The regulation of islet hormone secretion in vivo is likely to involve a complex interplay between circulating nutrients, hormones, and neurotransmitters (1). A new study by Hauge-Evans et al. (2) in this issue provides interesting new insights into the contribution of an intraislet paracrine role for somatostatin in the control of insulin, and more especially glucagon, release.
Glucagon is the principal counterregulatory hormone that opposes the anabolic effects of insulin, notably on the liver (3), and a relative excess of glucagon is a hallmark of all forms of diabetes. However, failure to secrete adequate quantities of glucagon in response to insulin-induced hypoglycemia characterizes longstanding type 1 diabetes (4) and is an important contributor to mortality in this disease, accounting for 2–4% of all deaths (5).
Glucagon is stored alongside insulin in the islet, albeit in a discrete cellular compartment, the pancreatic α-cell. Just as the metabolic actions of glucagon oppose those of insulin, the regulators of insulin's release (1) tend to exert opposing effects on glucagon secretion (6). Thus, elevated concentrations of glucose suppress glucagon release, while catecholamines stimulate the secretion of this hormone. Acting independently of these mechanisms, neuronal inputs into the islet exert a further important level of control over glucagon release (7).
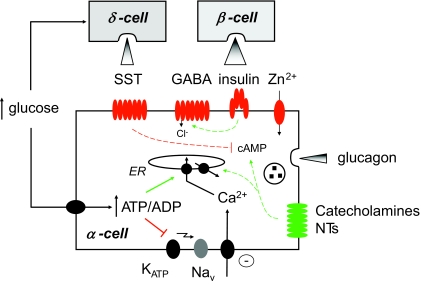
Despite being a subject under investigation for more than 35 years (6), just how the effects of glucose are achieved at the level of individual α-cells is still disputed and has become an area of vigorous research in recent times (Figure 1). As yet, however, a consensus has not been reached. Several laboratories (e.g., 8), including the author's (9), have concluded that glucose acts directly on isolated mouse α-cells to suppress oscillations in intracellular free Ca2+ concentration in the absence of paracrine influences from β-cells (the latter parameter is usually taken in these excitable cells as an adequate surrogate for electrical and secretory activity). The Ca2+ changes were associated with increases in intracellular free ATP concentration (9), which might be “decoded” via 1) the partial closure of ATP-sensitive K+ channels (KATP), resulting in the inactivation of N-type Ca2+ and voltage-gated Na+ channels, suppression of electrical activity, and Ca2+ influx through L-type Ca2+ channels (1,10); 2) through the activation of Ca2+ uptake by the endoplasmic reticulum, the consequent inactivation of a store-operated current that results in plasma membrane hyperpolarization, and decreased Ca2+ influx through voltage-gated Ca2+ channels (11); and 3) through changes in the activity of nutrient-regulated protein kinases including AMP kinase (12).
FIG. 1.
Multiple mechanisms of control of glucagon secretion. For details, see the text. ER, endoplasmic reticulum; Nav, Cav, voltage-gated Na+ and Ca2+ channels, respectively; NTs, neurotransmitters.
An alternative model has become known as the “intraislet” or “switch off” hypothesis. Informed by the strikingly “anti-parallel” regulation of insulin and glucagon secretion, this posits that factors released from the β-cell as glucose levels rise, including insulin itself (13) and cosecreted species such as γ-aminobutyric acid (GABA) (14–16) and Zn2+ ions (1,17,18), suppress the release of glucagon in a paracrine manner. This idea is supported by the fact that the intraislet circulation appears to be from β- to α-cell (19) and by the clinical observation that treatment of type 1 diabetic subjects with insulin usually lowers glucagon levels. Finally, purified rat α-cells have been reported to respond to elevated glucose concentrations with enhanced glucagon secretion (20) (though the impact of the fluorescence-activated cell sorting on the functional integrity of these preparations is uncertain). However, the “switch off” hypothesis has been challenged (8,9) on the grounds that the concentrations of glucose that almost fully inhibit glucagon release from isolated rodent (8) and human islets (3–4 mmol/l) are significantly below those that elicit detectable depolarization of the β-cell or the release of insulin and costored regulators (5.5–6 mmol/l). The existence of highly local “microdomains” of the regulators in the interstitial space between local β- and α-cells must therefore be invoked to sustain this hypothesis.
