Abstract
OBJECTIVE—SIRT1, a class III histone/protein deacetylase, is known to interfere with the nuclear factor-κB (NF-κB) signaling pathway and thereby has an anti-inflammatory function. Because of the central role of NF-κB in cytokine-mediated pancreatic β-cell damage, we postulated that SIRT1 might work in pancreatic β-cell damage models.
RESEARCH DESIGN AND METHODS—RINm5F (RIN) cells or isolated rat islets were treated with interleukin-1β and interferon-γ. SIRT1 was activated by resveratrol, a pharmacological activator, or ectopic overexpression. The underlying mechanisms of SIRT1 against cytokine toxicity were further explored.
RESULTS—Treatment of RIN cells with cytokines induced cell damage, and this damage was well correlated with the expression of the inducible form of nitric oxide (NO) synthase (iNOS) and NO production. However, SIRT1 overexpression completely prevented cytokine-mediated cytotoxicity, NO production, and iNOS expression. The molecular mechanism by which SIRT1 inhibits iNOS expression appeared to involve the inhibition of the NF-κB signaling pathway through deacetylation of p65. In addition, SIRT1 activation by either resveratrol or adenoviral-directed overexpression of SIRT1 could prevent cytokine toxicity and maintain normal insulin-secreting responses to glucose in isolated rat islets.
CONCLUSIONS—This study will provide valuable information not only into the mechanisms underlying β-cell destruction but also into the regulation of SIRT1 as a possible target to attenuate cytokine-induced β-cell damage.
Diabetes, which is characterized at the cellular level by a deficit in β-cell mass, is increasing to epidemic proportions. However, the mechanisms underlying β-cell destruction are not clear, although it has been suggested that cytokines may be involved. For example, in type 1 diabetes, cytokines are important mediators in the impaired function and destruction of pancreatic β-cells. In pancreatic islets, interleukin (IL)-1β, either alone or in combination with interferon (IFN)-γ, causes the production of nitric oxide (NO) through the inducible form of NO synthase (iNOS) (1–4).
IL-1β exerts its primary effects through the transcriptional nuclear factor-κB (NF-κB) pathway. NF-κB is initially located in the cytoplasm in an inactive form complexed with inhibitor of κB (IκB), an inhibitory factor of NF-κB. Various inducers can cause dissociation of this complex, presumably by phosphorylation of IκB, resulting in NF-κB being released from the complex. NF-κB then translocates to the nucleus, where it interacts with specific DNA recognition sites to mediate gene transcription such as iNOS and cyclooxygenase-2 (5–7). NF-κB signaling is modulated by posttranslational modifications, including reversible acetylation of the p65 subunit (8). Five main acetylation sites have been identified within p65, and the modification of these sites modulates both the DNA-binding and transcriptional activities. Acetylation at Lys221 enhances DNA binding by p65 and impairs its assembly with IκBα, whereas acetylation of Lys310 is required for full transcriptional activity of p65 (9). Therefore, NF-κB–dependent transactivation depends on the balance between acetylation and deacetylation status of NF-κB.
SIRT1 belongs to class III histone/protein deacetylases (HDACs) and is a member of the silent information regulator (Sir2) family. Unlike class I and II HDACs, which use zinc as a cofactor (10), SIRT1 consumes one NAD+ for every acetyl group removed from a protein substrate (11). SIRT1, through its deacetylase activity, plays pivotal roles in various cellular processes, including gene silencing, metabolism, stress resistance, and life span extension in response to caloric restriction (11,12). SIRT1 also promotes cell survival or inhibits apoptotic cell death by deacetylating the p53 (13), Ku70 (14), and forkhead transcription factor (15,16). Thus, SIRT1 is regarded as a key regulator of cell defense and survival under various stress conditions.
One of the hallmarks of calorie restriction is reduced inflammation, and a key regulator of inflammation is NF-κB (17–19). Recently, Yang et al. (20) reported that cigarette smoke decreased the SIRT1 level and increased acetylation of p65, which was concomitant to increased NF-κB–dependent proinflammatory mediator release. Considering NF-κB as a direct target of SIRT1, their results imply that SIRT-1 may play an important role in the inflammatory process. SIRT1 is constitutively expressed in the endocrine cells of the islets of Langerhans. To our knowledge, the only known function of SIRT1 in the islets is improving insulin secretion by repressing uncoupling protein 2 (21,22). In this study, we have uncovered a new role of SIRT1 in the protection of pancreatic β-cells from cytokine toxicity.
RESEARCH DESIGN AND METHODS
Cell culture and reagents.
RINm5F (RIN) cells were purchased from the American Type Culture Collection. IL-1β and IFN-γ were obtained from R&D Systems (Minneapolis, MN). The retroviral vector expressing human SIRT1 (wild-type [WT]-SIRT1 and pYESir2), the retroviral vector expressing a dominant-negative form of human SIRT1 (dominant-negative [DN]-SIRT1 and pYESir2HY), and the backbone vector for pYESir2 and pYESir2HY, pBABE, were purchased from Addgene (Cambridge, MA). Amphophenix cells for packaging retrovirus were provided by G. Nolan (Stanford University, Stanford, CA). All reagents were purchased from Sigma (St. Louis, MO) unless otherwise noted.
Animals.
Five-week-old male ICR mice were purchased from the Orientbio (Seoungnam, Korea). To induce multiple low-dose streptozotocin (MLDS)-induced diabetes, once a day for 5 consecutive days, the mice received an injection of freshly prepared streptozotocin (50 mg/kg body wt i.p.; 0.2 ml) dissolved in sodium citrate buffer (pH 4.0). Eight-week-old female NOD mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed at our animal facility for 8 weeks. All experimental procedures were approved by the Institutional Animal Care and Use Committee at Chonbuk National University.
Isolation of islets and glucose-stimulated insulin secretion assay.
