Abstract
OBJECTIVE—To quantitate plasma ceramide subspecies concentrations in obese subjects with type 2 diabetes and relate these plasma levels to the severity of insulin resistance. Ceramides are a putative mediator of insulin resistance and lipotoxicity, and accumulation of ceramides within tissues in obese and diabetic subjects has been well described.
RESEARCH DESIGN AND METHODS—We analyzed fasting plasma ceramide subspecies by quantitative tandem mass spectrometry in 13 obese type 2 diabetic patients and 14 lean healthy control subjects. Results were related to insulin sensitivity measured with the hyperinsulinemic-euglycemic clamp technique and with plasma tumor necrosis factor-α (TNF-α) levels, a marker of inflammation. Ceramide species (C18:1, 18:0, 20:0, 24:1, and 24:0) were quantified using electrospray ionization tandem mass spectrometry after separation with high-performance liquid chromatography.
RESULTS—Insulin sensitivity (mg · kg−1 · min−1) was lower in type 2 diabetic patients (4.90 ± 0.3) versus control subjects (9.6 ± 0.4) (P < 0.0001). Type 2 diabetic subjects had higher (P < 0.05) concentrations of C18:0, C20:0, C24:1, and total ceramide. Insulin sensitivity was inversely correlated with C18:0, C20:0, C24:1, C24:0, and total ceramide (all P < 0.01). Plasma TNF-α concentration was increased (P < 0.05) in type 2 diabetic subjects and correlated with increased C18:1 and C18:0 ceramide subspecies.
CONCLUSIONS—Plasma ceramide levels are elevated in type 2 diabetic subjects and may contribute to insulin resistance through activation of inflammatory mediators, such as TNF-α.
Type 2 diabetes is an insulin-resistant state characterized by impaired glucose tolerance (1) and inflammation (2). Much evidence has demonstrated the role of increased circulating free fatty acids and tissue fat accumulation in the development of muscle and liver insulin resistance (1,3,4). The disturbances in plasma and tissue lipid metabolism result from an oversupply of lipid substrates, both exogenously and endogenously (increased lipolysis secondary to adipocyte insulin resistance), and perturbations in fat oxidation and utilization by muscle and liver, resulting in the accumulation of ectopic fat (4). Ectopic fat is “lipotoxic” and has been linked to the severity of insulin resistance and pancreatic β-cell dysfunction, i.e., the core defects in type 2 diabetes (1,4). Ectopic fat comprises various lipid species, including long-chain fatty acyl CoAs, diacylglycerol, and ceramide. It is well documented that ceramide accumulates within insulin-resistant tissues of animals (5–7) and humans (8–10) and inhibits insulin action and subsequent glucose uptake through inactivation of Akt. Ceramide also induces inflammation through activation of the nuclear factor-κB–tumor necrosis factor-α (TNF-α) axis (5–7).
TNF-α is released from adipocytes and circulating mononuclear cells (MNCs) in response to stimuli, such as lipid infusion, lipopolysaccharide, reactive oxygen species, and hyperglycemia, and elevated TNF-α concentrations have been shown to induce insulin resistance (11–14). TNF-α also activates the plasma membrane enzyme sphingomyelinase (SMase) that hydrolyzes sphingomyelin to ceramide, allowing ceramides to accumulate within the cell (5,6,15–17). This accumulation of ceramide within tissues is thought to initiate a positive feedback mechanism, leading to enhanced production of proinflammatory cytokines (5), resulting in further inhibition of insulin-stimulated glucose uptake. Both plasma TNF-α concentrations and intracellular lipid intermediates, such as ceramides, are elevated in subjects with type 2 diabetes (8,18). Thus, ceramide is a bioactive lipid and putative mediator of insulin resistance that could link nutrient (fat) oversupply and cytokine-induced inflammation in tissues (5–7).
Plasma ceramide levels also have been shown to correlate with coronary artery disease, independent of the plasma cholesterol concentration (19,20). However, the role of circulating ceramides has received little attention with respect to the development of insulin resistance and type 2 diabetes. Conflicting reports exist as to whether total circulating ceramides are elevated in obese (21) and type 2 diabetic subjects (22). Subspecies of plasma ceramides have been demonstrated to be increased in patients with sepsis and atherosclerosis (23–25), but the relationship between plasma ceramide subspecies levels and insulin resistance has not been investigated in patients with type 2 diabetes.
Given their central role in the induction of insulin resistance and inflammation, elevated plasma ceramide levels may serve as a biomarker or direct perpetuator of insulin resistance and lipid-induced inflammation. Elevated plasma ceramide concentrations also may serve to identify individuals who are at risk to develop type 2 diabetes. The objective of this study was to quantify the concentration of individual ceramide subspecies in the circulation of patients with type 2 diabetes and healthy control subjects and to examine the correlation between plasma levels of ceramide subspecies and insulin sensitivity, measured with the euglycemic-hyperinsulinemic clamp, and plasma TNF-α concentration, a marker of inflammation.
