Abstract
OBJECTIVE—The aim of this study was to find an effective treatment for the genetic form of diabetes that is present in some Huntington's disease patients and in Huntington's disease mouse models. Huntington's disease is a neurodegenerative disorder caused by a polyglutamine expansion within the huntingtin protein. Huntington's disease patients exhibit neuronal dysfunction/degeneration, chorea, and progressive weight loss. Additionally, they suffer from abnormalities in energy metabolism affecting both the brain and periphery. Similarly to Huntington's disease patients, mice expressing the mutated human huntingtin protein also exhibit neurodegenerative changes, motor dysfunction, perturbed energy metabolism, and elevated blood glucose levels.
RESEARCH DESIGN AND METHODS—Huntington's disease mice were treated with an FDA-approved antidiabetic glucagon-like peptide 1 receptor agonist, exendin-4 (Ex-4), to test whether euglycemia could be achieved, whether pancreatic dysfunction could be alleviated, and whether the mice showed any neurological benefit. Blood glucose and insulin levels and various appetite hormone concentrations were measured during the study. Additionally, motor performance and life span were quantified and mutant huntingtin (mhtt) aggregates were measured in both the pancreas and brain.
RESULTS—Ex-4 treatment ameliorated abnormalities in peripheral glucose regulation and suppressed cellular pathology in both brain and pancreas in a mouse model of Huntington's disease. The treatment also improved motor function and extended the survival time of the Huntington's disease mice. These clinical improvements were correlated with reduced accumulation of mhtt protein aggregates in both islet and brain cells.
CONCLUSIONS—Targeting both peripheral and neuronal deficits, Ex-4 is an attractive agent for therapeutic intervention in Huntington's disease patients suffering from diabetes.
Huntington's disease is an inherited neurodegenerative disorder typified by involuntary body movements and psychiatric and cognitive abnormalities. The incidence of Huntington's disease is ∼5–10 cases per 100,000 worldwide, making it one of the most common inherited neurodegenerative disorders (1). The genetic defect underlying Huntington's disease involves expansion of CAG trinucleotide repeats in exon 1 of the Huntington's disease gene, resulting in polyglutamine expansions in the huntingtin (htt) protein (2). Polyglutamine expansion in htt leads to its abnormal processing and deleterious intracellular aggregation. The number of polyglutamine repeats in htt is inversely correlated with the age of onset, with 70–100 repeats leading to juvenile onset (1). The wild-type htt protein is thought to be a scaffolding protein involved in multiple processes, including vesicle movement and cell metabolism. Mutant htt forms abnormal intracellular aggregates in degenerating neurons in the striatum and cerebral cortex (3).
Despite being considered primarily a neurological disorder, Huntington's disease patients also exhibit peripheral symptoms, including progressive weight loss, appetite dysfunction, and poor glycemic control (4). Huntington's disease patients suffer from an unusual combination of a hypermetabolic state due in part to continuous body movements (5), and despite this, glucose metabolism is paradoxically impaired in both brain and periphery (6,7). Dietary supplementation with creatine has been shown to reduce brain damage and delay the onset of motor dysfunction in huntingtin mutant mice (8), which suggests a potential benefit of increasing brain energy availability in Huntington's disease. High levels of mutant htt have been documented in peripheral tissues, including muscle and gonads (9), and in the pancreatic islet cells of the R6/2 Huntington's disease mice, which exhibit decreased β-cell mass and impaired insulin release capacity (10,11). However, the “diabetic-like” condition in the Huntington's disease mice is not improved by treatment with hypoglycemic agents, such as insulin or metformin (12,13). Disruption of glycemic homeostasis is likely to affect nutrient availability to neurons and could alter neuronal function and contribute to neurodegeneration and motor deficits in Huntington's disease. The emerging view of Huntington's disease as a “body-wide” disorder supports the increasing evidence that the maintenance of a healthy nervous system is tightly linked with peripheral metabolic health (14). Therefore, treatment of both the peripheral and central pathophysiologies of Huntington's disease could form the basis of a more effective Huntington's disease therapeutic strategy.
Glucagon-like peptide-1 (GLP-1), a hormone secreted by intestinal enteroendocrine L-cells in response to food ingestion and the natural ligand of the GLP-1 receptor, acts on multiple target tissues to enhance energy metabolism; it stimulates the production and release of insulin from β-cells in islets of Langerhans in the pancreas and increases insulin sensitivity by multiple mechanisms (15,16). These actions, however, appear to be highly complicated and context specific because several studies have not demonstrated a link between GLP-1 activity and increases in insulin sensitivity (17). The antidiabetic actions of GLP-1 have been shown to improve glucose regulation in human subjects (18), and a long-acting GLP-1 receptor agonist, exendin-4 (Ex-4), is now a treatment for type 2 diabetes (19). In addition to peripheral actions, GLP-1 and Ex-4 have been shown to act on neurons in the brain. GLP-1 receptors are widely expressed in neurons throughout the brain (20), and Ex-4 readily crosses the blood-brain barrier (21). GLP-1 and Ex-4 have been shown to exert neuroprotective actions in experimental models of excitotoxic brain injury (22) and peripheral neuropathy (23). The antidiabetic and direct neuroprotective activities of Ex-4 suggested to us its potential to ameliorate both the central and peripheral abnormalities in Huntington's disease. We therefore determined whether daily administration of Ex-4 could attenuate disease progression and alleviate metabolic abnormalities in the N171-82Q mouse model of Huntington's disease. Our findings show that Ex-4 treatment suppresses the development of mutant huntingtin (mhtt) inclusions in the pancreas and brain, ameliorates metabolic defects and motor dysfunction, and extends the survival of Huntington's disease mice.
RESEARCH DESIGN AND METHODS
Animals and drug administration.
Forty male B6C3-Tg(HD82Gln)81Dbo/J (common name N171-82Q) mice (24) and 43 age-matched wild-type mice were used in this study. Transgenic mice were identified by PCR analysis of tail DNA. All procedures using these Huntington's disease mice and their wild-type littermates were approved by the institutional Animal Care and Use Committee of the National Institute on Aging. The N171-82Q mice express 82 CAG repeats and display many Huntington's disease–like symptoms, including huntingtin aggregate formation in and degeneration of striatal and cortical neurons, motor impairment, progressive weight loss, and significantly elevated plasma glucose levels (24). All mice were group housed on a 12-h light/12 h dark cycle and had ad libitum access to food and water. Either Ex-4 (Bachem, Torrance, CA) or saline (PBS; Sigma, St. Louis, MO) was administered by a once-daily subcutaneous injection (300 μl 0.1-μmol/l solution of Ex-4). This study was repeated in multiple cohorts of the N171-82Q mice.
Body weight and glucose measurements.