Pancreatic islets were isolated from male Sprague-Dawley rats using the collagenase digestion method, as described previously (23). Islets were pretreated with resveratrol or infected with Ad-SIRT1 and then cultured for 24 h with 2 ng/ml IL-1β and 100 units/ml IFN-γ. The islets were then washed three times in Krebs-Ringer bicarbonate buffer (25 mmol/l HEPES, 115 mmol/l NaCl, 24 mmol/l NaHCO3, 5 mmol/l KCl, 1 mmol/l MgCl2, 2.5 mmol/l CaCl2, and 0.1% BSA, pH 7.4) containing 3 mmol/l d-glucose, and insulin secretion assays were then performed in the presence of either 5.5 or 20 mmol/l d-glucose. The insulin content of the medium was then determined by ELISA kit (Linco Research, St. Charles, MO).
Retroviral infection of WT-SIRT1 and DN-SIRT1.
Amphophenix cells were transfected with retroviral vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), and on the following day, the media were changed. Thirty hours after media change, retroviral particle-containing supernatants were harvested and filtered through a 0.45-μm low-protein binding filter (Millipore, Bedford, MA). RIN cells were transduced with virus supernatants on a nontissue culture plate coated with recombinant human fibronectin fragment CH296 (Retronectin, Takara Shuzo, Japan). Two days later, the transduced cells were selected with 5 μg/ml puromycin (Sigma) for 7 days.
Preparation of the recombinant adenovirus.
To prepare SIRT1-expressing adenovirus, the mouse SIRT1 cDNA was cloned into the KpnI and XhoI sites of pENTR 2B (Invitrogen), and the SIRT1 cDNA insert was transferred to the pAd/CMV/V5-DEST vector (Invitrogen) by the Gateway system using LR Clonase (Invitrogen). The plasmids linearized with PacI (Promega, Madison, WI) were transfected into 293A cells using Lipofectamine 2000. Then 293A cells were cultured for 1–2 weeks in RPMI 1640 containing 10% fetal bovine serum (FBS), with replacement of the medium every 2 days. As a control, the pAd/CMV/V5-GW/lacZ vector (Invitrogen) was used to produce lacZ-bearing adenovirus.
SIRT1 HDAC assay.
Cells (5 × 106) were homogenized in 100 μl CytoBuster Protein Extraction Buffer (Novagen, Madison, WI). For immunoprecipitation of SIRT1, primary antibody for SIRT1 (1 μg) was mixed with precleared lysates for 1 h at 4°C before the addition of 20 μl protein agarose A/G, and reactions were tumbled overnight at 4°C. The agarose beads were extensively washed until the next day and followed by HDAC assay. Equal amounts of lysates were analyzed for enzyme activity using the HDAC fluorescence activity assay kit from Millipore.
Cell viability of RIN cells and islets.
The viability of RIN cells was determined by assaying the reduction of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) to formazan. A cell proliferation enzyme-linked immunosorbent assay (5-bromo-2-deoxyuridine [BrdU] kit; Amersham Biosciences, Piscataway, NJ) was used to measure the incorporation of BrdU during DNA synthesis following the manufacturer's protocols. Islet viability was evaluated by acridine orange and propidium iodide staining under fluorescence microscopy. Briefly, islets that had been treated with cytokines were stained with 10 μg/ml acridine orange and 1 μg/ml propidium iodide for 10 min at 37°C. After careful washing, islets were placed on coverslips and examined by fluorescence microscopy. Live cells were identified by a green staining (acridine orange staining), whereas dead cells were brown-red (propidium iodide staining). For histological analysis, islets were fixed overnight in a solution of 6.5% glutaraldehyde. The islets were then centrifuged in 1% agarose solution and embedded in paraffin. Four-micrometer sections were prepared, and cell viability was determined by hematoxylin-eosin staining. The sections were labeled for anti-insulin antibody (Santa Cruz Biochemicals, Santa Cruz, CA) or anti-glucagon antibody (Dako, Carpinteria, CA) and then examined by microscopy.
Nitrite measurement.
After treatment with cytokine for 24 h, 100-μl aliquots of the culture supernatants were incubated with 100 μl modified Griess reagent (1:1 mixture of 1% sulfanilamide in 30% acetic acid and 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride in 60% acetic acid) at room temperature for 5 min, at which time the absorbance at 540 nm was measured using a spectrophotometer.
Whole-cell and nuclear protein extracts.
Cells were washed with PBS and lysed in CytoBuster Protein Extraction Buffer (Novagen). The lysate was centrifuged at 10,000g for 5 min at 4°C, and the supernatant was used as the whole-cell protein extract. Cytoplasmic and nuclear extracts were prepared from cells using the NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL).
Western blot analysis.
RIN cells or islets were homogenized in 100 μl ice-cold lysis buffer (20 mmol/l HEPES, pH 7.2, 1% Triton X-100, 10% glycerol, 1 mmol/l phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin). The homogenates, which contained 20 μg protein, were then separated by SDS-PAGE and transferred to nitrocellulose membranes. The blot was probed with a 1 μg/ml primary antibodies for SIRT2,3,4, p65, β-actin, proliferating cell nuclear antigen (PCNA; Santa Cruz Biochemicals), SIRT1,5,7 (Lifespan Biosciences, Seattle, WA), or acetyl-lysine p65 (Abcam, Cambridge, MA) and then detected with horseradish peroxidase–conjugated IgG (Zymed, South San Francisco, CA).
RNA isolation and real-time PCR.