RESEARCH DESIGN AND METHODS
Twenty-seven subjects were recruited from the greater San Antonio metropolitan area. Thirteen subjects had type 2 diabetes, and 14 were healthy nondiabetic control subjects. Descriptive data from these subjects has been presented in previous reports (26,27) (Table 1). Type 2 diabetic subjects were older and more obese than nondiabetic subjects and were characterized by dyslipidemia and hyperglycemia (Table 1). Normal glucose tolerance was confirmed in all control subjects by a 75-g oral glucose tolerance test using American Diabetes Association criteria (28). After a 10- to 12-h overnight fast, body composition was assessed with bioelectrical impedance in all subjects (29). Type 2 diabetic subjects were in reasonably good glycemic control, as reflected by a mean A1C of 7.5%. Type 2 diabetic patients were treated with diet (n = 8) or sulfonylureas (n = 5). No type 2 diabetic subject had received treatment with metformin, thiazolidinediones, or insulin. The mean duration of diabetes was <5 years. Five type 2 diabetic subjects had normal fasting plasma glucose concentrations and were diagnosed with an oral glucose tolerance test (2-h plasma glucose ≥200 mg/dl). Oral antidiabetic agents were discontinued 24 h before the study. Other than diabetes, none of the subjects had any medical problems, and none were taking any medications (other than sulfonylureas) known to affect glucose metabolism. None of the participants smoked, and none of the women were on hormone replacement therapy. Weight was stable (±3 lb) in all subjects for the 3 months before study, and no subject participated in an excessively heavy exercise program. The purpose, nature, and potential risks of the study were explained to all subjects, and written consent was obtained before their participation. The protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio.
TABLE 1.
Subject characteristics
| Control subjects | Type 2 diabetic patients | P value | |
|---|---|---|---|
| n | 14 | 13 | — |
| Sex | 9 men/5 women | 6 men/7 women | — |
| Age (years) | 40 ± 4 | 50 ± 3 | 0.04 |
| BMI (kg/m2) | 26.3 ± 1 | 32.9 ± 1 | <0.01 |
| LBM (%) | 72 ± 2 | 64 ± 2 | <0.01 |
| FPG (mg/dl) | 91 ± 2 | 151 ± 17 | <0.01 |
| FPI (μU/ml) | 5.6 ± 0.6 | 14.0 ± 1.4 | <0.01 |
| F-FFA (μmol/l) | 495 ± 48 | 887 ± 77 | <0.01 |
| TG (mg/dl) | 86 ± 12 | 188 ± 23 | <0.01 |
| Total cholesterol (mg/dl) | 159 ± 8 | 204 ± 10 | <0.01 |
| HDL (mg/dl) | 47 ± 3 | 45 ± 2 | NS |
| LDL (mg/dl) | 98 ± 6 | 119 ± 8 | NS |
Data are means ± SE. FPG, fasting plasma glucose; FPI, fasting plasma insulin; F-FFA, fasting plasma free fatty acids; LBM, lean body mass; TG, triglycerides.
All studies were conducted in the General Clinical Research Center of the University of Texas Health Science Center at San Antonio and began at 0700 h after a 12-h overnight fast. Before the start of the euglycemic insulin clamp study, an anticubital vein was cannulated for infusion of all test substances. A second catheter was inserted retrogradely into a dorsal hand vein, and the hand was placed in a heated box (60°C) to obtain arterialized blood samples. A primed (25 μCi × fasting plasma glucose/100)-continuous infusion (0.25 μCi/min) of 3-[3H]glucose was started 2 h (3 h for type 2 diabetes) before the start of the insulin clamp to allow for isotopic equilibration. The priming dose of tritiated glucose was increased in the type 2 diabetic subjects in proportion to the increase in their fasting plasma glucose concentration. At the end of the tracer equilibration period, blood was obtained for determination of plasma ceramide and TNF-α levels. At time 0, a primed-continuous infusion (80 mU · m−2 · min−1) of insulin was started and continued for 4 h. During insulin infusion, the plasma glucose concentration was measured every 5 min with a Glucose Oxidase Analyzer (Beckman Instruments, Fullerton, CA). Based on the negative-feedback principle, a variable infusion of 20% glucose was adjusted to maintain the plasma glucose concentration constant at each subject's fasting plasma glucose level in the control group. In type 2 diabetic subjects, the plasma glucose concentration was allowed to decrease during the insulin infusion to 100 mg/dl, at which level it was maintained.
Analytical determinations.
