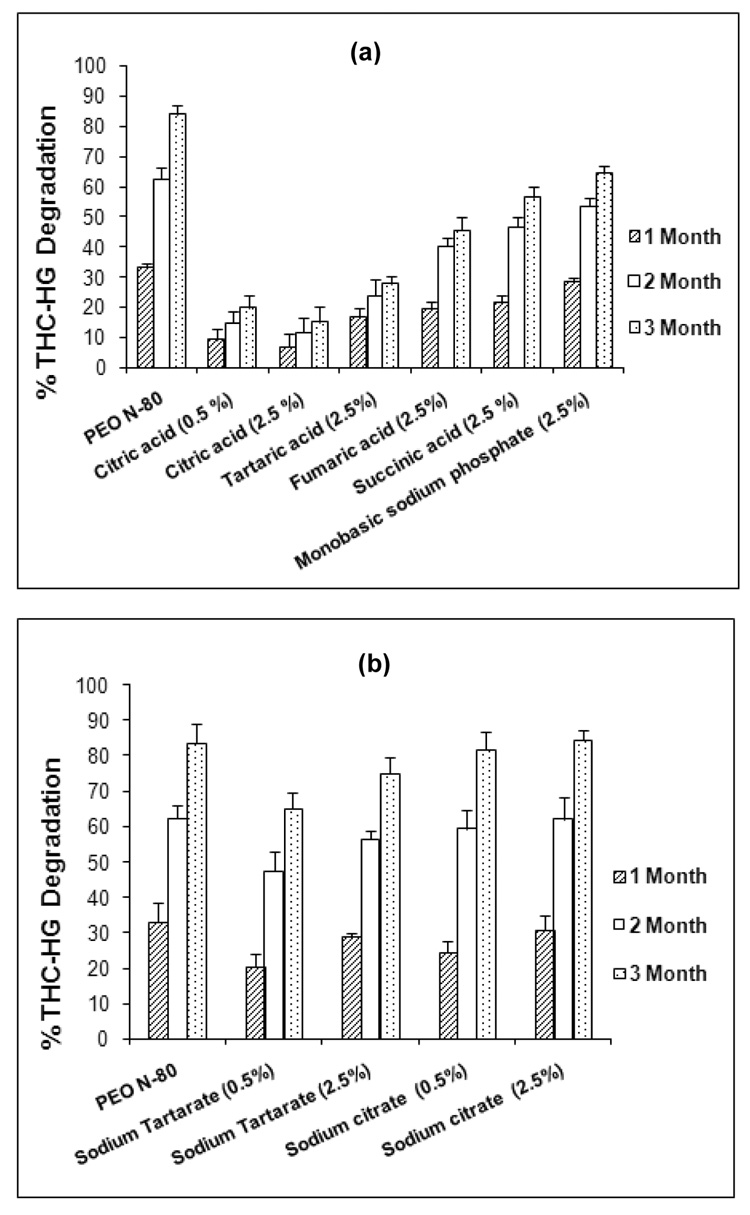
Preformulation studies conducted by our research group on THC-HG indicated that the prodrug exhibited significant degradation when stored at 40 °C/75% RH (90.3 %) at the end of 3 months (Thumma et al.). An examination of the HPLC chromatograms of these samples depicted THC as the major degradant peak indicating that the degradation is most likely the result of hydrolysis of the ester bond. Studies on protecting the drug against hydrolysis by incorporating pH modifiers into the formulation have been reported. Stability of acetyl salicylic acid in a tablet formulation was enhanced by addition of acidic agents (Delonca et al., 1975). The rate of the hydrolysis of ester prodrug, DMP-754 was decreased by modulating the micro-environmental pH to ~4.0, in the solid-state (Badawy et al., 1999). In the present study, a hot-melt method was utilized to fabricate the THC-HG-PEO matrices incorporated with various pH modifiers. The results of the effect of acidic and basic pH modifiers on THC-HG stability are presented in Figures 6a and 6b, respectively. The acidic pH modifiers were found to be more effective in reducing the degradation of the prodrug in PEO matrices as compared to basic pH modifiers. These agents reduced the degradation of the prodrug by 20–70% as compared to PEO-only matrices which demonstrated 83.9 % degradation at the end of 3 months at 40 ° C/75% RH. The basic pH modifiers however exhibited the extent of instability similar to the PEO-only matrices during the same period. Of all of the pH modifiers investigated, citric acid was found to be the most effective in enhancing the prodrug stability (85.3 % THC-HG remaining at the end of 3 months). From the pH measurements, it was observed that the degradation of the active increased with an increase in pH of the matrix system (Table 1). The relatively stable system (THC-HG-PEO-citric acid) exhibited a pH range of 3.4–3.8 while the other pH modifiers produced a pH greater than 4.0 indicating that the drug is relatively stable in the pH range of 3.0–4.0. This corresponded well with the pH-rate profile of THC-HG in solution showing a maximum stability in the range 3.0–4.0 (Thumma et al.).