RNA was isolated using Trizol reagent (Invitrogen). RNA was precipitated with isopropanol and dissolved in diethyl pyrocarbonate–treated distilled water. Total RNA (2 μg) was then treated with RNase-free DNase, and first-strand cDNA was generated using the random hexamer primer provided in the first-strand cDNA synthesis kit (Applied Biosystems, Foster City, CA). Specific primers for each gene (Table 1) were designed using primer express software (Applied Biosynthesis). The control 18S ribosomal RNA was purchased from Applied Biosystems and used as the invariant control. The real-time PCR, which was contained in a final volume of 10 μl, consisted of 10 ng reverse-transcribed total RNA, 167 nmol/l forward and reverse primers, and 2× PCR master mix. The PCR was carried out in 384-well plates using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems). All reactions were conducted in triplicate.
TABLE 1.
Sequences and accession numbers for primers used in real-time RT-PCR
| Gene | Sequences for primers | Accession no. |
|---|---|---|
| iNOS | FOR: TGGTGAAAGCGGTGTTCTTTG | NM_012611 |
| REV: ACGCGGGAAGCCATGA | ||
| COX-2 | FOR: TGCTCACTTTGTTGAGTCATTCAC | NM_017232 |
| REV: CATTCCTTCCCCCAGCAA | ||
| HO-1 | FOR: CAGCCCCACCAAGTTCAAA | BC010757 |
| REV: CAGCCCCACCAAGTTCAAA | ||
| MnSOD | FOR: GGGCTGGCTTGGCTTCA | NM_017051 |
| REV: AGCAGGCGGCAATCTGTAA | ||
| HSP70 | FOR: GGCTGATCGGACGGAAGTT | NM_021863 |
| REV: ACGGCCAGTGCTTCATATCC |
COX-2, cyclooxygenase-2; FOR, forward; HO-1, heme oxygenase-1; HSP70, heat shock protein 70; MnSOD, mitochondrial superoxide dismutase; REV, reverse.
Electrophoretic mobility shift assay.
The activation of NF-κB was assayed by a gel mobility shift assay using nuclear extracts from control and treated cells. An oligonucleotide containing the κ-chain binding site (κB, 5′-CCGGTTAACAGAGGGGGCTTTCCGAG-3′) was synthesized and used as a probe for the gel retardation assay. The two complementary strands were then annealed and labeled with [α-32P]dCTP. Labeled oligonucleotides (10,000 cpm), 10 μg nuclear extracts, and binding buffer [10 mmol/l Tris-HCl, pH 7.6, 500 mmol/l KCl, 10 mmol/l EDTA, 50% glycerol, 100 ng poly(dI·dC), and 1 mmol/l dithiothreitol] were then incubated for 30 min at room temperature in a final volume of 20 μl. Next, the reaction mixtures were analyzed by electrophoresis on 4% polyacrylamide gels in 0.5 × Tris-borate buffer, and the gels were then dried and examined by autoradiography.
NF-κB luciferase assay.
Cells were seeded on 24-well culture plates at 2 × 104 cells/well and adapted for 12 h. Cells were incubated for 1 h with a total of 170 ng plasmids (85 ng NF-κB–dependent luciferase reporter and 85 ng pcDNA3–β-gal), 1 μl Tfx-50 reagent (Promega), and 200 μl serum-free RPMI. In all, 800 μl RPMI containing FBS was then added, and incubation continued. After 24 h of incubation, cells were treated with cytokine for 1 h. Luciferase activity was measured using a luciferase assay system and normalized against β-galactosidase activity.
Statistical analysis.
Statistical analysis of the data was performed using ANOVA and Duncan's test. Differences with a P < 0.05 were considered statistically significant.
RESULTS
Decrease of SIRT1 protein levels in cytokine-treated RIN cells and islets.
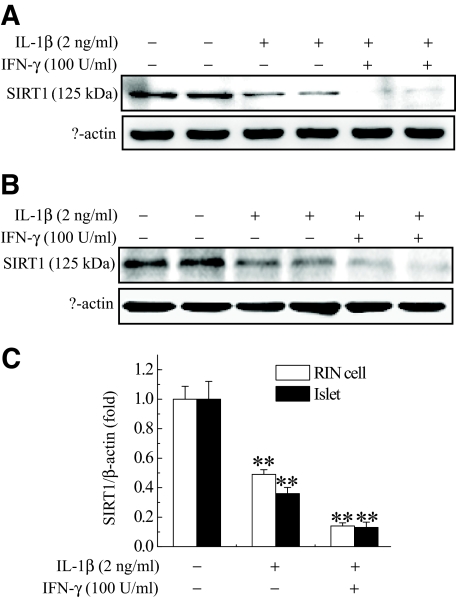
Western blotting data showed that various isoforms of SIRT proteins were expressed in RIN cells with varying degrees (supplementary Fig. S1, available in an online appendix at http://dx.doi.org/10.2337/db07-1795). To investigate whether SIRT1 is involved in cytokine toxicity in pancreatic β-cells, we compared SIRT1 protein levels in control and cytokine-treated RIN cells (Fig. 1A). Moderate levels of SIRT1 were detected in RIN cells, and SIRT1 protein level was decreased by 2-ng/ml IL-1β treatment. When RIN cells were treated with 2 ng/ml IL-1β plus 100 units/ml IFN-γ, SIRT1 protein level was almost completely lost. We further investigated this decline in isolated rat islets. Similar level of SIRT1 was expressed in isolated rat islets, and its expression was also decreased by cytokine treatment (Fig. 1B). To examine whether SIRT1 protein level was also decreased in diabetic animal models, we immunostained SIRT1 on pancreatic sections of MLDS-treated (supplementary Fig. S2A, available in the online appendix) or NOD mice (supplementary Fig. S2B). As Moynihan et al. (22) reported, SIRT1 was expressed in pancreatic islets of control mice; however, a relatively lower level of SIRT1 was detected in our diabetic animal models. These results suggest that the decrease of SIRT1 in pancreatic β-cells is related to the type 1 diabetes development.
FIG. 1.