Plasma tritiated glucose specific activity was determined using barium hydroxide/zinc sulfate extracts of plasma. Plasma insulin concentration was determined by radioimmunoassay (Diagnostic Products, Los Angeles, CA). Plasma free fatty acid concentration was determined by colorimetric assay (Wako, Neuss, Germany). Plasma TNF-α concentration was determined by UltraSensitive ELISA (Biosource International, Camarillo, CA).
Ceramide subspecies analysis
Sample preparation.
Calibration curves (0–1,000 ng) for each ceramide standard (purity >99%; Avanti Polar Lipids, Alabaster, AL) were prepared in 100 μl plasma matrix. C17:0 ceramide was used as a nonnaturally occurring internal standard. Plasma samples (100 μl), in parallel with standard solutions, were spiked with 100 ng C17:0 ceramide and were extracted with 2 ml chloroform:methanol (1:2) mixture according to the protocol of Bligh and Dyer (30). Phases were broken by adding 0.5 ml chloroform and 0.5 ml water. The lower organic fraction was removed, and the remainder was extracted with an additional 1 ml chloroform. The pooled organic phase was dried under nitrogen gas, and the residue was reconstituted in 500 μl methylene chloride and loaded onto a silica gel column packed with 2 ml silica gel suspension in methylene chloride. Columns were washed with 1 ml methylene chloride, and ceramides were eluted with 2 × 2 ml 30% isopropanol in methylene chloride. Eluent was dried under nitrogen gas and the residue was reconstituted in high-performance liquid chromatography (HPLC) elution buffer and analyzed by mass spectrometry.
Mass spectrometry.
Ceramide species were quantified by HPLC on-line electrospray ionization tandem mass spectrometry (MS/MS). For optimization, the mixture of ceramide standards was infused directly into the mass spectrometer, and all source parameters and ionization conditions were adjusted to improve the sensitivity of the assay. Extracted samples (40 μl) were injected onto a Waters HPLC (2690 Separations Module; Waters, Franklin, MA) and separated through an Ascentis C18 column (2.1 × 50 mm, 5 μm; SUPELCO, Bellefonte, PA) using a gradient starting from 15% mobile phase A (water containing 0.2% formic acid) at a flow rate of 0.3 ml/min for 1 min, to 100% mobile phase B (methanol containing 0.2% formic acid) over 3 min, and then with 100% B for 22 min. The HPLC column effluent was introduced onto a Micromass triple quadruple mass spectrometer (Quattro Ultima; Waters) and analyzed using electrospray ionization in positive mode. A potential difference of 3 keV was applied between the electrospray needle and the interior of the ion source. Hot nitrogen gas (250°C) was used to help evaporate the solvent from the charged droplets and argon was used as the collision gas. All ceramides were quantified using multiple reaction monitoring. The MS/MS transitions (m/z) were 552→264 for C17:0, 564→264 for C18:1, 566→264 for C18:0, 594→264 for C20:0, 648→264 for C24:1, and 650→264 for C24:0. Ceramide subspecies were quantified (nmol/ml) by taking the ratios of the integrated peak areas (MassLynx 3.5; Manchester, U.K.) for each subspecies to the area of C17:0. Total ceramide was calculated from the sum of C18:1, C18:0, C20:0, C24:1, and C24:0 ceramide subspecies.
Calculations.
During the postabsorptive period, the rate of glucose appearance equals the rate of glucose disappearance and was calculated as the tritiated glucose infusion rate (dpm/min) divided by the plasma tritiated glucose specific activity (dpm/mg). During the euglycemic insulin clamp, non–steady-state conditions prevail, and the rate of glucose appearance was calculated with Steele's non–steady-state equation, using a glucose distribution volume of 0.65. The rate of endogenous (primarily hepatic) glucose production during the insulin clamp was calculated by subtracting the exogenous glucose infusion rate from the tracer-derived rate of glucose appearance. The rate of total body (primarily reflects muscle) insulin-stimulated glucose disposal was calculated by adding the rate of residual hepatic glucose production to the cold glucose infusion rate. The basal hepatic insulin resistance index (IRI) was calculated as the product of basal hepatic glucose production and the fasting plasma insulin concentration (31).
Statistical analysis.
Data are presented as mean ± SE. Comparisons between groups for the ceramide species was performed using the Mann-Whitney U test, and correlations were performed using Spearman's rank correlation coefficient. For all other comparisons, a two-sample t test was performed. In all tests, P < 0.05 was considered significant. For overall P value comparison, parametric versus nonparametric tests were consistent with means. All statistical analyses were performed using StatView version 5.0.1 (SAS Institute).
RESULTS
Metabolic characteristics.