Body weight (g) and blood glucose levels (mg/dl) were measured weekly. Glucose levels were measured in blood collected from the tail vein blood using a Bayer Glucometer Elite XL blood glucose meter.
In vivo insulin sensitivity test.
An insulin tolerance test was performed on a cohort of mice (six to eight animals per group) to investigate insulin sensitivity. Human, rapid-acting insulin (Novo Nordisk) was diluted to 0.1 unit/ml in isotonic NaCl 0.05% BSA (Sigma). The mice were injected subcutaneously in the neck region with 1 mU/g body wt insulin, and blood glucose levels were measured at various time points (t = 0, 15, 30, 45, 60, 90, and 120 min) from the tail vein using a Bayer Glucometer Elite XL blood glucose meter.
Motor performance assessment.
Motor coordination was tested using an accelerating rotarod (Med. Associates, Georgia, VT). Mice were trained to use the rotarod apparatus during a 2-min habituation trial (4 rpm) on the day before the first day of testing. On test days the rotarod apparatus was gradually accelerated from 4 to 40 rpm over 5 min. The latency to fall was measured and averaged over two trials per test day. Testing was started the week before initial treatment and continued bi-weekly throughout the course of the study.
Tissue collection.
Mice were killed by isofluorene overdose inhalation and decapitation. Brains were removed, and the cerebral cortex was isolated by dissection. Additionally, the pancreas was removed from each animal, and blood was collected for analyses of energy metabolism hormones. Blood was centrifuged at 3,000 rpm for 30 min at 4°C, and plasma was aspirated off. The pancreata were fixed in 4% formalin for 48 h and stored in PBS until processing. The pancreatic tissue was processed and embedded in paraffin wax. Pancreatic sections were cut at 5 μm thickness using a microtome, and the sections were adhered to poly-l-lysine coated microscope slides (Fisher, Springfield, NJ).
Insulin and glucagon immunohistochemistry.
Pancreatic sections were immunostained according to a previously described protocol (25). Briefly, tissue was incubated with the primary insulin antibody (guinea pig anti-swine insulin; 1:300; DakoCytomation, Carpinteria, CA) or glucagon antibody (1:500; guinea pig anti-glucagon; Millipore, Billerica, MA) for 2 h at room temperature and then incubated with secondary antibody (Alexa Fluor 488 goat anti–guinea pig, 1:200; or Alexa Fluor 568 goat anti–guinea pig, 1:1,000; Invitrogen, Carlsbad, CA) for 1 h at room temperature. Sections were imaged with an Olympus Fluoview IX70 confocal microscope (Olympus America, Center Valley, PA).
Pancreatic islet image analysis.
Quantification of immunohistochemistry images was performed in Matlab (Mathworks) using novel software in conjunction with the image processing toolbox. Intensity readings of each image ranged from 0 to 256, with 256 being the greatest pixel density and hence the highest staining intensity. The region of interest (ROI) was drawn around each islet after background subtraction. The pixels within the bounds of the ROI and above the set threshold of 8 were selected, from which actual islet area was calculated. The normalized variance of the ROI was used to calculate an artificial ellipse from which the major and minor axes were determined. The major axis is the longest diameter that can be drawn in the ellipse, and the minor axis is the shortest diameter, both giving an accurate approximation for the range of the actual islet diameter. Islet morphometry and sizing analyses were performed in an unbiased, random fashion.
Adipokine and hormone measurements.
These were measured by ELISA and radioimmunoassay (RIA) methods according to the kit manufacturers’ instructions: adiponectin (ELISA; Linco Research, St. Charles, MO), leptin (ELISA; Crystal Chem, Downers Grove, IL), insulin (ELISA; Crystal Chem), and ghrelin (RIA; Phoenix Pharmaceuticals, Belmont, CA). Homeostatic model of insulin resistance values (HOMA) were calculated from glucose and insulin values using the HOMA2 software available from the Oxford Centre for Diabetes, Endocrinology, and Metabolism Diabetes Trials Unit (www.dtu.ox.ac.uk).
Western immunoblotting.
Cortex samples were homogenized and sonicated in a Nonidet P-40–based lysis buffer, as described previously (26). Subsequently, the samples were centrifuged at 14,000 rpm for 15 min, and the supernatant was removed for protein analysis. Samples were diluted 1:1 with Laemmli buffer and resolved by SDS-PAGE. Proteins were then transferred onto a polyvinylidine fluoride membrane for immunoblotting. Membranes were blocked by soaking in methanol, allowed to air dry, and then incubated with primary antibody diluted in a 4% BSA, 50 mmol/l Tris-HCl, pH 7.0, 0.05% Tween 20, and 0.05% Nonidet P-40 blocking solution for 1 h at room temperature (S830 anti-HD exon 1 transgene protein, goat polyclonal, 1:3,000; anti–heat shock protein-70 [anti–Hsp-70] mouse monoclonal [Stressgen, Ann Arbor, MI], 1:1,000; anti-actin, mouse monoclonal [Sigma], 1:3,000). Membranes were probed with secondary alkaline phosphatase antibody diluted in 4% BSA/TBS-T solution for 1 h (anti-goat or anti-mouse IgG; 1:3,000; Sigma) and then developed with ECF Substrate (GE Healthcare). A Typhoon 9410 Variable Mode Imager (Amersham Biosciences) was used for signal detection, and band intensities were calculated using ImageQuant software (Molecular Dynamics).
mhtt immunohistochemistry.
Pancreatic sections were immunostained as previously described (27). S830, a goat polyclonal antibody to the Huntington's disease exon 1 transgene protein, was used at a dilution of 1:2,000. A biotinylated secondary rabbit anti-goat antibody was used at 1:500 (Vector Laboratories, Burlingame, CA). Slices were complexed with avidin-biotin using a Vectastain Elite ABC kit (Vector Laboratories) and developed using a Dako Liquid DAB Substrate Chromogen System (DakoCytomation). Images were acquired using an Olympus Fluoview IX70 confocal microscope and Nikon Coolpix P5000 digital camera.
Statistical analyses.
The data represent the means ± SE. Differences between mean values for variables within individual experiments were compared statistically by Student's t test and ANOVA. Comparisons were performed by using Graphpad Prism (GraphPad Software, San Diego, CA) and Excel. P < 0.05 was considered statistically significant.
RESULTS
Ex-4 treatment induces euglycemia in Huntington's disease mice.