Figure 6.
Stability of THC-HG in PEO matrices in presence of (a) acidic pH modifiers and (b) basic pH modifiers. The matrices (n = 3) were fabricated at 110 ° C and stored at 40° C/75% RH.
Table 1.
Microenvironmental pH values of the polymeric patch formulations containing THC-HG (5 % w/w).
| Patch Formulation | % pH modifier | pH |
|---|---|---|
| PEO N-80 | 0 | 6.7 |
| PEO-citric acid | 0.5 | 3.8 |
| PEO-citric acid | 2.5 | 3.4 |
| PEO-tartaric acid | 2.5 | 4.2 |
| PEO-fumaric acid | 2.5 | 4.5 |
| PEO-succinic acid | 2.5 | 4.7 |
| PEO-monobasic sodium phosphate | 2.5 | 5.4 |
| PEO-sodium tartarate | 0.5 | 5.8 |
| PEO- sodium tartarate | 2.5 | 6.0 |
| PEO- sodium citrate | 0.5 | 6.3 |
| PEO- sodium citrate | 2.5 | 6.9 |
3.5.2. Effect of Antioxidants
Drugs may undergo oxidation due to reactive impurities that are added during polymer manufacturing or generated upon exposure of the excipient or formulation to light, heat or metals. In addition, electrophilic or nucleophilic oxidants such as peracids and hydroperoxides can also cause oxidation of an active substance (Puz et al., 2005). Preformulation studies conducted on the prodrug by our research group revealed its susceptibility to oxidation. An examination of HPLC chromatograms of these samples showed the presence of cannabinol (oxidative degradant of THC) along with the parent drug (THC) indicating that oxidation is one of the mechanisms by which the prodrug degrades, in addition to hydrolysis of the ester bond. Various classes of antioxidants were incorporated into the PEO matrices along with the drug utilizing the aforementioned hot-melt method and stored at 25 °C/ 60% RH to assess their role in preventing prodrug degradation. The results of the stability of THC-HG in the presence of antioxidants investigated are presented in Table 2. Incorporation of the prodrug into the PEO matrix reduced its degradation significantly (p <0.05). For example, the prodrug exhibited 74.5 % degradation at the end of 3 months at 25 °C/ 60% RH, while incorporation into PEO matrices reduced its degradation by 20%. All of the antioxidants investigated further reduced the degradation of the active observed in PEO-only matrices. Degradation was observed to be least in presence of BHT and ascorbic acid (1% w/w). Only 7.6 % and 8.2 % of THC-HG degraded in these matrices, respectively as compared to the PEO-only matrices (59.4 %). Propyl gallate and EDTA-containing matrices induced the highest decomposition of the prodrug. The results of these studies indicated that the degradation of drug in PEO matrices can be controlled by employing suitable antioxidants such as ascorbic acid and BHT.
Table 2.