Type 2 diabetic subjects were older, had a higher BMI, and increased fasting plasma glucose, insulin, triglyceride, total cholesterol, and free fatty acid concentrations (Table 1). Insulin-stimulated glucose disposal (mg · kg−1 · min−1) was approximately twofold lower in type 2 diabetic subjects (4.9 ± 0.3) versus control subjects (9.6 ± 0.4) (P < 0.0001). Basal endogenous glucose production (EGP) (mg · kg−1 · min−1) was increased in type 2 diabetic patients (2.1 ± 0.1) versus control subjects (1.7 ± 0.1) (P = 0.002), and the basal hepatic IRI was markedly increased in type 2 diabetic patients versus control subjects (30.3 ± 5.1 vs. 9.4 ± 1.2 mg · kg−1 · min−1 × μU/ml) (P = 0.0001).
Plasma ceramide concentrations.
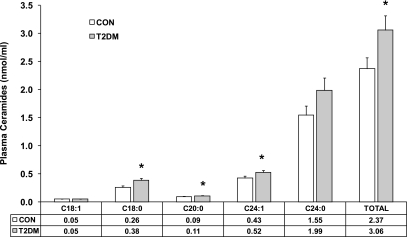
Fig. 1 displays the distribution of ceramide subspecies in the plasma of type 2 diabetic and control subjects. Consistent with previous reports for patients with sepsis and atherosclerosis (23,24), the major ceramides in plasma were C24:1 and C24:0. Type 2 diabetic subjects had increased (P < 0.05–0.01) concentrations (nmol/ml) of C18:0 (control, 0.26 ± 0.03 vs. type 2 diabetes, 0.38 ± 0.03), C20:0 (control, 0.09 ± 0.004 vs. type 2 diabetes, 0.11 ± 0.004), C24:1 (control, 0.43 ± 0.03 vs. type 2 diabetes, 0.52 ± 0.04), and total ceramide (control, 2.37 ± 0.19 vs. type 2 diabetes, 3.06 ± 0.26).
FIG. 1.
Plasma concentration of ceramide subspecies in obese type 2 diabetic patients (n = 13) compared with lean healthy control subjects (n = 14). Plasma concentrations were determined by quantitative tandem mass spectrometry. Data are expressed as means ± SE. *P < 0.05 type 2 diabetes vs. control; Mann-Whitney unpaired test.
Correlation between plasma ceramide subspecies and insulin sensitivity.
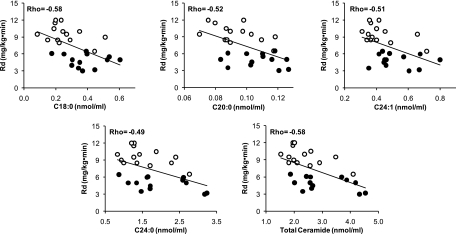
Spearman rank correlations were used to determine potential relationships between individual ceramide subspecies and insulin-stimulated glucose disposal. Insulin sensitivity (mg · kg−1 · min−1) was inversely correlated with C18:0 (ρ = −0.58, P = 0.003), C20:0 (ρ = −0.52, P = 0.008), C24:1 (ρ = −0.51, P = 0.009), C24:0 (ρ = −0.49, P = 0.01), and total ceramide (ρ = −0.58, P = 0.003) concentrations (Fig. 2).
FIG. 2.
Correlation of individual ceramide subspecies with the insulin-stimulated rate of glucose disposal (Rd). ○, control subjects; •, type 2 diabetic patients. Spearman's rank correlation was used to access the relationships between datasets. All correlations were significant, P < 0.05.
Plasma TNF-α concentration.
Fasting plasma TNF-α concentration (pg/ml), a marker of inflammation, was increased in type 2 diabetic subjects compared with control subjects (control, 2.81 ± 0.13 vs. type 2 diabetes, 4.30 ± 0.76) (P = 0.03).
Correlation between plasma ceramide subspecies and plasma TNF-α and triglyceride concentrations.
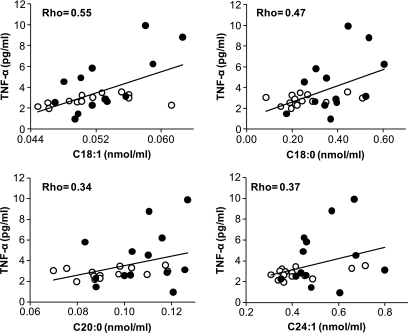
Plasma TNF-α was correlated with C18:1 (ρ = 0.55, P = 0.005) and C18:0 (ρ = 0.47, P = 0.02) ceramide subspecies (Fig. 3). There was also a trend for ceramide subspecies C20:0 (P = 0.08) and C24:1 (P = 0.06) to be correlated with TNF-α (Fig. 3). Fasting triglyceride was correlated with C18:0 (ρ = 0.48, P = 0.02) and C20:0 (ρ = 0.48, P = 0.02) ceramide subspecies. There was a trend for ceramide subspecies C24:1 (P = 0.07) and total ceramide (P = 0.06) to be correlated with the fasting plasma triglyceride concentration.