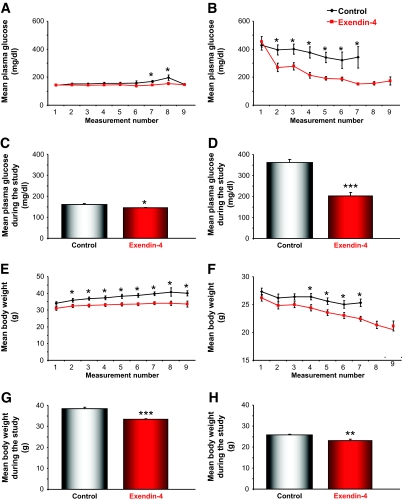
Presymptomatic male N171-82Q Huntington's disease mice (which express cDNA encoding an NH2-terminal fragment [171 amino acids] of huntingtin with 82 glutamines) and age-matched male wild-type littermates were injected daily with either Ex-4 or saline (control). Body weight and nonfasting plasma glucose levels were measured weekly, and motor coordination was measured bi-weekly (Fig. 1A). Daily Ex-4 treatment in the wild-type mice resulted in a small reduction in resting glucose levels, compared with the saline-treated control wild-type mice (Fig. 2A). The Huntington's disease mice exhibited significantly elevated plasma glucose levels compared with the wild-type mice (Fig. 2B), which corroborates with previous reports of elevated peripheral blood glucose levels in Huntington's disease mouse models (10,13). Daily treatment with Ex-4 resulted in a significant and progressive reduction in glucose levels in the Huntington's disease mice (P < 0.05; Fig. 2B). Mean blood glucose levels across the whole study period were significantly reduced in the wild-type Ex-4–treated mice (P < 0.05; Fig. 2C) and to a much greater extent in the Ex-4–treated Huntington's disease mice (P < 0.001; Fig. 2D). We attempted to achieve euglycemia in the Huntington's disease mice by administering daily or twice-daily injections of long-acting insulin (Glargine); however, we were unable to affect plasma glucose levels in the Huntington's disease mice, even with a high dose of insulin (up to 8 IU/kg), and animals died more quickly than nontreated Huntington's disease mice (data not shown). The insensitivity of the N171-82Q mice to insulin is similar to that reported for R6/2 mice, another Huntington's disease mouse model, treated with insulin or metformin (12,13).
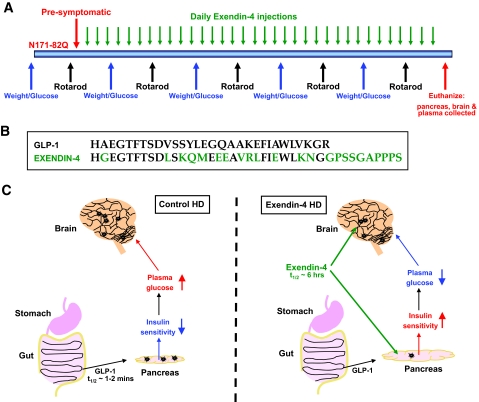
FIG. 1.
Experimental design. A: Experimental timeline of the study. Male N171-82Q and age-matched wild-type mice were injected daily with either Ex-4 or saline (control). Body weight and glucose measurements were recorded weekly, and rotarod performance was assessed bi-weekly. On euthanization, cortex, pancreas, and plasma were collected for further analyses. B: Ex-4 is an agonist of the GLP-1 receptor. Several amino acids differ between GLP-1 and Ex-4 sequences, most importantly in the NH2-terminal region where the substitution of alanine to glycine renders Ex-4 resistant to proteolysis by dipeptidyl peptidase-IV. Whereas GLP-1 has a half-life of <2 min in circulation, Ex-4 has biological activity for ∼6 h. This ensures that Ex-4 has potent and long-acting effects on both the periphery (pancreas) and the brain. C: Proposed mechanisms of action of Ex-4 in peripheral tissues and the brain. Ex-4 promotes pancreatic β-cell growth and insulin production and secretion and increases insulin sensitivity of muscle and liver cells. Ex-4 crosses the blood-brain barrier and acts on neurons in the brain to promote their survival and support their high energy demands.
FIG. 2.
Ex-4 normalizes plasma glucose levels in Huntington's disease mice (right panel). Weekly (A and B) and average (C and D) glucose levels of saline- and Ex-4–treated wild-type (left panel) and Huntington's disease mice are shown throughout the treatment period. Ex-4 treatment significantly reduced plasma glucose levels in Huntington's disease mice from the first week of treatment, and euglycemia was maintained throughout the course of the study period. Ex-4 also significantly reduced plasma glucose levels in wild-type mice. Weekly (E and F) and average (G and H) body weight measurements for saline- and Ex-4–treated wild-type and Huntington's disease mice throughout the study are shown. Ex-4 treatment caused a significant reduction in body weight in both wild-type and Huntington's disease mice. Values are means ± SE, n = 18–24 animals per group. *P < 0.05, **P < 0.01, ***P < 0.001.
Severe and progressive weight loss is a common symptom of Huntington's disease that also occurs in many mouse models of Huntington's disease (8,10). The control Huntington's disease mice lost a significant amount of body weight throughout the study (Fig. 2F and H), and their weight was significantly lower than the control wild-type mice (P < 0.001). Ex-4 treatment exacerbated this weight loss, and both the Huntington's disease and wild-type Ex-4–treated mice weighed significantly less than their saline-treated control counterparts (P < 0.001, P < 0.01; Fig. 2E–H). This reduction in body weight is in accord with previous reports that have shown that Ex-4 treatment leads to progressive weight loss in human subjects (28).
Energy- and appetite-regulating hormones are altered by Ex-4 treatment.
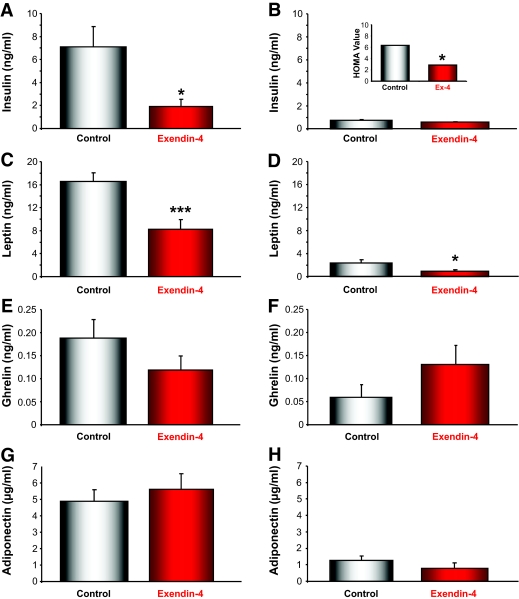
An alteration in energy homeostasis has been reported in Huntington's disease patients and in several of the Huntington's disease mouse models (29,30). A disruption in energy balance could contribute to some of the Huntington's disease symptomology, including extreme weight loss and alterations in appetite. We measured plasma levels of the main energy-regulating hormones in the Huntington's disease and wild-type mice to determine whether Ex-4 had caused any alterations in these hormones. Ex-4 treatment induced a significant reduction in plasma insulin levels in the wild-type mice (P < 0.05) and a small reduction in plasma insulin levels in the Huntington's disease mice (Fig. 3A and B). Ex-4 is a proven and effective treatment for type 2 diabetes, because it not only protects pancreatic β-cell function, but it also significantly increases insulin sensitivity (31,32). Ex-4 treatment increased insulin sensitivity by ∼50% in the Huntington's disease mice (Fig. 3B, inset), as judged by the HOMA. Because the HOMA index was created to measure classic diabetic states and not the idiosyncratic diabetic pathophysiology in Huntington's disease mice, we also used an insulin tolerance test in a separate cohort of animals to assess insulin resistance. This demonstrated that Ex-4 improved insulin-stimulated glucose uptake in both Huntington's disease and wild-type animals (supplementary Fig. 1, available in an online appendix at http://dx.doi.org/10.2337/db08-0799).