Decreased expression of SIRT1 by cytokine in RIN cells (A) and isolated rat islets (B). RIN cells (5 × 106) or islets (30) were treated with IL-1β (2 ng/ml) alone or IL-1β and IFN-γ (100 units/ml). After 48 h of incubation, Western blotting for SIRT1 was conducted. C: Protein bands were detected using the enhanced chemiluminescence detection system and analyzed by imaging densitometry. Each value represents the mean ± SE of six independent experiments. **P < 0.01 vs. untreated control.
Prevention of IL-1β–and IFN-γ–induced cell death by SIRT1 overexpression.
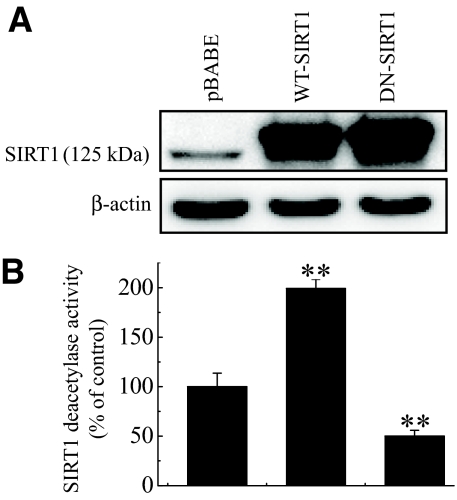
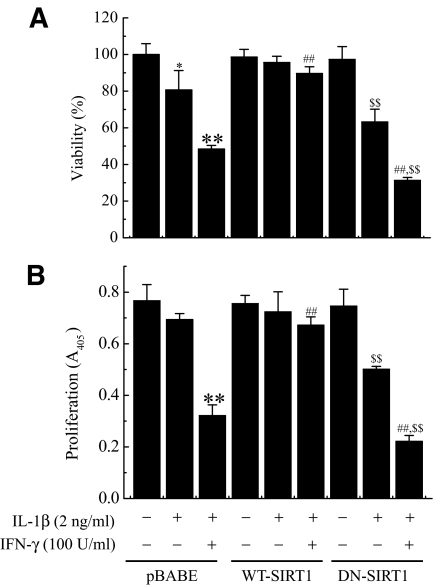
A reduction in SIRT1 protein levels in cytokine-induced pancreatic β-cell toxicity model raises the possibility that overexpression of WT- or DN-SIRT1 might modulate the cytokine toxicity. To address this question, RIN cells were infected with retroviruses expressing WT-SIRT1 or DN-SIRT1. Overexpression of SIRT1 was demonstrated by Western blotting and HDAC activity assay (Fig. 2A and B). Next, we compared control and SIRT1-overexpressing RIN cells on cytokine toxicity. RIN cells were exposed to cytokine for 48 h, and their viabilities were assessed using an MTT assay (Fig. 3A). Treatment with IL-1β alone or IL-1β and IFN-γ significantly reduced the cell viability to 80.6 ± 10.6 and 48.4 ± 1.7% of that of the control, respectively. Conversely, SIRT1-overexpressing RIN cells were resistant against cytokine, whereas DN-SIRT1–overexpressing RIN cells were more sensitive to cytokine. The protective effect of SIRT1 overexpression on cytokine-induced cytotoxicity was further confirmed by measuring the BrdU incorporation in RIN cells (Fig. 3B). Results were similar to those of MTT assay.
FIG. 2.
Ectopic overexpression of SIRT1 regulates HDAC activity in RIN cells. A: RIN cells (5 × 106) were transduced with retroviruses expressing WT-SIRT1 or DN-SIRT1, and RIN cell extracts prepared 48 h after viral infection were subjected to Western blot analysis with anti-SIRT1 antibody. B: Deacetylase activity of overexpressed SIRT1 was measured using fluorogenic substrate as described in research design and methods. Each value represents the mean ± SE of three independent experiments. **P < 0.01 vs. pBABE.
FIG. 3.
Effects of SIRT1 overexpression on cytokine-induced cytotoxicity. Control or SIRT1-overexpressing RIN cells (1 × 105) were treated with IL-1β alone or IL-1β and IFN-γ for 48 h. The cell viability (A) and cell proliferative potential (B) were then determined using an MTT and BrdU incorporation assay, respectively. Each value represents the mean ± SE of three independent experiments. *P < 0.05, **P < 0.01 vs. untreated pBABE; ##P < 0.01 vs. IL-1β + IFN-γ–treated pBABE; $$P < 0.01 vs. untreated DN-SIRT1.
Effect of SIRT1 overexpression on IL-1β–and IFN-γ–induced iNOS expression and NO production.
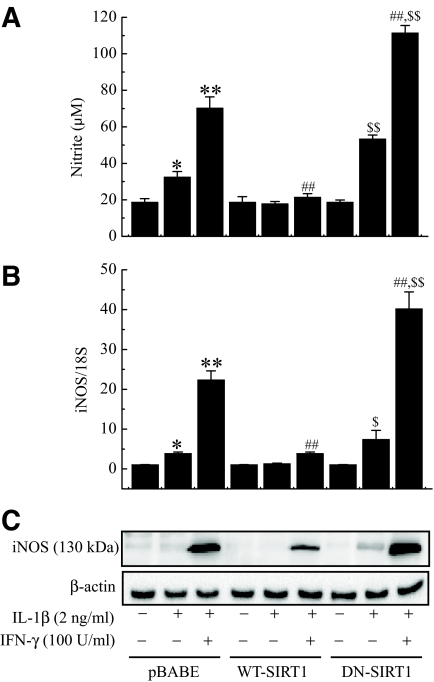
It has been reported that IL-1β–and IFN-γ–mediated destruction of β-cells is caused by an increase of NO (24,25). Incubation of RIN cells with cytokine for 24 h resulted in significant production of nitrite (a stable oxidized product of NO) by these cells (Fig. 4A). However, SIRT1 overexpression significantly diminished the cytokine-mediated nitrite production, and this reduction was well correlated with the lowered cytotoxicity of the cells (Fig. 3). To examine whether SIRT1 overexpression inhibited NO production via suppression of iNOS expression, the changes in iNOS mRNA and proteins were investigated by real-time PCR and Western blot analysis, respectively (Fig. 4B and C). iNOS mRNA and protein expressions were markedly increased in cells treated with cytokine, whereas SIRT1-overexpressing cells showed suppressed expression of both the mRNA and protein levels of iNOS. In contrast, nitrite production and iNOS expression were significantly increased in DN-SIRT1–overexpressing RIN cells compared with control cells (P < 0.01).