FIG. 3.
Correlation between individual ceramide species and plasma TNF-α concentration. Spearman's rank correlation was used to access the relationships between datasets. ○, control subjects; •, type 2 diabetic patients. TNF-α correlations between C18:1 and C18:0 ceramide subspecies were significant, P < 0.05. TNF-α correlations between C20: and C24:1 ceramide subspecies did not reach significance but displayed a trend toward significance: P = 0.08 and 0.06, respectively.
No significant correlations were found between age, basal EGP, fasting plasma glucose, plasma insulin, free fatty acids, HDL, LDL, or total cholesterol concentrations versus ceramide subspecies or total ceramide. Separate group (control subjects and type 2 diabetic patients) correlations were also performed between each of the ceramide subspecies and total ceramide to age, insulin sensitivity, plasma TNF-α, and fasting triglycerides. No additional individual group correlation was found to be significant (P > 0.05).
DISCUSSION
Increased tissue (muscle and liver) and plasma fat content, i.e., lipotoxicity, plays a central role in the pathogenesis of type 2 diabetes (1,4,8,27,32–34). Elevated bioactive lipids in the circulation, including lipoproteins, triglycerides, and fatty acids (27), and excessive tissue lipid deposits of long-chain fatty acyl CoAs, diacylglycerol, and ceramide (35,36) have been implicated in the phenomenon of lipotoxicity. Much evidence supports a role for circulating free fatty acids in the development of insulin resistance, inflammation, and β-cell dysfunction (1,3,4,33). Recently, elevated plasma sphingolipids have been implicated in the pathogenesis of obesity-induced cardiovascular and metabolic disease (37). Sphingolipid and ceramide formation are stimulated by inflammatory cytokines, such as TNF-α, which is released from adipocytes and elevated in the plasma of type 2 diabetic and obese subjects (5,6).
The present study shows for the first time that total and specific plasma ceramide subspecies concentrations are elevated in type 2 diabetic subjects (Fig. 1) and that these elevated lipid moieties are associated with the severity of insulin resistance (Fig. 2) and with elevated plasma TNF-α levels (Fig. 3). These findings suggest that elevated plasma ceramide levels in obese type 2 diabetic subjects may be an important mediator of insulin resistance and inflammation in these insulin-resistant states.
The present results are consistent with those in animals that demonstrate increased plasma levels of sphingomyelin and ceramide subspecies in ob/ob mice compared with lean controls (37). All detectable species of ceramide were elevated in the plasma of obese mice, with the greatest increase (86%) being observed for C18:0 ceramide (37). Our data demonstrate that all but one ceramide subspecies (C18:1) are elevated in obese type 2 diabetic subjects, with C18:0 showing the greatest increase (32%) (Fig. 1). C24:0 was found to be the most abundant ceramide subspecies in plasma, constituting 65% of the total. This is consistent with both animal (37) and human (8,23,24) reports. From the present and other studies (8,37), the plasma C18:0 subspecies concentration appears to be increased the most in conditions of excess adiposity. In muscle, however, Adams et al. (8) demonstrated that the greatest increase in ceramide subspecies in obese subjects was for C16:0 and C20:0 (increased 76 and 83%, respectively). Taken collectively, these observations suggest that there may be selective regulation or formation of ceramide species in the circulation versus tissue and that the accumulation of ceramide in each respective compartment may serve different biological functions and/or involve different metabolic/inflammatory responses.
The etiological mechanisms responsible for the elevated plasma ceramide levels measured in the present study were not identified. However, one could postulate that lipid spillover from excess fat deposits in adipocytes in the obese type 2 diabetic subjects was a contributing source. Ceramides also may be derived from macrophages that have infiltrated adipocytes in obese and type 2 diabetic subjects (38). Delogu et al. (39) have suggested that MNCs produce ceramides in response to tissue inflammation and hypothesized that the increased ceramide production by MNCs may induce apoptosis in adipocytes that become dysfunctional from chronic inflammation.