FIG. 3.
Modification of plasma levels of energy-regulating hormones by Ex-4 treatment in wild-type (left panel) and Huntington's disease (right panel) mice. Plasma concentrations of insulin (A and B), leptin (C and D), ghrelin (E and F), and adiponectin (G and H) were measured in wild-type and Huntington's disease mice treated with either saline (control) or Ex-4. Ex-4 treatment significantly reduced plasma insulin levels in wild-type mice, compared with control wild-type animals (P < 0.05). In Huntington's disease mice, Ex-4 treatment did not significantly alter circulating levels of insulin compared with Huntington's disease control animals. Thus, despite having lower circulating levels of insulin, the Ex-4–treated wild-type and Huntington's disease mice exhibit improved insulin sensitivity, as demonstrated by the reduction in plasma glucose levels (A and B). B, inset: Using plasma insulin and glucose measurements, HOMA values were calculated. C and D: Ex-4 treatment significantly reduced leptin levels in both wild-type (P < 0.001) and Huntington's disease (P < 0.05) mice, consistent with the decrease in the body weight in the Ex-4–treated mice. E and F: There were no significant effects of Ex-4 on plasma ghrelin levels in wild-type and Huntington's disease mice, although there were trends toward decreased levels in the wild-type mice and increased levels in the Huntington's disease mice. G and H: There were no significant alterations in the plasma adiponectin levels with Ex-4 treatment for both the wild-type and Huntington's disease mice, and Huntington's disease mice had significantly lower plasma adiponectin levels compared with wild-type mice (P < 0.01). Values are means ± SE, n = 18–24 animals per group. *P < 0.05, ***P < 0.001.
The extreme and uncontrolled weight loss in Huntington's disease patients and mice has been linked to alterations in hypothalamic function (29), including perturbations of the gastric hormone ghrelin and the adipocyte hormone leptin (30). Compared with wild-type animals, the Huntington's disease mice had significantly lower leptin, ghrelin, and adiponectin levels (Fig. 3C–H). In both wild type and Huntington's disease mice, Ex-4 treatment significantly lowered plasma leptin levels (Fig. 3C and D; wild type, P < 0.001; Huntington's disease, P < 0.05). Plasma ghrelin levels were reduced in the Ex-4–treated wild-type mice compared with the control wild-type mice (Fig. 3E), and this effect has also been observed in human subjects (33). The control Huntington's disease mice had significantly lower ghrelin levels compared with the wild-type mice, and Ex-4 treatment unexpectedly restored ghrelin levels in the Huntington's disease mice to that of wild-type mice (Fig. 3F). This suggests that Ex-4 treatment might ameliorate some of the appetite-related dysfunctions in Huntington's disease patients. Ex-4 treatment did not affect plasma adiponectin levels in Huntington's disease or wild-type mice (Fig. 3G and H), although adiponectin levels were significantly lower in the Huntington's disease mice compared with the wild-type mice (P < 0.001). Decreased adiponectin levels are a universal finding in insulin-resistant states (34).
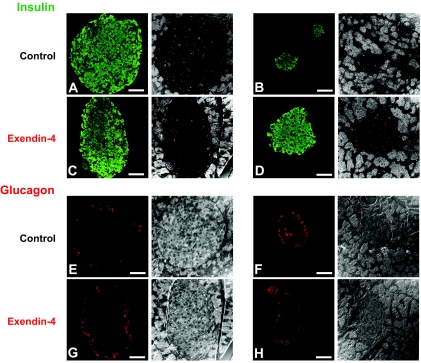
Ex-4 improves pancreatic islet structure in Huntington's disease mice.
In the N171-82Q mice and in other Huntington's disease mouse models, there is a build-up of mhtt aggregates within the islets of Langerhans, which may contribute to their “diabetic-like” condition (11). Huntington's disease mice showed significant alterations in islet structure, evidenced by greatly reduced numbers of insulin immunoreactive β-cells compared with their wild-type control counterparts (Fig. 4A and B). Ex-4 treatment restored numbers of β-cells in the Huntington's disease mice to levels close to those of wild-type mice (Fig. 4C and D; Table 1). The alterations in islet size and structure were also confirmed by glucagon staining. In control and Ex-4–treated wild-type mice, the glucagon-expressing α-cells were situated on the periphery of the islets (Fig. 4E and G). In Huntington's disease mice, some of the α-cells were displaced into the center of the islets (Fig. 4F), causing abnormal islet structure. Ex-4 treatment largely restored the α-cell topography of islets in the Huntington's disease mice (Fig. 4H).
FIG. 4.
Treatment with Ex-4 improves pancreatic islet physiology in Huntington's disease mice (right panel). Immunostaining of pancreatic tissue for the β-cell–derived hormone insulin and corresponding phase contrast images. A and B: Saline-treated Huntington's disease mice had small, significantly diminished islets compared with saline-treated wild-type mice (left panel). C and D: Treatment with Ex-4 restored islet size in Huntington's disease mice but did not significantly affect islet size in wild-type mice. Immunostaining for the α-cell–derived hormone glucagon confirmed these improvements in islet physiology with Ex-4 treatment. E and G: In nondiabetic mice, glucagon-positive cells are typically arranged in a “halo” around the edge of the islet. F: In the Huntington's disease mice, the islet structure was altered, and α-cells were displaced into the center of the islet. H: This α-cell abnormality was improved with Ex-4 treatment in Huntington's disease mice. Values are means ± SE, n = 6–8 animals per group. (Please see http://dx.doi.org/10.2337/db08-0799 for a high-quality digital representation of this figure.)
TABLE 1.