FIG. 4.
Effect of SIRT1 overexpression on cytokine-induced NO production and iNOS mRNA and protein expressions. Control or SIRT1-overexpressing RIN cells were treated with IL-1β alone or IL-1β and IFN-γ for 24 h, and nitrite production (A) and iNOS mRNA (B) and protein (C) expressions were determined. The results of three independent experiments are expressed as the means ± SE. *P < 0.05, **P < 0.01 vs. untreated pBABE; ##P < 0.01 vs. IL-1β + IFN-γ–treated pBABE; $P < 0.05, $$P < 0.01 vs. untreated DN-SIRT1.
Effect of SIRT1 overexpression on IL-1β–and IFN-γ–induced activation of NF-κB pathway.
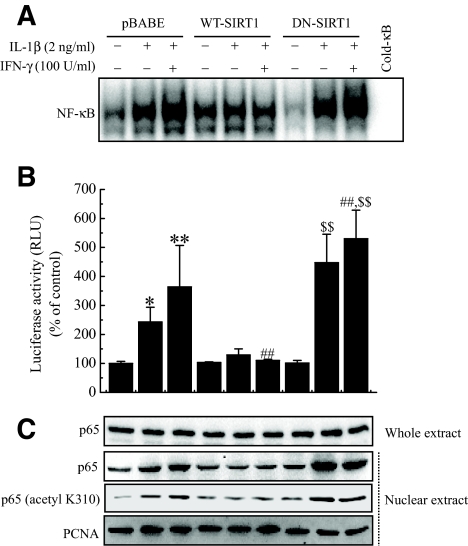
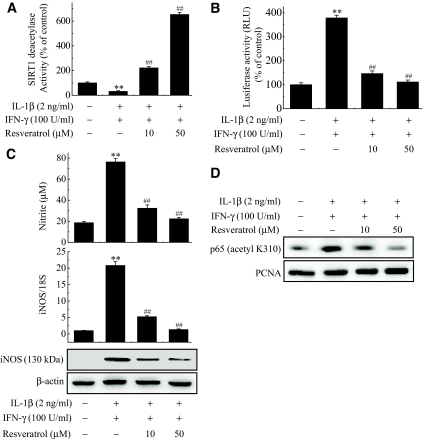
NF-κB has been implicated in the transcriptional regulation of cytokine-induced iNOS expression. Therefore, we studied the effect of SIRT1 on the cytokine-stimulated NF-κB activation pathway. Cytokine-treated RIN cells showed increased DNA binding and transcriptional activities of NF-κB, as demonstrated by electrophoretic mobility shift assay (EMSA) and luciferase reporter assay, respectively (Fig. 5A and B). Additionally, Western blot analysis confirmed the increased nuclear translocation of the p65 subunit (Fig. 5C). However, SIRT1-overexpressing cells showed significant decrease of NF-κB activity compared with control cells. Much evidence has shown that acetylation of p65 at lysine residues (particularly at 310) is required for the full transactivation function of p65 (9,26,27). We therefore determined whether the SIRT1 overexpression has any role on NF-κB acetylation. To address this question, cytokine was added for 1 h, and whole-cell lysates were prepared and analyzed by Western blotting using anti–acetyl-lysine p65 antibody. The addition of cytokine effectively acetylated p65 in RIN cells. On overexpression, SIRT1 suppressed the cytokine-induced acetylation of p65, whereas DN-SIRT1–overexpressing cells increased the acetylation of p65 compared with control cells (Fig. 5C). Because SIRT1 overexpression protected cytokine-induced β-cell death, we tested whether resveratrol, a SIRT1 activator, modulated the cytokine toxicity. We first confirmed the activation of HDAC activity in RIN cells after treatment with resveratrol (Fig. 6A). Similar to the results obtained using the SIRT1-overexpressing RIN cells, pretreatment with resveratrol abolished the effects of the cytokines and resulted in levels of NO production and iNOS expression in terms of both mRNA and protein levels that were similar to those of the controls (Fig. 6B). Additionally, NF-κB transcriptional activity and acetylation of p65 induced by cytokine treatment was markedly suppressed by resveratrol (Fig. 6C and D).
FIG. 5.
Effect of SIRT1 overexpression on cytokine-induced NF-κB activation and translocation and acetylation of p65. Control or SIRT1-overexpressing RIN cells were treated with IL-1β alone or IL-1β and IFN-γ. After 1 h of incubation, DNA binding (A) and transcriptional (B) activities of NF-κB were analyzed by EMSA and luciferase reporter assay, respectively. C: The nuclear translocation of p65 and acetylation of p65 at K310 were determined by Western blotting. PCNA was used as loading control for nuclear protein. Each value represents the mean ± SE of three independent experiments. *P < 0.05, **P < 0.01 vs. untreated pBABE; ##P < 0.01 vs. IL-1β + IFN-γ–treated pBABE; $$P < 0.01 vs. untreated DN-SIRT1. PCNA, proliferating cell nuclear antigen; RLU, relative light unit.
FIG. 6.
Inhibition of cytokine-induced activation of the NF-κB pathway by resveratrol in RIN cells. RIN cells were treated with IL-1β and IFN-γ with or without a 3-h pretreatment with resveratrol. SIRT1 deacetylase activity was determined 1 h later (A), and nitrite production and iNOS mRNA and protein expressions were determined 24 h later (C). Transcriptional activity of NF-κB (B) and acetylation of p65 (D) were analyzed by luciferase reporter assay and Western blotting, respectively. Each value represents the mean ± SE of three independent experiments. **P < 0.01 vs. untreated control; ##P < 0.01 vs. IL-1β + IFN-γ.