The release of an inhibitor of glucagon release over a range of glucose concentrations that more closely match that which regulates glucagon secretion would potentially provide a more attractive means of controlling α-cell activity. Enter somatostatin. Stored in pancreatic δ-cells, release of somatostatin is controlled similarly to that of insulin, but with the crucial difference that the secretion of somatostatin is already substantially stimulated by glucose concentrations as low as 3 mmol/l (half-maximal effects are observed at ∼6 mmol/l) (8). Existing evidence that somatostatin may be involved in controlling the release of glucagon in response to changes in glucose concentration is mixed. Supporting this view, exogenously added somatostatin potently inhibits glucagon release from the pancreas (21), while anti-somatostatin antibodies activate glucagon release from isolated islets (22). However, studies with the isolated perfused pancreas have argued both for (23) and against (24) a role for locally acting somatostatin on glucagon release. Finally, the selective somatostatin receptor type 2 (SSTR2) antagonist DC-41-33 only weakly enhanced glucagon secretion from the perfused rat pancreas in the presence of 3.3 mmol/l glucose while strongly potentiating the response to arginine (25).
The important new study by Hauge-Evans et al. (2) provides a key step forward by exploring the effects on glucagon and insulin release of the deletion, through homologous recombination, of the somatostatin gene in mice. SST−/− mice have previously been described to show a relatively modest phenotype including changes in the release of pituitary hormones (26). The new study shows first that arginine-induced release of insulin and glucagon is markedly stimulated in vivo when somatostatin is absent. Moreover, in islets isolated from SST−/− mice, the normal inhibition of glucagon release by glucose was eliminated, while the stimulation of insulin release by the sugar was enhanced. On the other hand, the rapid inhibition of insulin secretion upon glucose lowering was unaltered in SST−/− mice, questioning a role for somatostatin in this process.
These studies thus further emphasize the contribution of somatostatin as a tonic inhibitor of glucagon release during stimulation by low glucose concentrations or after challenge with arginine. The work also provides a powerful adjunct to earlier studies using pharmacological approaches, as well as those using SSTR2−/− mice (27). However, there remain uncertainties: SST was deleted unconditionally throughout the body in the present studies, providing the possibility for an indirect developmental effect on the regulation of glucagon secretion by other mechanisms (Figure 1); microarray profiling of islets from SST−/− mice may be informative to explore whether other changes occur in the expression of key genes in either β- or α-cells to limit the ability of glucose to control glucagon release normally.
The studies raise several important new questions: 1) Is the whole panoply of putative regulatory mechanisms (Figure 1) really important in vivo, and if so, under what conditions? 2) Do they same mechanisms play a role(s) in humans as well as rodents? 3) Given the effects of somatostatin deletion to enhance insulin and glucagon secretion, respectively, might somatostatin receptor antagonists prove useful to treat hyperglycemia in type 2 diabetes and hypoglycemia in type 1 diabetes? Imaginative new approaches seem likely to be needed to address these points: studies of SST−/− mice under hypoglycemic clamp may be particularly informative to confirm or refute the importance of a decrease in somatostatin release to the activation of glucagon secretion in vivo. Additionally, a more detailed molecular exploration of other proposed mechanisms, for example, by α-cell–specific deletion of insulin or GABA receptors, or the islet-specific Zn2+ transporter, ZnT8 (28), in mice is also needed.
Although understanding of the regulation of glucagon release still remains incomplete, the new insights about the importance of somatostatin provided by Hauge-Evans et al. (2) may allow the more rational design and use of drugs to modulate glucagon (and insulin) release in all forms of diabetes.
Acknowledgments
The author thanks the Wellcome Trust, Medical Research Council, European Union, National Institutes of Health, and Diabetes UK for financial support.
No potential conflicts of interest relevant to this article were reported.
The author thanks Dr. Isabelle Leclerc for discussion.