Pancreatic islet size analysis of wild-type and N171-82Q Huntington's disease mice treated with either saline or Ex-4
| Wild type
|
N171-82Q Huntington's disease
|
|||||
|---|---|---|---|---|---|---|
| Control | Ex-4 | P value | Control | Ex-4 | P value | |
| Islet area (μm2) | 15,101 ± 2,546 | 12,606 ± 2,652 | 0.501 | 2,590 ± 601 | 13,193 ± 2,496 | <0.001* |
| Major axis (μm) | 211.5 ± 19.0 | 185.3 ± 21.5 | 0.369 | 91.6 ± 11.6 | 203.9 ± 31.9 | 0.003† |
| Minor axis (μm) | 154.1 ± 13.5 | 132.7 ± 13.6 | 0.272 | 65.8 ± 8.6 | 126.1 ± 13.1 | <0.001* |
Data are means ± SEM unless otherwise indicated.
P < 0.001;
P < 0.01.
Motor coordination is improved in Ex-4–treated Huntington's disease mice.
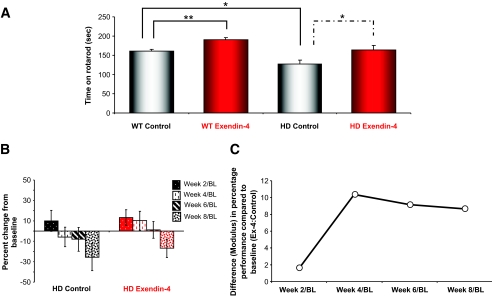
Motor coordination was measured on a bi-weekly basis using an accelerating rotarod (Fig. 5A and B). As Huntington's disease patients and Huntington's disease mice become symptomatic, they exhibit impaired motor coordination (8,35). The Huntington's disease control mice spent significantly less time on the rotarod than the wild-type control mice, which indicates that motor function was significantly impaired in the Huntington's disease control mice. Ex-4 treatment significantly enhanced the ability to stay on the rotarod for both wild-type (P < 0.05) and Huntington's disease (P < 0.05) mice. Generally, the wild-type and Huntington's disease mice that were treated with Ex-4 showed increased levels of general activity in their home cages (data not shown). We propose that the improved performance of the wild-type Ex-4–treated mice on the rotarod could be due to their increased activity levels. In humans, nausea is often observed with Ex-4 treatment; however, in our paradigm it is unlikely that this was in effect because this would likely have negatively affected the animals’ rotarod performance.
FIG. 5.
Ex-4 treatment improves motor coordination in Huntington's disease mice. Motor performance was measured bi-weekly using an accelerating rotarod apparatus. Mice were placed on the rotarod, which accelerated from 4 to 40 rpm over a 5-min test period, and latency to fall was recorded. A: Average time spent on the rotarod during the course of treatment for each study group is shown. Treatment with Ex-4 significantly increased the rotarod times of Huntington's disease mice (P < 0.05). Ex-4 treatment also increased the rotarod latencies of wild-type mice (P < 0.01). B: Analysis of percent change in rotarod performance from baseline confirmed that the performance of the Ex-4–treated Huntington's disease mice showed a much slower rate of motor control decline than the saline-treated Huntington's disease mice. Hence, at each time trial, Ex-4–treated animals showed a greater time on the rotarod compared with saline-treated animals. C: The relative week-to-week differences (modulus) in rotarod performance (percent change from baseline) between Ex-4–and control-treated Huntington's disease animals expressed as a ratio. Hence, an increase in the ratio denotes a relative increase in maintenance of rotarod performance in the Ex-4–treated animals compared with control. Values are means ± SE, n = 19–24 animals per group.
Ex-4–treated Huntington's disease mice showed superior motor control compared with the Huntington's disease control animals, and treatment with Ex-4 recovered Huntington's disease rotarod performance times to a similar proficiency as the wild-type control mice (Fig. 5A). As Huntington's disease symptoms progressed in the N171-82Q mice, the control and Ex-4–treated Huntington's disease mice performed progressively worse on each rotarod test. However, Ex-4 treatment significantly attenuated this decline in rotarod performance in the Huntington's disease mice, which indicates that Ex-4 can ameliorate motor symptoms in Huntington's disease mice (Fig. 5B). Comparing the differences in performance (percent change from baseline) from week to week of treatment, we demonstrated that as a ratio of Ex-4 to control animals, there was a consistent superiority of the Ex-4–treated Huntington's disease animals compared with saline-treated Huntington's disease animals. Hence, both saline- and Ex-4–treated animals declined in performance over time, but the Ex-4–treated animals each week had a better performance than control animals., i.e., greater positive change to baseline at week 4 and then lesser decreases from baseline at weeks 6 and 8 (Fig. 5B and C).
mhtt aggregates are reduced with Ex-4 treatment.
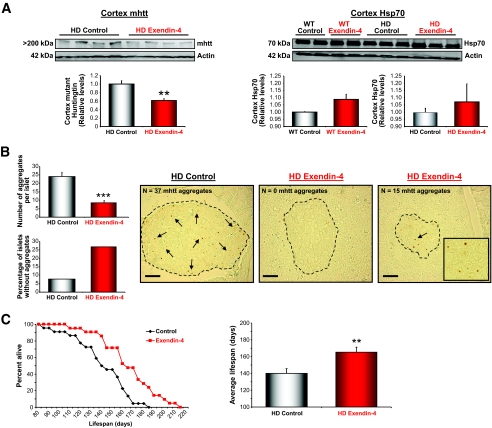
Inclusions of aggregated huntingtin protein in cortical, striatal, and hippocampal neurons are present in the Huntington's disease mice and in Huntington's disease patients. In the N171-82Q mice, intranuclear inclusions and neuritic aggregates [all immunoreactive with an antibody to the NH2 terminus (amino acids 1–17) of huntingtin] have been shown to be present in multiple populations of neurons (24). We determined whether Ex-4 treatment had any efficacy at reducing the number of mhtt aggregates in both the brain (cortex) and pancreas (islets of Langerhans). Ex-4 treatment caused a reduction in the amount of mhtt aggregates in the cortex of the Huntington's disease mice (Fig. 6A; P < 0.01). Additionally, there was a small, but nonsignificant, increase in Hsp-70 levels in the cortex of both the wild-type and Huntington's disease Ex-4–treated mice compared with vehicle-treated mice. In the pancreas, Ex-4 treatment caused a significant reduction (Fig. 6B; P < 0.001) in the number of mhtt aggregates in the islets of Langerhans. The Huntington's disease control mice showed a large number of mhtt aggregates in the pancreatic islets. Typically, the Huntington's disease control mice had two types of islets; very small islets containing a large number of mhtt aggregates and somewhat larger irregular-shaped islets containing a large number of mhtt aggregates. The Ex-4–treated Huntington's disease mice on the other hand demonstrated that only the smaller islets contained a low number of aggregates and that larger islets had few or no aggregates.
FIG. 6.