Prevention of IL-1β–and IFN-γ–induced β-cell damage in islets by resveratrol or Ad-SIRT1.
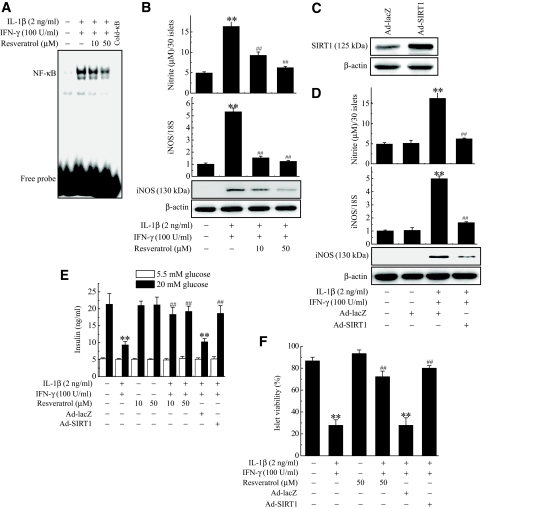
We further assessed the preventive effects of SIRT1 activation using rat pancreatic islets isolated from male Sprague-Dawley rats to support the physiological importance of the results obtained in the cell line studies. Incubation of rat islets with cytokines resulted in increases of DNA binding of NF-κB, NO production, and iNOS expression in rat islets, and pretreatment of the islets with resveratrol abolished all of the effects of the cytokine (Fig. 7A and B). Next, overexpression of SIRT1 was achieved by infection of islets with Ad-SIRT1 (Fig. 7C). Again, SIRT1-overexpressing islets showed significant decrease of nitrite production and iNOS expression compared with control islets (Fig. 7D). To add functional data, insulin secretion was observed in response to 20 mmol/l glucose. Control islets secreted insulin at a concentration of 21.3 ± 3.1 ng/ml, whereas insulin secretion from cytokine-treated islets decreased significantly to 9.3 ± 1.0 ng/ml (P < 0.01) (Fig. 7E). As expected, pretreatment with resveratrol or SIRT1 overexpression blocked the effect of the cytokine and restored islet cell insulin secretion to the control level. Enhanced SIRT1 function by resveratrol or SIRT1 overexpression increased the resistance of β-cells to cytokine toxicity, which resulted in the increased viability of islets (Fig. 7F). Histological analysis stained for insulin or glucagon antibody confirmed that protective effect of SIRT1 was β-cell specific (supplementary Fig. S3, available in the online appendix).
FIG. 7.
Inhibition of NF-κB–mediated cytotoxic pathway and restoration of glucose-stimulated insulin secretion by resveratrol and Ad-SIRT1 in rat islets. Rat islets (30) were treated with IL-1β and IFN-γ with or without a 3-h pretreatment with resveratrol. DNA binding of NF-κB (A) was then determined 1 h later, and nitrite production and iNOS mRNA and protein expressions (B) were determined 24 h later. Rat islets (50) were infected with 1 × 109 pfu Ad-SIRT1 or Ad-lacZ for 12 h and exposed to IL-1β and IFN-γ for 24 h. C: Islets extracts prepared 24 h after viral infection were subjected to Western blot analysis with anti-SIRT1 antibody. D: The effects of overexpressed SIRT1 on IL-1B–and IFN-γ–induced nitrite production, iNOS mRNA expression, and protein expression were determined. Rat islets (10) were treated with IL-1β and IFN-γ with or without a 3-h pretreatment with resveratrol or Ad-SIRT1 infection. E: After 24 h of incubation, glucose-stimulated insulin secretion was quantified. F: Islet viability was analyzed by microscopic analysis after staining with acridine orange and propidium iodide as described in research design and methods. Viable islets were counted manually and represented as percentage viability. Results of triplicate samples are expressed as the mean ± SE. **P < 0.01 vs. untreated control; ##P < 0.01 vs. IL-1β + IFN-γ.
DISCUSSION
SIRT1, a class III histone/protein deacetylase, interferes with the NF-κB signaling pathway and thereby has an anti-inflammatory function (20). Because of the central role of NF-κB in cytokine-mediated pancreatic β-cell damage, we postulated that SIRT1 might work in cytokine-induced pancreatic β-cell damage models. Our results provide evidence that SIRT1 has a protective effect against cytokine in RIN cells and islets for the following reasons. First, addition of cytokine to RIN cells and islets decreased SIRT1 protein levels. Second, SIRT1 overexpression protected RIN cells from cytokine, whereas DN-SIRT1 overexpression rather aggravated its cytotoxicity. SIRT1 overexpression suppressed the NF-κB transactivation potential, NF-κB–dependent iNOS expression and NO formation, and cytotoxicity induced by cytokine in RIN cells. Third, resveratrol and Ad-SIRT1 were also capable of protecting islets by stimulating SIRT1 and maintaining normal insulin secretion capacity. These results lead us to suggest the hypothesis that SIRT1 plays a cytoprotective role against cytokine in pancreatic β-cells.
We first showed that SIRT1 protein levels were decreased by cytokine both in RIN cells and islets. In this regard, a recent report by Sun et al. (28) showed the downregulated SIRT1 protein levels in insulin-resistant C2C12 myotubes and high-fat diet–fed mice. Using different approaches, other groups also found that stress-induced cell damage or dysfunction was also associated with SIRT1 depletion (29,30). Thus, our findings together with their results imply that SIRT1 may modulate the sensitivity of cells against various types of stress response through regulation of its protein levels or enzyme activities.