Stability of THC-HG (5% w/w) in PEO matrices containing various antioxidants. The matrices (n = 3) were fabricated at 110 ° C and stored at 25 ° C/ 60% RH.
| % THC-HG Degradation | ||||
|---|---|---|---|---|
| Antioxidant/ Drug | % Antioxidant | 1 Month | 2 Months | 3 Months |
| Pure THC-HG | - | 53.3 ± 2.1 | 62.2 ±1.3 | 74.5 ±2.5 |
| PEO N-80 | 0.0 | 39.1 ± 2.3 | 45.6 ±3.6 | 55.4±1.4 |
| BHT | 0.2 | 2.6 ±0.4 | 3.7 ±1.2 | 7.6 ±1.4 |
| BHA | 0.2 | 5.7 ±1.4 | 6.5 ±0.5 | 10.7 ±2.4 |
| Propyl gallate | 0.5 | 18.2 ±3.4 | 25.8 ±5.9 | 27.2 ±4.1 |
| Ascorbic acid | 0.5 | 7.7 ±5.1 | 11.6 ±4.3 | 14.1 ±3.5 |
| Ascorbic acid | 1.0 | 5.3 ±1.1 | 6.6 ±0.4 | 8.2 ±1.4 |
| EDTA | 0.05 | 15.3 ± 1.5 | 21.6 ± 2.1 | 29.3±4.2 |
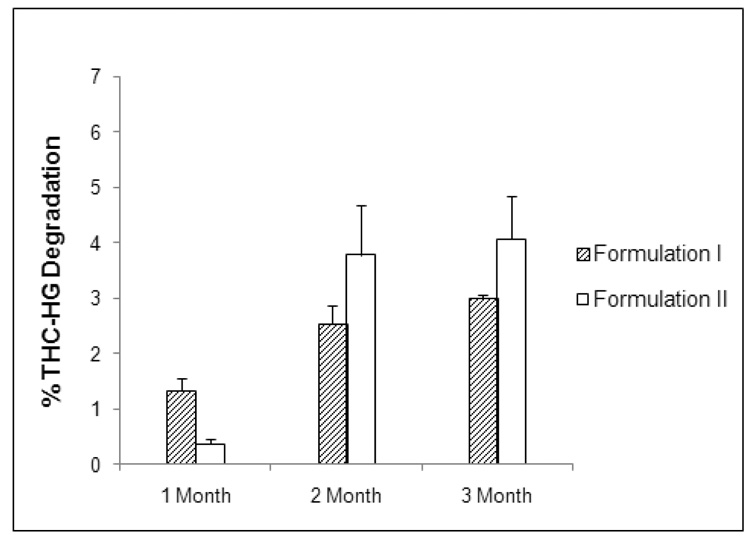
Based on the results of the stability studies, two final formulations were produced and the stability of the drug in these matrices was assessed by storage at 40 °C/ 75% RH for 3 months. Formulation I consisted of THC-HG (5.0 % w/w), PEO N-80 (78.3 % w/w), VES (10.0 % w/w), citric acid (2.5 % w/w), Noveon® AA-1 (4.0 % w/w) and BHT (0.2 % w/w), while formulation II consisted of THC-HG (5.0 % w/w), PEO N-80 (78.3 % w/w), VES (10.0 % w/w), citric acid (2.5 % w/w), Carbopol® 971p (4.0 % w/w) and BHT (0.2 % w/w). VES was incorporated as a plasticizer based on our previous studies (Thumma et al.). The results of the stability study are demonstrated in Figure 7. The prodrug was very stable in both of these formulations at the end of the 3 months. Less than 5% drug degradation was observed at the conditions employed. The results of the present study clearly demonstrate the utility of formulation excipients in stabilizing the ester prodrug. Future studies will employ further formulation and processing techniques to enhance the long term stability.
Figure 7.
Stability of THC-HG in the final formulations. The matrices (n = 3) were fabricated at 110 ° C and stored at 40° C/ 75% RH.