Ex-4 reduces mhtt aggregates in both the cortex and islets of Langerhans and significantly increases life span of Huntington's disease mice. A, left: Western immunoblot analysis with S830, an antibody against the Huntington's disease exon 1 transgene protein, showed that the amount of mhtt aggregation was significantly reduced in cortical tissue of Ex-4–treated Huntington's disease mice, compared with saline-treated controls (P < 0.01). Hsp-70 is a molecular chaperone whose expression is increased in times of cellular stress, and it acts to prevent protein misfolding and aggregation in response to environmental insults or disease. A, right: Western immunoblotting showed that cortical levels of Hsp-70 were slightly elevated with Ex-4 treatment in both Huntington's disease and wild-type mice. Immunostaining of pancreatic tissue with S830 antibody showed that Ex-4 treatment caused a significant decrease in the number of mhtt aggregates in the pancreatic islets of Langerhans. B: There was a significant decrease in the average number of mhtt aggregates per islet (P < 0.001) and an increase in the number of islets containing no mhtt aggregates in the Ex-4–treated Huntington's disease mice (bar charts). Representative images of both Huntington's disease control and Huntington's disease Ex-4–treated islets are shown. The Huntington's disease control mice generally had two types of islets, some very small and others large and irregularly shaped, and both types containing large numbers of mhtt aggregates. B: The small islets in Ex-4–treated Huntington's disease mice had a small number of mhtt aggregates, whereas large islets had a more regular structure and little or no mhtt aggregates. Ex-4 treatment caused a significant increase in the survival of Huntington's disease mice (P < 0.01). C: Ex-4–treated Huntington's disease mice lived an average of 25.3 days longer than saline-treated controls, an 18% increase in life span. Values are means ± SE, n = 21–24 animals per group. **P < 0.01, ***P < 0.001.
Ex-4 treatment extends the survival of Huntington's disease mice.
Any effective therapeutic agent for the treatment of Huntington's disease symptomology would ideally delay the onset of symptoms (i.e., motor dysfunction) and extend life span. In addition to ameliorating motor dysfunction (Fig. 5), Ex-4 treatment resulted in a highly significant increase in the survival of the Huntington's disease mice compared with vehicle-treated control Huntington's disease mice (Fig. 6C). The onset of mortality in Huntington's disease mice treated with Ex-4 was significantly delayed compared with the control Huntington's disease mice, and the mean life span was significantly increased from 140 to 165 days (P < 0.01; Fig. 6C). This life span–extending effect of Ex-4 of nearly 1 month represents an 18% increase over the life span of vehicle-treated control Huntington's disease mice.
DISCUSSION
We have shown that Ex-4, a potent long-acting agonist of GLP-1 used to treat type 2 diabetes, significantly improved glycemic control and pancreatic cellular architecture, attenuated motor performance decline, reduced mhtt aggregation in the brain and pancreas, and significantly increased life span in the N171-82Q mouse model of Huntington's disease. This is the first study to report that euglycemia can be achieved and maintained and that pancreatic islets can be preserved in a mouse model of Huntington's disease by daily administration of a widely used drug. The beneficial effects of Ex-4 on abnormalities of energy metabolism (glycemic control) and brain pathology (mhtt-associated degeneration and motor dysfunction) are likely due to a combination of peripheral and central actions of Ex-4. The global effects of Ex-4 are clearly beneficial to the animal even with the presence of reduced body mass; thus, the treatment is still effective even with this unwanted side effect. In human patients, cognizance of this action may be offset by an increase in input calories in their diet, which may be actually facilitated by Ex-4 treatment, because in the animals, it reduced leptin levels and increased ghrelin levels. A simultaneous reduction in satiety (lower leptin) and increase in hunger (increased ghrelin) would help foster elevated caloric intake in the Huntington's disease patients.
The high basal glucose levels in Huntington's disease mice may be associated with accumulation of mhtt and, therefore, disruption of function in pancreatic β-cells, reductions in numbers of β-cells, and perturbed α-cell topography. Others have reported the presence of mhtt aggregates in pancreatic cells associated with perturbed structure and endocrine function of the pancreas in Huntington's disease mouse models (11). We found that Ex-4, based on the morphological findings, obviously ameliorates mhtt-related β-cell pathologies, suggesting that the beneficial effects of Ex-4 on the pancreas are, at least in part, responsible for its ability to restore glycemic control in the Huntington's disease mice. Of Huntington's disease patients, ∼20% are reported to have diabetes (10,36). This incidence may prove to be higher if all Huntington's disease patients were tested. Insulin treatment (up to 8 IU/kg daily or twice daily) of long-acting insulin, a dose that typically induces lethal hypoglycemia in normal mice, did not control blood glucose in the Huntington's disease mice, illustrating a severe insulin resistance, and actually appeared to shorten the life span of the Huntington's disease mice.
Ex-4 had profound effects on insulin resistance, as demonstrated by the marked improvement in the HOMA index. Plasma glucose levels of the Ex-4–treated Huntington's disease mice were significantly lower than those of the nontreated Huntington's disease animals, for the same prevailing plasma insulin levels, clearly indicating improved insulin action. We propose that this is due to direct effects of Ex-4 on the brain as a result of reducing mutant htt aggregates, and we suggest that the therapeutic locus of improvement may be at the level of the hypothalamus. Evidence indicates that while insulin is not a major regulator of glucose use by the brain (37), the brain is clearly not insulin insensitive. In normal rats, blockade of insulin receptor signaling in the hypothalamus by phosphoinositide 3-kinase (PI 3-kinase) inhibitors leads to hepatic insulin resistance and its inevitable consequence of increased hepatic glucose production (38,39). Centrally acting insulin may regulate glucose metabolism via neuronal systems that are partially independent of one another (39). Defective insulin receptor substrate/PI 3-kinase signaling is implicated in insulin resistance in peripheral tissues (40) and presumably may be causing neuronal/hypothalamic insulin resistance in Huntington's disease patients. In addition to insulin control of PI 3-kinase activity in the hypothalamus, the satiety-controlling hormone leptin also activates PI 3-kinase in the hypothalamus (41). Therefore, defective insulin/PI 3-kinase signaling in hypothalamus would be compounded by the presence of low leptin levels, as we found to be the case in the Huntington's disease mice. Intriguing data also point to centrally acting carnitine palmitoyl transferase-1 (CPT-1), the mitochondrial protein that regulates the rate of fatty acid oxidation, being a regulator of peripheral insulin sensitivity. Its inhibition centrally led to increased hepatic insulin sensitivity that was functionally blocked by vagotomy (42). Lipid sensing by the brain appears to regulate hepatic glucose metabolism via activation of vagal afferent fibers. We therefore suggest that in Huntington's disease, severe peripheral insulin resistance may develop via insulin resistance in the hypothalamus. This may also lead to upregulation of CPT-1 and increased fatty acid oxidation, further decreasing signaling via the vagus to the liver. The defects related to glucose metabolism in the Huntington's disease mice were not corrected by exogenous insulin (and presumably also could not be corrected by endogenous insulin action), suggesting that mutant htt aggregates are directly impacting the insulin receptor downstream signaling pathways. Because Ex-4 lessened aggregates in both the brain and the pancreas in our Huntington's disease mice, we hypothesize that this is the primary mechanism underlying the ability to markedly improve insulin action. Taken together, such a mechanism further reinforces the potential impact of considering hormonal periphery-central interactions with respect to therapeutics for neurodegenerative disorders.