Several cytokines regulate inflammatory responses in pancreatic β-cells by modulating the NF-κB signaling pathway. One of these is IL-1β, a proinflammatory cytokine that has been implicated in early events in β-cell destruction. Suppression of IL-1β production or inhibition of its interaction with the corresponding cellular receptors significantly inhibits IL-1β–mediated deleterious effects on β-cells (31–33). IL-1β activates the extracellular signal-regulated kinase-1/2, p38 kinase, and c-Jun NH2-terminal kinase in pancreatic β-cells (34,35). However, as we and others have found, IL-1β exerts its main effects through the NF-κB pathway (1,3,23). Therefore, IL-1β–NF-κB signaling pathway is considered as the central in cytokine-induced β-cell destruction, and NF-κB has been targeted for studies of the prevention of islet destruction (36–38). Because NF-κB is a molecular target for cytokine toxicity and deacetylation by SIRT1, we investigated the changes of NF-κB signaling pathways in cytokine-treated SIRT1-overexpressing RIN cells. SIRT1 overexpression led to inhibition of NF-κB pathway. Compared with control cells, SIRT1 overexpression decreased DNA binding and transcriptional activities, as demonstrated by Western blot analysis, and decreased nuclear translocation of p65 protein, as demonstrated by EMSA and immunofluorescence staining. Consistent with this, nuclear translocation of p65 and DNA binding were increased in DN-SIRT1–overexpressing RIN cells than control cells, which is associated with increased susceptibility to cytokine toxicity. We found that SIRT1 overexpression could block cytokine-induced NF-κB downstream gene iNOS expression. Besides iNOS, various other genes, including cyclooxygenase-2, heat shock protein 70, heme oxygenase-1, and mitochondrial superoxide dismutase, are also regulated by NF-κB in pancreatic β-cells (39,40). As expected, SIRT1 overexpression moderately decreased cytokine-induced expression of all of the aforementioned genes (supplementary Fig. S4, available in the online appendix). Therefore, we cannot exclude the possibility that SIRT1 induces the beneficial effect by regulating those proteins. Interestingly, protective effects of SIRT1 in pancreatic β-cells were cytokine specific. When SIRT1-overexpressing RIN cells were exposed to palmitate (lipotoxicity), H2O2 (oxidative stress), or thapsigargin (ER stress), protective effects of SIRT1 were not observed in our experimental conditions (supplementary Fig. S5, available in the online appendix). This is in contrast with a recent study obtained from the high-fat diet–fed β-cell–specific SIRT1 (BESTO) mice, where they still maintained enhanced glucose-stimulated insulin secretion and improved glucose tolerance (41). These differences might result from the methodological differences. We used cultured cells and primary rat islets (type 1 diabetes model), whereas they obtained in vivo results from a type 2 diabetes model. No clear insights could be provided at present, and further studies are required to understand the exact mechanism.
The mechanism of cytoprotection involves a number of factors, and SIRT1 could participate in this pathway by affecting target protein levels by transcriptional silencing through histone modification. Alternatively, it might deacetylate nonhistone proteins such as NF-κB in a context-specific manner. In proving the molecular mechanisms underlying NF-κB inactivation by SIRT1, we focused on the acetylation status of p65. Yeung et al. (9) demonstrated that p65 could be acetylated at five lysine residues: Lys122, Lys123, Lys218, Lys221, and Lys310. Among these, SIRT1 physically interacts and deacetylates Lys310 of p65 without affecting the other lysine residues. In support of this, our data also showed that NF-κB activation by cytokine was associated with acetylation of p65 at Lys310. In contrast, SIRT1 overexpression induced deacetylation of p65, which impedes this protein from controlling the inflammatory response. It is not clear whether SIRT1 affects other lysine residues. These results are in accordance with the hypothesis that deacetylation of NF-κB by SIRT1 is an important mechanism for cytoprotection. In addition to SIRT1 overexpression, we further studied whether the pharmacological activation of SIRT1 by resveratrol could protect islets from cytokine toxicity. Resveratrol mimicked the effects of SIRT1 overexpression, leading to inactivation of NF-κB and downregulation of iNOS expression in response to cytokine treatment. In addition, resveratrol-treated cells produced a lower amount of NO than control cells, which leads to resistance to cytokine toxicity. Considering the similarities between resveratrol and SIRT1 overexpression with regard to the inhibition of NF-κB in both RIN cells and pancreatic islets, SIRT1 activation could be a potential target for the development of a type 1 diabetes drug.
Until now, the only known function of SIRT1 in pancreas was the regulation of insulin secretion through suppression of uncoupling protein 2. Pancreatic β-cell–specific SIRT1-transgenic mice showed enhanced glucose-stimulated insulin secretion (21,22). Here, we showed an additional effect of SIRT1: Through inhibition of NF-κB by deacetylating p65, it could protect β-cells from cytokine. The precise mechanism by which SIRT1 inhibits cytokine-mediated β-cell death needs to be further elucidated; however, our results suggest that SIRT1 may have diverse roles in addition to regulating insulin secretion in pancreatic β-cells and that it constitutes a novel mechanism for treating type 1 diabetes.
Supplementary Material
Acknowledgments
This work was supported by the Ministry of Science and Technology/Korea Science and Engineering Foundation through the Diabetes Research Center at Chonbuk National University (R13-2008-005-0000-0).
No potential conflicts of interest relevant to this article were reported.
We thank Roger H. Unger (University of Texas Southwestern Medical Center at Dallas) for critically reading the manuscript.
Published ahead of print at http://diabetes.diabetesjournals.org on 13 November 2008.