4. Conclusions
The results of the present study indicate that the bioadhesion of PEO matrices may vary based on the chemical nature, molecular structure as well as the hydration status of the bioadhesive polymer utilized. Hence, a judicious choice of an appropriate bioadhesive polymer is necessary to optimize this parameter. Contact time and wetting time also exhibited an influence on bioadhesion. In addition, incorporation of THC-HG beyond 5 wt % in PEO matrices led to an increase in bioadhesive strength of these systems, demonstrating the prodrug’s inherent mucoadhesive nature. Incorporation of citric acid in the PEO matrices enhanced the stability of the prodrug considerably by modulating the microenvironmental pH of the matrix. Also, the degradation of the drug was considerably reduced in the presence of various antioxidants utilized. The two final formulations utilized the previous data to incorporate optimized excipients for stabilization of the prodrug in the PEO matrices. The findings of this research clearly demonstrate that the stability of the ester prodrug could be optimized in the solid-state by employing appropriate formulation excipients and also by adjusting the microenvironmental pH of the formulation to coincide with the pH of maximum stability of the drug. These studies are relevant to the development of a stable bioadhesive transmucosal matrix system for the therapeutic delivery of THC and its prodrugs.
Acknowledgment
This project was supported by Grant Number P20RR021929 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anlar S, Capan Y, Guven O, Gogus A, Dalkara T, Hincal AA. Formulation and in vitro-in vivo evaluation of buccoadhesive morphine sulfate tablets. Pharm Res. 1994;11:231–236. doi: 10.1023/a:1018951323522. [DOI] [PubMed] [Google Scholar]
- Badawy SIF, Williams RC, Gilbert DL. Effect of different acids on solid-state stability of an ester prodrug of a IIb/IIIa glycoprotein receptor antagonist. Pharm Dev Technol. 1999;4:325–331. doi: 10.1081/pdt-100101368. [DOI] [PubMed] [Google Scholar]
- Challapalli PVN, Stinchcomb AL. In vitro experiment optimization for measuring tetrahydrocannabinol skin permeation. Int J Pharm. 2002;241:329–339. doi: 10.1016/s0378-5173(02)00262-4. [DOI] [PubMed] [Google Scholar]
- Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Battu S, McGinity JW, Martin C. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33:909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- Delonca H, Joachim J, Joachim G, Cantos A. Granulation de l’acide acetylsalicylique par voie humide. Influence du pH et de certains adjuvants de stabilization. Farmaco. 1975;30 [PubMed] [Google Scholar]
- ElSohly MA, Little TLJ, Hikal A, Harland E, Stanford DF, Walker L. Rectal bioavailability of delta-9-tetrahydrocannabinol from various esters. Pharmacol Biochem Behav. 1991;40:497–502. doi: 10.1016/0091-3057(91)90353-4. [DOI] [PubMed] [Google Scholar]
- Fairbairn JW, Liebmann JA, Rowan MG. The stability of cannabis and its preparations on storage. J Pharm Pharmacol. 1976;28:1–7. doi: 10.1111/j.2042-7158.1976.tb04014.x. [DOI] [PubMed] [Google Scholar]
- Guy GW, Flint ME. Symposium on the Cannabinoids. 2000:115. (Abstract) [Google Scholar]
- Harris AS, Ohlin M, Lethagen S, Nilsson IM. Effects of concentration and volume on nasal bioavailability and biological response to desmopressin. J. Pharm. Sci. 1988;77:337–339. doi: 10.1002/jps.2600770412. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: the next generation. J Pharm Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Marihuana chemistry. Science. 1970;168:1159–1166. doi: 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- Mortazavi SA, Smart JD. An in-vitro method for assessing the duration of mucoadhesion. J. Controlled Release. 1994;31:207–212. [Google Scholar]