Neither the normal function of huntingtin nor the mechanism whereby the polyglutamine expansions result in selective loss of striatal neurons is fully understood, although impaired energy metabolism (43), excitotoxicity (44), and oxidative stress (45) have all been implicated. Mutant htt may cause neuronal dysfunction and death by inducing oxidative stress, impairing energy metabolism, inhibiting neurotrophic factor expression, and triggering apoptosis (46). Deficits in striatal and cortical glucose metabolism have been shown to precede the appearance of symptoms in Huntington's disease patients (47), and mhtt impairs neuronal energy metabolism in cultured neurons and transgenic mice (48,49). The ability of Ex-4 to suppress mhtt accumulation in brain cells and improve motor performance in Huntington's disease mice indicates that Ex-4 counteracts the adverse effects of mhtt on neurons at a relatively early stage in the neurodegenerative process. Previous studies have shown that Ex-4 crosses the blood-brain barrier (21) and that small fragments of Ex-4 have been shown in one study to mediate neuronal protection against excitotoxic and metabolic insults in culture and in vivo (22). Our findings suggest that Ex-4 can protect neurons against the pathogenic actions of mhtt, thereby delaying disease onset and extending the survival of the Huntington's disease mice.
Our understanding of neurodegenerative brain disorders has evolved to a point at which brain health and somatic health are intimately associated in the disease process. The contribution of peripheral and glycemic health is critical to the maintenance of a healthy brain (14). In searching for an effective therapeutic strategy for Huntington's disease, previous research has focused only on the detrimental effects of aberrant mhtt processing in brain cells, while largely overlooking the fact that peripheral pathophysiology (such as altered glucose control, disrupted energy expenditure, and severe uncontrolled weight loss) could contribute to Huntington's disease symptomology and exacerbate neuronal dysfunction and disease progression. Our findings show that Ex-4, an agent that targets both the central (i.e., neuronal dysfunction) and peripheral (abnormal energy and glucose regulation, appetite dysregulation) pathological processes in Huntington's disease, is a therapeutic agent in Huntington's disease mice. When taken together with the increasing evidence that GLP-1 and Ex-4 can protect neurons against a range of insults (22) and can stimulate neurogenesis (50), our findings suggest that Ex-4 might also prove efficacious in other complex neurodegenerative disorders that involve metabolic disturbances, including Alzheimer's and Parkinson's diseases. Regardless of all else, our data suggest that Ex-4, now marketed as exenatide, should be seriously considered for treating metabolic deficits in Huntington's disease patients.
Supplementary Material
Acknowledgments
B.M. has received a postdoctoral fellowship awarded by the Huntington's Disease Society of America. This research was supported by the Intramural Research Program of the National Institute on Aging.
No potential conflicts of interest relevant to this article were reported.
We thank the National Institute on Aging animal facility staff and Marveia Daniel for the expert animal care, Alfred May for microscopy assistance, and Dr. David Borchelt for advice during the study.
Published ahead of print at http://diabetes.diabetesjournals.org on 4 November 2008.
S.M., M.P.M., and J.M.E. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Landles C, Bates GP: Huntingtin and the molecular pathogenesis of Huntington's disease: fourth in molecular medicine review series. EMBO Rep 5: 958–963, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, Graham RK, Hayden MR: The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet 4: 398–403, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Bates G: Huntingtin aggregation and toxicity in Huntington's disease. Lancet 361: 1642–1644, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Aziz NA, Swaab DF, Pijl H, Roos RA: Hypothalamic dysfunction and neuroendocrine and metabolic alterations in Huntington's disease: clinical consequences and therapeutic implications. Rev Neurosci 18: 223–251, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Gaba AM, Zhang K, Marder K, Moskowitz CB, Werner P, Boozer CN: Energy balance in early-stage Huntington disease. Am J Clin Nutr 81: 1335–1341, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Powers WJ, Videen TO, Markham J, McGee-Minnich L, Antenor-Dorsey JV, Hershey T, Perlmutter JS: Selective defect of in vivo glycolysis in early Huntington's disease striatum. Proc Natl Acad Sci U S A 104: 2945–2949, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lodi R, Schapira AH, Manners D, Styles P, Wood NW, Taylor DJ, Warner TT: Abnormal in vivo skeletal muscle energy metabolism in Huntington's disease and dentatorubropallidoluysian atrophy. Ann Neurol 48: 72–76, 2000 [PubMed] [Google Scholar]
- 8.Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Friedlich A, Browne SE, Schilling G, Borchelt DR, Hersch SM, Ross CA, Beal MF: Creatine increase survival and delays motor symptoms in a transgenic animal model of Huntington's disease. Neurobiol Dis 8: 479–491, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Sathasivam K, Hobbs C, Turmaine M, Mangiarini L, Mahal A, Bertaux F, Wanker EE, Doherty P, Davies SW, Bates GP: Formation of polyglutamine inclusions in non-CNS tissue. Hum Mol Genet 8: 813–822, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Hurlbert MS, Zhou W, Wasmeier C, Kaddis FG, Hutton JC, Freed CR: Mice transgenic for an expanded CAG repeat in the Huntington's disease gene develop diabetes. Diabetes 48: 649–651, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Bjorkqvist M, Fex M, Renstrom E, Wierup N, Petersen A, Gil J, Bacos K, Popovic N, Li JY, Sundler F, Brundin P, Mulder H: The R6/2 transgenic mouse model of Huntington's disease develops diabetes due to deficient beta-cell mass and exocytosis. Hum Mol Genet 14: 565–574, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Hunt MJ, Morton AJ: Atypical diabetes associated with inclusion formation in the R6/2 mouse model of Huntington's disease is not improved by treatment with hypoglycaemic agents. Exp Brain Res 166: 220–229, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, Hoyt KR: Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci Lett 411: 98–103, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Martin B, Golden E, Keselman A, Stone M, Mattson MP, Egan JM, Maudsley S: Therapeutic perspectives for the treatment of Huntington's disease: treating the whole body. Histol Histopathol 23: 237–250, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandhu H, Wiesenthal SR, MacDonald PE, McCall RH, Tchipashvili V, Rashid S, Satkunarajah M, Irwin DM, Shi ZQ, Brubaker PL, Wheeler MB, Vranic M, Efendic S, Giacca A: Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes 48: 1045–1053, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Doyle