Tissue accumulation of ceramides in insulin-resistant subjects has been shown to inhibit insulin action by decreasing phosphorylation and activation of Akt (5), whereas elevated plasma ceramide levels have been demonstrated to be related to the development of atherosclerosis (19,20,24). However, the role of plasma ceramide subspecies has not been examined with regard to insulin resistance nor have plasma ceramide subspecies been related to the excess intracellular ceramide accumulation in obesity and type 2 diabetes. If the elevated plasma ceramide concentrations are the result of the spillover phenomenon, then plasma ceramide levels may be a marker of intracellular ceramide accumulation and thus insulin resistance. Consistent with this scenario, we found an inverse relationship between C18:0, C20:0, C24:1, C24:0, and total ceramide versus whole-body insulin-stimulated glucose disposal, as determined with the euglycemic insulin clamp (Fig. 2). Although the mechanism(s) via which individual ceramide subspecies in the circulation contribute to insulin resistance has yet to be investigated, some insights are available from cell-permeable ceramide analog experiments.
In C2C12 myotubes incubated with a cell-permeable C2-ceramide analog, insulin-stimulated glucose uptake, glycogen synthesis, and Akt serine phosphorylation were decreased (40) in association with an increase in intracellular ceramide concentration. Similar results have been demonstrated in L6 muscle cells (41) and other cell types (42–46) preincubated with C2- or C6-ceramide analogs. The results of these experiments indicate that there is a mechanism for cellular ceramide uptake. However, these studies used short-chain ceramide analogs, whereas circulating ceramides in humans and animals are of the long-chain variety (5). It also should be noted that most circulating ceramides and sphingolipids do not exist in a free form but are bound to plasma proteins (47). With regard to this, Serlie et al. (22) measured plasma and intramuscular ceramides in response to a 6-h lipid infusion in lean and obese subjects and found that although plasma ceramides were significantly increased, intramuscular ceramides were unchanged. These results indicate that there is no active uptake of ceramides into the muscle (21). In summary, it appears unlikely that plasma ceramide levels contribute to peripheral (muscle) insulin resistance through an uptake mechanism, and further inquiry is required to identify the role and source of both the elevated plasma (Fig. 1) and muscle (4) ceramide concentrations observed in obese and diabetic subjects. It should be noted that, although the results of Serlie et al. (22) demonstrated that the insulin resistance associated with short-term lipid infusion cannot be explained by increased muscle ceramide content, this does not exclude a role for excessive muscle ceramide accumulation in the development of insulin resistance in obesity and diabetes (4,7).
Chronic and acute inflammation have been shown to play a role in the intracellular accumulation of ceramides (15–17), which in turn have been implicated in the excess production of inflammatory cytokines, including TNF-α, interleukin (IL)-1, and IL-6. These proinflammatory cytokines also have been reported to be potent inducers of de novo ceramide synthesis (5,6). Given that diabetes, obesity, and other insulin-resistant states are associated with excess production of proinflammatory cytokines, it is reasonable to hypothesize that circulating ceramides may contribute to insulin resistance through local or systemic stimulation of the innate immune response. Consistent with such a hypothesis, the plasma concentration of TNF-α, a marker of inflammation, was increased and correlated with each plasma ceramide subspecies (Fig. 3). TNF-α stimulates ceramide formation by activating acidic and basic SMase isoforms (48), and acute systemic inflammation has been shown to upregulate a secretory form of SMase, which has been linked to atherogenesis (15). Furthermore, human vascular endothelial cells secrete SMase on stimulation by IL-1β and interferon-γ (49), and SMase is increased in the urine of patients with peritonitis, hepatitis, and after surgery or trauma (50). These findings are consistent with the results of Delogu et al. (39) who found a strong correlation between MNC-derived total ceramide concentration and plasma TNF-α concentrations in septic patients.
Study limitations.
The current study was limited to those subspecies that could be analyzed by the standards that were commercially available at the time of investigation. We recognize the potential importance of the ceramide subspecies C16:0, which contributes up to 10% of the total ceramide pool in human plasma (23,24), and other ceramide subspecies, including C22:0, C22:1, C23:0, and C24:2 (23,24). Furthermore, the correlation between plasma C18:1 ceramide and TNF-α may not be of any physiological or clinical relevance, because the concentration of this ceramide was close to the lower limit of detection and C18:1 was not found to be different between control subjects and type 2 diabetic subjects. In addition, the measures of plasma ceramide subspecies were obtained in the postabsorptive state, and it should be noted that feeding has been shown to influence plasma ceramide levels (5,51,52). Future studies will have the opportunity to measure additional plasma ceramide subspecies in the postprandial condition and to quantitate simultaneously both plasma and intracellular ceramide subspecies with concomitant measurement of insulin sensitivity, inflammation, and other metabolic variables.
Conclusions.
In summary, this is the first examination of plasma ceramide subspecies in obese subjects with type 2 diabetes. The increase in total and ceramide subspecies concentrations correlated closely with the severity of insulin resistance and elevated TNF-α levels. The increased circulating ceramide levels may be the result of lipid spillover from skeletal muscle, liver, or adipose tissues. Elevated plasma ceramide levels may be a marker of insulin resistance, atherosclerotic risk, and/or obesity-induced inflammation. Further study will be required to establish mechanistic links between specific plasma ceramide subspecies identified herein and insulin resistance.