- Munjal M, ElSohly MA, Repka MA. Chemical stabilization of a Delta-9-tetrahydrocannabinol prodrug in polymeric matrix systems produced by a hot-melt method: role of microenvironment pH. AAPS PharmSciTech. 2006;7:71. doi: 10.1208/pt070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm. 2003;264:1–14. doi: 10.1016/s0378-5173(03)00371-5. [DOI] [PubMed] [Google Scholar]
- Nafee NA, Ismail FA, Boraie NA, Mortada LM. Mucoadhesive delivery systems. I. Evaluation of mucoadhesive polymers for buccal tablet formulation. Drug Dev Ind Pharm. 2004;30:985–993. doi: 10.1081/ddc-200037245. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- Ponchel G, Touchard F, Wouessidjewe D, Duchene D, Peppas NA. Bioadhesive analysis of controlled-release systems. III. Bioadhesive and release behavior of metronidazole-containing poly(acrylic acid)-hydroxypropyl methylcellulose systems. Int. J. Pharm. 1987;38:65–70. [Google Scholar]
- Prodduturi S, Manek RV, Kolling WM, Stodghill SP, Repka MA. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J Pharm Sci. 2005;94:2232–2245. doi: 10.1002/jps.20437. [DOI] [PubMed] [Google Scholar]
- Puz M, Johnson B, Murphy B. Use of the antioxidant BHT in asymmetric membrane tablet coatings to stabilize the core to the acid catalyzed peroxide oxidation of a thioether drug. Pharm Dev Technol. 2005;10:115–125. doi: 10.1081/pdt-49690. [DOI] [PubMed] [Google Scholar]
- Repka MA, ElSohly MA, Munjal M, Ross SA. Temperature stability and bioadhesive properties of delta9-tetrahydrocannabinol incorporated hydroxypropylcellulose polymer matrix systems. Drug Dev Ind Pharm. 2006;32:21–32. doi: 10.1080/03639040500387914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repka MA, Gerding TG, Repka SL, McGinity JW. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot-melt extrusion. Drug Dev Ind Pharm. 1999;25:625–633. doi: 10.1081/ddc-100102218. [DOI] [PubMed] [Google Scholar]
- Repka MA, McGinity JW. Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials. 2000;21:1509–1517. doi: 10.1016/s0142-9612(00)00046-6. [DOI] [PubMed] [Google Scholar]
- Repka MA, McGinity JW. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot- melt extrusion. J Control Release. 2001;70:341–351. doi: 10.1016/s0168-3659(00)00365-5. [DOI] [PubMed] [Google Scholar]
- Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–1691. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Seymour RB, Carraher CE. In: Thermal properties of polymers. Seymour RB, Carraher CE, editors. New York: Plenum Publishing Corporation; 1984. pp. 83–93. [Google Scholar]
- Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J. Pharm. Pharm. Sci. 1998;1:15–30. [PubMed] [Google Scholar]
- Shojaei AH, Zhuo SL, Li X. Transbuccal delivery of acyclovir (II): feasibility, system design, and in vitro permeation studies. J Pharm Pharm Sci. 1998;1:66–73. [PubMed] [Google Scholar]
- Smart JD. An in vitro assessment of some mucoadhesive dosage forms. Int. J. Pharm. 1991;73:69–74. [Google Scholar]
- Solomonidou D, Cremer K, Krumme M, Kreuter J. Effect of carbomer concentration and degree of neutralization on the mucoadhesive properties of polymer films. J Biomater Sci Polym Ed. 2001;12:1191–1205. doi: 10.1163/156856201753395743. [DOI] [PubMed] [Google Scholar]
- Thumma S, Majumdar S, ElSohly MA, Gul W, Repka MA. Preformulation Studies of a Pro-Drug of Δ9-Tetrahydrocannabinol. AAPS PharmSci Tech. doi: 10.1208/s12249-008-9136-7. (in communication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth EA, Schwartz RH. Medicinal applications of delta-9-tetrahydrocannabinol and marijuana. Ann Intern Med. 1997;126:791–798. doi: 10.7326/0003-4819-126-10-199705150-00008. [DOI] [PubMed] [Google Scholar]
- Wong CF, Yuen KH, Peh KK. An in-vitro method for buccal adhesion studies: importance of instrument variables. Int J Pharm. 1999;180:47–57. doi: 10.1016/s0378-5173(98)00402-5. [DOI] [PubMed] [Google Scholar]
- Wu S. Formation of adhesive bond, Polymer Interface and Adhesion. New York: Marcel Dekker Inc; 1982. [Google Scholar]