ME, Egan JM: Glucagon-like peptide-1. Recent Prog Horm Res 56: 377–399, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Vilsboll T, Brock B, Perrild H, Levin K, Lervang HH, Kolendorf K, Krarup T, Schmitz O, Zdravkovic M, Le-Thi T, Madsbad S: Liraglutide, a once daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycemia in patients with type 2 diabetes mellitus Diabet Med 25: 152–156, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Meneilly GS, Greig N, Tildesley H, Habener JF, Egan JM, Elahi D: Effects of 3 months of continuous subcutaneous administration of glucagon-like peptide 1 in elderly patients with type 2 diabetes. Diabetes Care 26: 2835–2841, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Egan JM, Meneilly GS, Elahi D: Effects of 1-mo bolus subcutaneous administration of exendin-4 in type 2 diabetes. Am J Physiol Endocrinol Metab 284: E1072–E1079, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Dunphy JL, Taylor RG, Fuller PJ: Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol 141: 179–186, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Kastin AJ, Akerstrom V: Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord 27: 313–318, 2003 [DOI] [PubMed] [Google Scholar]
- 22.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN: Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9: 1173–1179, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Perry T, Holloway HW, Weerasuriya A, Mouton PR, Duffy K, Mattison JA, Greig NH: Evidence of GLP-1-mediated neuroprotection in an animal model of pyridoxine-induced peripheral sensory neuropathy. Exp Neurol 203: 293–301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilling G, Becher MW, Sharp AH, Jinnah HA, Duan K, Kotzuk JA, Slunt HH, Ratovitski T, Cooper JK, Jenkins NA, Copeland NG, Price DL, Ross CA, Borchelt DR: Intranuclear inclusions and neuritic aggregates in transgenic mice expressing a mutant N-terminal fragment of huntingtin. Hum Mol Genet 8: 397–407, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Doyle ME, McConville P, Theodorakis MJ, Goetschkes MM, Bernier M, Spencer RG, Holloway HW, Greig NH, Egan JM: In vivo biological activity of exendin (1–30). Endocrine 27: 1–9, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Maudsley S, Pierce KL, Zamah AM, Miller WE, Ahn S, Daaka Y, Lefkowitz RJ, Luttrell LM: The beta(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor. J Biol Chem 275: 9572–9580, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Smith DL, Portier R, Woodman B, Hockly E, Mahal A, Klunk WE, Li XJ, Wanker E, Murray KD, Bates GP: Inhibition of polyglutamine aggregation in R6/2 HD brain slices-complex dose-response profiles. Neurobiol Dis 8: 1017–1026, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Egan JM, Clocquet AR, Elahi D: The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab 87: 1282–1290, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Petersen A, Bjorkqvist M: Hypothalamic-endocrine aspects in Huntington's disease. Eur J Neurosci 24: 961–967, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Popovic V, Svetel M, Djurovic M, Petrovic S, Doknic M, Pekic S, Miljic D, Milic N, Glodic J, Dieguez C, Casanueva FF, Kostic V: Circulating and cerebrospinal fluid ghrelin and leptin: potential role in altered body weight in Huntington's disease. Eur J Endocrinol 151: 451–455, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Ding X, Saxena NK, Lin S, Gupta NA, Anania FA: Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology 43: 173–181, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doyle ME, Egan JM: Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther 113: 546–593, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Tilve D, Gonzalez-Matias L, Alvarez-Crespo M, Leiras R, Tovar S, Dieguez C, Mallo F: Exendin-4 potently decreases ghrelin levels in fasting rats. Diabetes 56: 143–151, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Altinova AE, Toruner F, Bukan N, Yasar DG, Akturk M, Cakir N, Arslan M: Decreased plasma adiponectin is associated with insulin resistance and HDL cholesterol in overweight subjects. Endocr J 54: 221–226, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Young AB, Penney JB, Starosta-Rubinstein S, Markel DS, Berent S, Giordani B, Ehrenkaufer R, Jewett D, Hichwa R: PET scan investigations of Huntington's disease: cerebral metabolic correlates of neurological features and functional decline. Ann Neurol 20: 296–303, 1986 [DOI] [PubMed] [Google Scholar]
- 36.Farrer LA: Diabetes mellitus in Huntington disease. Clin Genet 27: 62–67, 1985 [DOI] [PubMed] [Google Scholar]
- 37.Adage T, Scheurink AJ, de Boer SF, de Vries K, Konsman JP, Kuipers F, Adan RA, Baskin DG, Schwartz MW, van Dijk G: Hypothalamic, metabolic, and behavioral responses to pharmacological inhibition of CNS melanocortin signaling in rats. J Neurosci 21: 3639–3645, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obici S, Zhang BB, Karkanias G, Rossetti L: Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8: 1376–1382, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L: Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5: 566–572, 2002 [DOI] [PubMed] [Google Scholar]
- 40.White MF: Insulin signaling in health and disease. Science 302: 1710–1711, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG Jr, Schwartz MW: Intracellular signalling: key enzyme in leptin-induced anorexia. Nature 413: 794–795, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Pocai A, Obici S, Schwartz GJ, Rossetti L: A brain-liver circuit regulates glucose homeostasis. Cell Metab 1: 53–61, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH: Mitochondrial defect in Huntington's disease caudate nucleus. Ann Neurol 39: 385–389, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz KJ, Martin JB: Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature 321: 168–171, 1986 [DOI] [PubMed] [Google Scholar]
- 45.Nakao N, Brundin P: Effects of alpha-phenyl-tert-butyl nitrone on neuronal survival and motor function following intrastriatal injections of quinolinate or 3-nitropropionic acid. Neuroscience 76: 749–761, 1997 [DOI] [PubMed] [Google Scholar]
- 46.Cha JH: Transcriptional dysregulation in Huntington's disease. Trends Neurosci 23: 387–392, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Kuwert T, Lange HW, Boecker H, Titz H, Herzog H, Aulich A, Wang BC, Nayak U, Feinendegen LE: Striatal glucose consumption in chorea-free subjects at risk of Huntington's disease. J Neurol 241: 31–36, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D: Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell 127: 59–69, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Lee JM, Ivanova EV, Seong IS, Cashorali T, Kohane I, Gusella JF, MacDonald ME: Unbiased gene expression analysis implicates the huntingtin polyglutamine tract in extra-mitochondrial energy metabolism. PLoS Genet 3: e135, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, Wikstrom L: Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res 86: 326–338, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.