Acknowledgments
This work was supported in part by National Institutes of Health Grant DK-24092, the National Center for Research Resources, Multidisciplinary Clinical Research Career Development Programs Grant 5K12RR023264, JPIC Grant AG-12834, University of Texas Health Science Center General Clinical Research Center Grant M01-RR-01346, Case Western Reserve University Grants T32 HL007887 and T32 DK007319.
R.A.D. has served on advisory boards for Bristol Myers Squibb, Amylin, Eli Lilly, Novartis, Pfizer, Takeda, Roche, Johnson & Johnson, and Merck; has received funding from Bristol Myers Squibb, Amylin, Eli Lilly, Novartis, Pfizer, Takeda, Roche, and Merck; and has participated in Speakers Bureau for Amylin, Eli Lilly, and Takeda.
J.M.H., S.R.K., T.K., R.Z., K.R.K., and J.P.K. report no other dualities of interest.
We are grateful to the General Clinical Research Center nurses at the Audie Murphy VA Hospital Medical Center (San Antonio, TX) for assisting in the performance of the metabolic studies.
Published ahead of print at http://diabetes.diabetesjournals.org on 13 November 2008.
J.M.H., and S.R.K. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.DeFronzo RA: Pathogenesis of type 2 diabetes mellitus. Med Clin N Am 88: 787–835, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS: Inflammation, stress, and diabetes. J Clin Invest 115: 1111–1119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger R: Minireview. Weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 144: 5159–5165, 2003 [DOI] [PubMed] [Google Scholar]
- 4.DeFronzo RA: Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl 9–21, 2004 [DOI] [PubMed]
- 5.Summers SA: Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Holland WL, Summers SA: Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev 29: 381–402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K: Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta 1485: 63–99, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ: Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Skovbro M, Baranowski M, Skov-Jensen C, Flint A, Dela F, Gorski J, Helge JW: Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 51: 1253–1260, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P, Adamska A, Blachnio A, Gorski J, Gorska M: Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia 50: 2366–2373, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez F, Rote NS, Minium J, Kirwan JP: In vitro evidence that hyperglycemia stimulates tumor necrosis factor-alpha release in obese women with polycystic ovary syndrome. J Endocrinol 188: 521–529, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez F, Minium J, Rote NS, Kirwan JP: Altered tumor necrosis factor alpha release from mononuclear cells of obese reproductive-age women during hyperglycemia. Metabolism 55: 271–276, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kirwan JP, Haugel-de Mouzon S, Lepercq J, Challier J-C, Huston-Presley L, Friedman JE, Kalhan SC, Catalano PM: TNF-α is a predictor of insulin resistance in human pregnancy. Diabetes 51: 2207–2213, 2002 [DOI] [PubMed] [Google Scholar]
- 14.del Aguila LF, Claffey KP, Kirwan JP: TNF-α impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol 276: E849–E855, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Wong ML, Xie B, Beatini N, Phu P, Marathe S, Johns A, Gold PW, Hirsch E, Williams KJ, Licinio J, Tabas I: Acute systemic inflammation up-regulates secretory sphingomyelinase in vivo: a possible link between inflammatory cytokines and atherogenesis. Proc Natl Acad Sci U S A 97: 8681–8686, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lightle SA, Oakly JI, Nilkolova-Karakashian MN: Activation of sphingolipid turnover and chronic generation of ceramide and sphingosine in liver aging. Mech Ageing Dev 120: 111–125, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I: Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH: implications for atherosclerotic lesion development. J Biol Chem 273: 2738–2746, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS, Spiegelman BM: Tumor necrosis factor α: a key component of the obesity-diabetes link. Diabetes 43: 1271–1278, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR: Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol 20: 2614–2618, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S: Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol 163: 903–912, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Serlie MJ, Meijer AJ, Groener JE, Duran M, Endert E, Fliers E, Aerts JM, Sauerwein HP: Short-term manipulation of plasma free fatty acids does not change skeletal muscle concentrations of ceramide and glucosylceramide in lean and overweight subjects. J Clin Endocrinol Metab 92: 1524–1529, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Serlie MJ, Allick G, Groener JE, Ackermans MT, Heijligenberg R, Voermans BC, Aerts JM, Meijer AJ, Sauerwein HP: Chronic treatment with pioglitazone does not protect obese patients with diabetes mellitus type II from free fatty acid-induced insulin resistance. J Clin Endocrinol Metab 92: 166–171, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G: Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res 44: 754–761, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, Maruyama T, Miwa Y, Harada-Shiba M, Tsushima M, Kojo S: Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids 41: 859–863, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Ichi I, Takashima Y, Adachi N, Nakahara