See accompanying original article, p. 403.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Rutter GA: Visualising insulin secretion: the Minkowski Lecture 2004. Diabetologia 47: 1861–1872, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson ICAF, Low MJ, Christie MR, Persaud SJ, Jones PM: Somatostatin secreted by islet δ-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes 58: 403–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger RH: Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 28: 574–578, 1985 [DOI] [PubMed] [Google Scholar]
- 4.Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH: Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 182: 171–173, 1973 [DOI] [PubMed] [Google Scholar]
- 5.Cryer PE: Hypoglycaemia: the limiting factor in the glycaemic management of type I and type II diabetes. Diabetologia 45: 937–948, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Gromada J, Franklin I, Wollheim CB: Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Havel PJ, Akpan JO, Curry DL, Stern JS, Gingerich RL, Ahren B: Autonomic control of pancreatic polypeptide and glucagon secretion during neuroglucopenia and hypoglycemia in mice. Am J Physiol 265: R246–R254, 1993 [DOI] [PubMed] [Google Scholar]
- 8.Vieira E, Salehi A, Gylfe E: Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50: 370–379, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ravier MA, Rutter GA: Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 54: 1789–1797, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gopel SO, Kanno T, Barg S, Weng X, Gromada J, Rorsman P: Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol 528: 509–520, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu YJ, Vieira E, Gylfe E: A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic alpha-cell. Cell Calcium 35: 357–365, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Leclerc I, Fernadez-Millan E, Nyirenda M, Rutter GA: Role of AMP-activated protein kinase in glucagon secretion. Diabet Med 24: 1, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, Liu S, Wendt A, Deng S, Ebina Y, Wheeler MB, Braun M, Wang Q: Intra-islet insulin suppresses glucagon release via GABA-GABA(A) receptor system. Cell Metab 3: 47–58, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Rorsman P, Berggren PO, Smith PA: Glucose in glucagon release. Nature 344: 716, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Gilon P, Bertrand G, Loubatieres-Mariani MM, Remacle C, Henquin JC: The influence of gamma-aminobutyric acid on hormone release by the mouse and rat endocrine pancreas. Endocrinology 129: 2521–2529, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Bailey SJ, Ravier MA, Rutter GA: Glucose-dependent regulation of γ-aminobutyric acid (GABA A) receptor expression in mouse pancreatic islet alpha-cells. Diabetes 56: 320–327, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB: Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 5: 330–335, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Zhou H, Tran PO, Yang S, Zhang T, LeRoy E, Oseid E, Robertson RP: Regulation of α-cell function by the β-cell during hypoglycemia in Wistar rats: the “switch-off” hypothesis. Diabetes 53: 1482–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Bonner Weir S, Orci L: New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31: 883–889, 1982 [DOI] [PubMed] [Google Scholar]
- 20.Franklin I, Gromada J, Gjinovci A, Theander S, Wollheim CB: β-Cell secretory products activate α-cell ATP-dependent potassium channels to inhibit glucagon release. Diabetes 54: 1808–1815, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Gerich JE, Lorenzi M, Schneider V, Kwan CW, Karam JH, Guillemin R, Forsham PH: Inhibition of pancreatic glucagon responses to arginine by somatostatin in normal man and in insulin-dependent diabetics. Diabetes 23: 876–880, 1974 [DOI] [PubMed] [Google Scholar]
- 22.Barden N, Lavoie M, Dupont A, Cote J, Cote JP: Stimulation of glucagon release by addition of anti-stomatostatin serum to islet of Langerhans in vitro. Endocrinology 101: 635–638, 1977 [DOI] [PubMed] [Google Scholar]
- 23.Brunicardi FC, Kleinman R, Moldovan S, Nguyen TH, Watt PC, Walsh J, Gingerich R: Immunoneutralization of somatostatin, insulin, and glucagon causes alterations in islet cell secretion in the isolated perfused human pancreas. Pancreas 23: 302–308, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kawai K, Ipp E, Orci L, Perrelet A, Unger RH: Circulating somatostatin acts on the islets of Langerhans by way of a somatostatin-poor compartment. Science 218: 477–478, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Cejvan K, Coy DH, Efendic S: Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes 52: 1176–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Luque RM, Kineman RD: Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology 148: 5998–6006, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM: Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141: 111–117, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Chimienti F, Devergnas S, Pattou F, Schuit F, Garcia-Cuenca R, Vandewalle B, Kerr-Conte J, Van LL, Grunwald D, Favier A, Seve M: In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci 119: 4199–4206, 2006 [DOI] [PubMed] [Google Scholar]