J.-H.L., M.-Y.S., and E.-K.S. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA: IL-1 produced and released endogenously within human islets inhibits β cell function. J Clin Invest 102: 516–526, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cetkovic-Cvrlje M, Eizirik DL: TNF-α and IFN-γ potentiate the deleterious effects of IL-1β on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine 6: 399–406, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Corbett JA, McDaniel ML: Intraislet release of interleukin 1 inhibits β cell function by inducing β cell expression of inducible nitric oxide synthase. J Exp Med 181: 559–568, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heitmeier MR, Scarim AL, Corbett JA: Interferon-γ increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem 272: 13697–13704, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle PA, Henkel T: Function and activation of NF-κB in the immune system. Annu Rev Immunol 12: 141–179, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Baldwin AS Jr: The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol 14: 649–683, 1996 [DOI] [PubMed] [Google Scholar]
- 7.May MJ, Ghosh S: Signal transduction through NF-κB. Immunol Today 19: 80–88, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Perkins ND: Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene 25: 6717–6730, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW: Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernick M, Fierke CA: Zinc hydrolases: the mechanisms of zinc-dependent deacetylases. Arch Biochem Biophys 433: 71–84, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Yang T, Fu M, Pestell R, Sauve AA: SIRT1 and endocrine signaling. Trends Endocrinol Metab 17: 186–191, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Michan S, Sinclair D: Sirtuins in mammals: insights into their biological function. Biochem J 404: 1–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W: Negative control of p53 by Sir2α promotes cell survival under stress. Cell 107: 137–148, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA: Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME: Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L: Mammalian SIRT1 represses forkhead transcription factors. Cell 116: 551–563, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharya A, Chandrasekar B, Rahman MM, Banu J, Kang JX, Fernandes G: Inhibition of inflammatory response in transgenic fat-1 mice on a calorie-restricted diet. Biochem Biophys Res Commun 349: 925–930, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Tellejohan R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP: Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med 42: 665–674, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim YJ, Kim HJ, No JK, Chung HY, Fernandes G: Anti-inflammatory action of dietary fish oil and calorie restriction. Life Sci 78: 2523–2532, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Yang SR, Wright J, Bauter M, Seweryniak K, Kode A, Rahman I: Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol 292: L567–L576, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L: Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol 4: e31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S: Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab 2: 105–117, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Kim EK, Kwon KB, Koo BS, Han MJ, Song MY, Song EK, Han MK, Park JW, Ryu DG, Park BH: Activation of peroxisome proliferator-activated receptor-γ protects pancreatic β-cells from cytokine-induced cytotoxicity via NFκB pathway. Int J Biochem Cell Biol 39: 1260–1275, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Eizirik DL, Flodstrom M, Karlsen AE, Welsh N: The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic β cells. Diabetologia 39: 875–890, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Flodstrom M, Welsh N, Eizirik DL: Cytokines activate the nuclear factor κB (NF-κB) and induce nitric oxide production in human pancreatic islets. FEBS Lett 385: 4–6, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Fischle W, Verdin E, Greene WC: Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293: 1653–1657, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Chen LF, Mu Y, Greene WC: Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J 21: 6539–6548, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q: SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab 6: 307–319, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Deng XQ, Chen LL, Li NX: The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int 27: 708–715, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Zhang J: The direct involvement of SirT1 in insulin-induced IRS-2 tyrosine phosphorylation. J Biol Chem 282: 34356–34364, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Amoli MM, Larijani B: Would blockage of cytokines improve the outcome of pancreatic islet transplantation? Med Hypotheses 66: 816–819, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Giannoukakis N, Rudert WA, Ghivizzani SC, Gambotto A, Ricordi C, Trucco M, Robbins PD: Adenoviral gene transfer of the interleukin-1 receptor antagonist protein to human islets prevents IL-1β–induced β-cell impairment and activation of islet cell apoptosis in vitro. Diabetes 48: 1730–1736, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Tellez N, Montolio M, Biarnes M, Castano E, Soler J, Montanya E: Adenoviral overexpression of interleukin-1 receptor antagonist protein increases β-cell replication in rat pancreatic islets. Gene Ther 12: 120–128, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Ammendrup A, Maillard A, Nielsen K, Aabenhus Andersen N, Serup P, Dragsbaek Madsen O, Mandrup-Poulsen T, Bonny C: The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic β-cells. Diabetes 49: 1468–1476, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Larsen CM, Wadt KA, Juhl LF, Andersen HU, Karlsen AE, Su MS, Seedorf K, Shapiro L, Dinarello CA, Mandrup-Poulsen T: Interleukin-1β-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem 273: 15294–15300, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Eldor R, Yeffet A, Baum K, Doviner V, Amar D, Ben-Neriah Y, Christofori G, Peled A, Carel JC, Boitard C, Klein T, Serup P, Eizirik DL, Melloul D: Conditional and specific NF-κB blockade protects pancreatic β cells from diabetogenic agents. Proc Natl Acad Sci U S A 103: 5072–5077, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giannoukakis N, Rudert WA, Trucco M, Robbins PD: Protection of human islets from the effects of interleukin-1β by adenoviral gene transfer of an IκB repressor. J Biol Chem 275: 36509–36513, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Mabley JG, Hasko G, Liaudet L, Soriano F, Southan GJ, Salzman AL, Szabo C: NFκB1 (p50)-deficient mice are not susceptible to multiple low-dose streptozotocin-induced diabetes. J Endocrinol 173: 457–464, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Sorli CH, Zhang HJ, Armstrong MB, Rajotte RV, Maclouf J, Robertson RP: Basal expression of cyclooxygenase-2 and nuclear factor-interleukin 6 are dominant and coordinately regulated by interleukin 1 in the pancreatic islet. Proc Natl Acad Sci U S A 95: 1788–1793, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strandell E, Buschard K, Saldeen J, Welsh N: Interleukin-1β induces the expression of hsp70, heme oxygenase and Mn-SOD in FACS-purified rat islet β-cells, but not in α-cells. Immunol Lett 48: 145–148, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Ramsey KM, Mills KF, Satoh A, Imai S: Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell 7: 78–88, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.