K, Kamikawa C, Harada-Shiba M, Kojo S: Effects of dietary cholesterol on tissue ceramides and oxidation products of apolipoprotein B-100 in ApoE-deficient mice. Lipids 42: 893–900, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Kashyap SR, Roman LJ, Lamont J, Masters BS, Bajaj M, Suraamornkul S, Belfort R, Berria R, Kellogg DL Jr, Liu Y, DeFronzo RA: Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metab 90: 1100–1105, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kashyap SR, Belfort R, Berria R, Suraamornkul S, Pratipranawatr T, Finlayson J, Barrentine A, Bajaj M, Mandarino L, DeFronzo R, Cusi K: Discordant effects of a chronic physiological increase in plasma FFA on insulin signaling in healthy subjects with or without a family history of type 2 diabetes. Am J Physiol Endocrinol Metab 287: E537–E546, 2004 [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 31: S55–S60, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Jackson AS, Pollock ML, Graves JE, Mahar MT: Reliability and validity of bioelectrical impedance in determining body composition. J Appl Physiol 64: 529–534, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Bligh EA, Dyer WJ: A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. Can J Biochem Physiol 37: 911–917, 1959. 13671378 [Google Scholar]
- 31.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA: Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 30: 89–94, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K: A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 52: 2461–2474, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Unger RH, Zhou YT: Lipotoxicity of β-cells in obesity and in other causes of fatty acid spillover. Diabetes 50 (Suppl. 1): S118–S121, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Unger RH: Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: genetic and clinical implications. Diabetes 44: 863–870, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Goodpaster BH, Kelley DE: Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr Diab Rep 2: 216–222, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM: Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Samad F, Hester KD, Yang G, Hannun YA, Bielawski J: Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 55: 2579–2587, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O'Rahilly S, Walker NI, Cameron DP: Tumor necrosis factor-α induces apoptosis of human adipose cells. Diabetes 46: 1993–1994, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Delogu G, Famularo G, Amati F, Signore L, Antonucci A, Trinchieri V, Di Marzio L, Cifone MG: Ceramide concentrations in septic patients: a possible marker of multiple organ dysfunction syndrome. Crit Care Med 27: 2413–2417, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Schmitz-Peiffer C, Craig DL, Biden TJ: Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202–24210, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Hajduch E, Balendran A, Batty IH, Litherland GJ, Blair AS, Downes CP, Hundal HS: Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia 44: 173–183, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Kanety H, Hemi R, Papa MZ, Karasik A: Sphingomyelinase and ceramide suppress insulin-induced tyrosine phosphorylation of the insulin receptor substrate-1. J Biol Chem 271: 9895–9897, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Zhou H, Summers SA, Birnbaum MJ, Pittman RN: Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J Biol Chem 273: 16568–16575, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Mei J, Wang CN, O'Brien L, Brindley DN: Cell-permeable ceramides increase basal glucose incorporation into triacylglycerols but decrease the stimulation by insulin in 3T3–L1 adipocytes. Int J Obes Relat Metab Disord 27: 31–39, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Brindley DN, Wang CN, Mei J, Xu J, Hanna AN: Tumor necrosis factor-alpha and ceramides in insulin resistance. Lipids 34 (Suppl.): S85–S88, 1999 [DOI] [PubMed] [Google Scholar]
- 46.Soeda S, Honda O, Shimeno H, Nagamatsu A: Sphingomyelinase and cell-permeable ceramide analogs increase the release of plasminogen activator inhibitor-1 from cultured endothelial cells. Thromb Res 80: 509–518, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Chatterjee S: Sphingolipids in atherosclerosis and vascular biology. Arterioscler Thromb Vasc Biol 18: 1523–1533, 1998 [DOI] [PubMed] [Google Scholar]
- 48.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM: Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 271: 13018–13022, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Marathe S, Schissel SL, Yellin MJ, Beatini N, Mintzer R, Williams KJ, Tabas I: Human vascular endothelial cells are a rich and regulatable source of secretory sphingomyelinase: implications for early atherogenesis and ceramide-mediated cell signaling. J Biol Chem 273: 4081–4088, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Quintern LE, Zenk TS, Sandhoff K: The urine from patients with peritonitis as a rich source for purifying human acid sphingomyelinase and other lysosomal enzymes. Biochim Biophys Acta 1003: 121–124, 1989 [DOI] [PubMed] [Google Scholar]
- 51.Nilsson A: Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim Biophys Acta 164: 575–584, 1968 [DOI] [PubMed] [Google Scholar]
- 52.Schmelz EM, Crall KJ, Larocque R, Dillehay DL, Merrill AH Jr: Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutr 124: 702–712, 1994 [DOI] [PubMed] [Google Scholar]